Translate this page into:
A Prospective Study to Evaluate the Reliability of Thyroid Imaging Reporting and Data System in Differentiation between Benign and Malignant Thyroid Lesions
Address for correspondence: Dr. M Naren Satya Srinivas, Department of Radiodiagnosis, MV Jayaram Medical College and Research Hospital, Hosakote, Bengaluru, Karnataka, India. E-mail: naren.amc@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
To evaluate diagnostic reliability of the daily use of thyroid imaging reporting and data system (TIRADS) classification proposed by Kwak et al., in differentiating between a benign and a malignant thyroid lesion, to calculate inter-observer variability in the interpretation of each of the TIRADS ultrasound features and to evaluate role of TIRADS system in reducing unnecessary biopsies of benign lesions.
Materials and Methods:
Three hundred and sixty-five patients with clinically suspected thyroid lesions during the period from November 1, 2011, to August 31, 2015, were prospectively scanned on gray-scale and Doppler imaging by six radiologists separately. We used GE VOLUSON 730 PRO machine (GE healthcare, Milwaukee, USA) equipped with a 7.5–12 MHz high-frequency linear array transducer with color and power Doppler capability. We evaluated five sonological features: Internal composition, echogenicity, margins, presence and type of calcification, and shape of the lesion. Based on the TIRADS proposed by Kwak et al., we determined categories of the thyroid lesions. The diagnostic performance of TIRADS classification system was evaluated by comparison with the fine-needle aspiration cytology (FNAC) reports which were subsequently obtained after taking informed consent from the patients. All follicular neoplasms on FNAC were further followed up with excision biopsy and histology. The cytopathological report was used as the standard final diagnosis for comparison. The P value and odds ratio were determined to quantify how strongly the presence or absence of a particular ultrasound feature was associated with benignity or malignancy in the study population. The risk of malignancy was stratified for each TIRADS category-based on the total number of benign and malignant lesions in that category. Cervical lymph nodes were also evaluated for their size, loss of the central, echogenic hilum, presence of irregular and indistinct margin, microcalcification, and necrotic changes. Cohen's Kappa coefficient was determined separately for each of the five TIRADS malignant features to study the inter-observer agreement. Furthermore, the percentage of benign cases that were accurately determined by TIRADS which could have avoided unnecessary FNAC was determined.
Results:
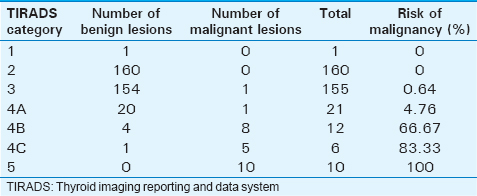
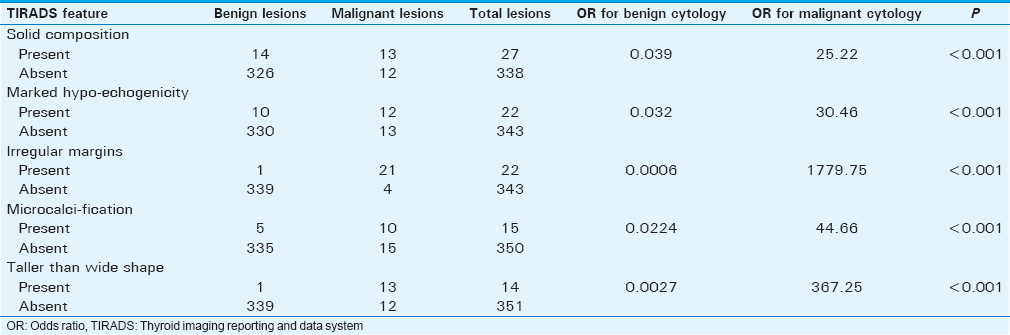
The risk of malignancy in TIRADS categories 1 and 2 was found to be 0%, 0.64% in category 3, 4.76% in category 4A, 66.67% in category 4B, 83.33% in category 4C, and 100% in category 5. Out of the five suspicious sonological features, irregular margins showed the highest positive predictive value (95.45%) for malignancy followed by taller than wide shape (92.86%), microcalcifications (66.67%), marked hypoechogenicity (54.55%), and solid composition (48.15%). The specificity of three sonological features (completely cystic structure, hyperechogenicity, and macrocalcification) in classifying a nodule as benign was 100%. Loss of central echogenic hilum, presence of an irregular and indistinct margin, microcalcification and necrosis were found to have sensitivity of 100%, 63.63%, 27.27%, and 9.09%, respectively and specificity of 95.7%, 98.5%, 100%, and 100%, respectively for cervical lymph node to be malignant. The Kappa value for taller than wide shape, microcalcification, marked hypoechogenicity, solid composition, and irregular margins was 1.0 (95% confidence interval [CI]: 1–1), 1.0 (95% CI: 1–1), 0.90 (95% CI: 0.82–1), 0.88 (95% CI: 0.77–0.92), and 0.82 (95% CI: 0.64–1), respectively. The estimated decrease in unnecessary FNACs was found to be 43.83–86.30%.
Conclusions:
TIRADS proposed by Kwak et al., combined with evaluation for sonological features of malignant lymph nodes is a valuable, safe, widely available, and easily reproducible imaging tool to stratify the risk of a thyroid lesion and helps in precluding unnecessary FNACs in a significant number of patients. TIRADS features convincingly show comparable results in the interpretation of TIRADS features more so, in the hands of radiologists experienced in thyroid imaging.
Keywords
Fine-needle aspiration cytology
thyroid
thyroid imaging reporting and data system
thyroid ultrasound

- M Naren Satya Srinivas
INTRODUCTION
High resolution ultrasound is a valuable, safe, nonionizing, cost effective, widely available, and easily reproducible imaging tool for diagnosis of clinically suspected thyroid lesions. Numerous studies suggest a prevalence of 2–6% of thyroid lesions with palpation, 19–35% with ultrasound, and 8–65% in autopsy data.[1] Although fine needle aspiration cytology (FNAC) is the standard method for evaluation of thyroid nodules, it is painful, incurs health care costs, and contains the risk of infection and bruising.[2] Approximately 10–20% of fine-needle aspiration biopsies are nondiagnostic, in which the thyroid nodule shows a high probability of malignancy, and aspiration needs to be repeated.[3] Furthermore, approximately only 3–7% of thyroid FNACs have conclusive features of malignancy.[4] Hence, there is a growing imperative need to devise and follow a reliable ultrasound classification for the evaluation of the thyroid lesions and differentiating between a benign and malignant lesion with a fair element of confidence, thereby reducing the number of unnecessary invasive biopsies.
Since 2009, many studies have suggested an thyroid imaging reporting and data system (TIRADS) and suitable modifications derived from the widely used and reliable breast imaging reporting and data system (BIRADS) and revealed a number of significant parameters for the quantitative analysis of the ultrasound features.[56] However, the significance of these parameters varied in each study. Most of the previous studies also threw less light on the inter-observer variability using the TIRADS system besides suggesting that the TIRADS system was difficult to apply in the clinical field due to its complexity.
In the TIRADS sonological lexicon, sonological features for the thyroid lesions are solid composition, marked hypoechogenicity, irregular margins, microcalcifications, and taller than wide shape. This study was performed to prospectively investigate the diagnostic reliability of the daily use of TIRADS classification system proposed by Kwak et al., in differentiating between a benign and a malignant lesion by stratifying the risk of malignancy separately for each of these ultrasound features, sonological features of malignant cervical lymphadenopathy, calculate the inter-observer variability in the interpretation of each of these features and also to estimate the decrease in the number of unnecessary biopsies.
MATERIALS AND METHODS
Subject profile
This prospective study was approved by our institutional review board. The study group comprised of 365 consecutive patients, equal to or above 18 years of age, referred to the Department of Radio-diagnosis from Surgical and Medical Departments during the period from November 1, 2011, to August 31, 2015, with clinically suspected thyroid lesions.All these patients underwent high resolution duplex sonography and subsequent ultrasound guided needle biopsy. In cases where surgery was done, these results were followed up with histopathology report. The study required an invasive investigation FNAC to be conducted on patients. Hence, an ethical clearance was obtained from the institution. All patients who have not given consent for FNAC and those with bleeding diathesis were excluded from the study. Cases with nondiagnostic or indeterminate cytology results were also excluded from our study.
Sonography technique
B-mode sonography and Doppler evaluation of the thyroid lesions were performed by 6 radiologists with 2–23 years of experience in thyroid ultrasound. All the ultrasound scans were performed on GE VOLUSON 730 PRO machine (GE healthcare, Milwaukee, USA) equipped with a 7.5–12 MHz high frequency linear array transducer. All images were examined on real-time two-dimensional gray-scale and Doppler imaging. All sonograms obtained were saved in a picture archiving and communication system.
Both lobes of the thyroid gland including the isthmus were evaluated. With the patient supine and neck hyper-extended, the entire gland was examined. Hyperextension of the neck was obtained by placing a pad under the shoulders. The superficial location of the thyroid permitted sonographic demonstration of any subtle anatomical changes.
The neck was scanned in sagittal, transverse, and oblique sections to optimally visualize both lobes of thyroid, isthmus, carotid arteries, as well as internal jugular veins. Imaging of the lower poles of thyroid was improved by making the patient swallow. This tended to raise the thyroid gland in the neck. The region of the carotid arteries and jugular veins laterally and supraclavicular fossa were also examined for any lymphadenopathy.
The image of the thyroid gland was zoomed adequately and suitably to fill the entire viewing monitor which included carotids and jugulars in the field. To obtain the image in the transverse plane, the scan was begun in the midpoint of the neck until thyroid tissue was identified. If the size of the thyroid mass was large and if it was not possible to image right and left lobes simultaneously, then the two lobes were examined separately and later compared bilaterally. To obtain the image in the longitudinal plane, the probe was inclined medially to view the thyroid gland after imaging the carotid artery longitudinally. If needed, the transducer was angulated 10-20° medially. This technique helped to determine if the mass was within the thyroid gland or extra thyroidal. The extra thyroidal masses displaced the carotid artery and internal jugular vein medially.
Image evaluation and data modulation
All the six radiologists separately scanned each of these 365 cases on B-mode sonography and Doppler imaging. Without the help of clinical or histologic information, they evaluated the sonographic features such as the internal composition, echogenicity, margins, presence and type of calcifications, and the shape of all these lesions. In a patient with more than one thyroid nodule, the nodule with the most number of suspicious sonographic features was considered. This nodule was selected after a consensus discussion amongst all the six participating radiologists. In this study, to evaluate the reliability of TIRADS, the findings from all the 365 ultrasound scans performed by the corresponding author were compared to the FNAC reports and histopathology reports whenever surgery was done. These sonological findings obtained by the corresponding author were also compared to the findings obtained from the ultrasound scans performed by the other five radiologists on the same patients to evaluate the inter-observer agreement of the TIRADS features.
The internal component of the suspected lesion was categorized as solid, cystic (anechoic), and mixed (partially solid with cystic component). A solid lesion was confirmed on gray-scale ultrasound when it showed no posterior acoustic enhancement on decreasing the ultrasound gain to a minimum and also when there was any amount of detectable vascularity on color Doppler and power Doppler imaging. The lesion was classified as cystic when the lesion was completely anechoic and displayed an obvious posterior acoustic enhancement on decreasing the ultrasound gain to a minimum. The mixed lesion revealed features of both solid and cystic components. Echogenicity was classified as hyperechogenicity (echogenicity of the nodule more than that of the adjacent thyroid parenchyma), isoechogenicity (echogenicity of the nodule similar to that of the adjacent thyroid parenchyma), hypoechogenicity (echogenicity less than that of the adjacent thyroid parenchyma but more than that of the surrounding strap muscle), or marked hypoechogenicity (echogenicity less than that of the surrounding strap muscle). The margins were classified as irregular (interface between the lesion and the adjacent thyroid parenchyma was indistinct, ill–defined, and not smooth) and regular (smooth, distinct, well-defined, and regular outline). Calcifications, when present, were categorized as microcalcifications (equal to or <1 mm in diameter and visualized as tiny, punctate, hyperechoic foci, either with or without acoustic shadowing, and without comet tail artifacts) or macrocalcifications (hyperechoic foci larger than 1 mm). If the lesion had both microcalcification and macrocalcification, it was classified under microcalcification. Shape was categorized as taller than wide (anteroposterior dimension greater than transverse dimension) or wider than tall (transverse dimension greater than anteroposterior dimension). All the six radiologists then separately reviewed their ultrasound findings and determined the category of a particular lesion according to the TIRADS classification suggested by Kwak et al., (who conducted a retrospective study) wherein, normal thyroid gland was classified under TIRADS 1 (negative), benign nodule under TIRADS 2, probably benign nodule (no suspicious ultrasound features) under TIRADS 3, a nodule with low suspicion for malignancy (one suspicious ultrasound feature) under TIRADS 4A, a nodule with intermediate suspicion for malignancy (two suspicious ultrasound features) under TIRADS 4B, a nodule with moderate concern but not classic for malignancy (three or four suspicious ultrasound features) under TIRADS 4C and a nodule highly suggestive of malignancy (five suspicious ultrasound features) under TIRADS 5. Cervical lymph nodes were evaluated for their size, loss of the central, echogenic hilum, presence of irregular and indistinct margin, microcalcification, and necrotic changes.
Ultrasound-guided fine needle aspiration cytology technique
Before the ultrasound-guided FNAC, the neck was hyper-extended and the skin was cleansed with povidone-iodine (Betadine) solution. The 7.5–12 MHz linear transducer was also cleansed with same solution. Sterile gel was used as a coupling agent. Then, the needle was held in one hand and the transducer in the other. The needle was inserted through the skin of thyroid region in front of the neck at an oblique angle within the image plane of transducer.
The needle used for thyroid FNAC was standard 1½" 25 gauge, non-cutting beveled edge needle. The needle was attached to a 10 ml syringe. After the needle was introduced, it was moved gently but rapidly through the nodule center under ultrasound guidance. Then, gentle suction was done by putting the piston of the syringe. If the specimen contained much blood, a non-aspiration technique was used. In this, 25 gauge needle was inserted under ultrasound guidance into the thyroid gland and no suction was applied and this needle was moved in back and forth excursions. Due to capillary action, the fluid of cells from the nodule moved into the needle. Such fluid specimen was often less bloody.
Two drops of the aspirate/fluid in the syringe were ejected over a clean slide and with the help of the other blank slide at 60° angle, the aspirate on the first slide was spread on it to form a film of coating on it. The slide making procedure was repeated once more and after the second slide was smeared, these slides were put in a jar containing absolute alcohol for fixation. These two slides in alcohol along with container were sent to Pathology department for cytopathological study.
Data and statistical analysis
Microsoft Word and Excel were used to generate the tables. For descriptive statistics, Statistical Package for Social Sciences (SPSS 15.0 for windows; SPSS Inc., Chicago, IL, USA) was used. Specificity, sensitivity, positive and negative predictive value, P value, and the odds ratio were calculated and used to evaluate the reliability of TIRADS method in differentiation between benign and malignant features. The odds ratio was determined to quantify how strongly the presence or absence of a particular ultrasound feature was associated with benignity or malignancy in the study population. The P values were measured using Student's t-test. In all analyses, P < 0.05 was taken to indicate statistical significance. Cohen's Kappa coefficient was determined separately to study the inter-observer agreement among each of the five TIRADS malignant features. Fleiss's guidelines and Landis and Koch's guidelines were applied to grade the degree of inter-observer agreement in our study. Finally, the number of benign cases determined by TIRADS which correlated correctly with the FNAC was calculated and subsequently, the reliability of TIRADS that could have avoided unnecessary FNACs was determined.
RESULTS
Of a total of 365 patients included in this study, there were 343 females (93.97%) and 22 males (6.03%) with a male to female ratio of 1:15.5. We observed a rather higher female to male ratio in our study but then, our study was not a multicentric study and only represented the values obtained from a single hospital. It could also be attributed to a speculative thought that the incidence of the thyroid nodules might be rising in females as compared to the males in the recent years. A total of 365 nodules were studied. The average age of the patients was 33.1 years (range: 18–68 years). The average size of nodules was 14.2 mm (range: 3–59 mm). The range of a benign nodule was 4–43 mm. The range of a malignant nodule was 3–59 mm.
Thyroid imaging reporting and data system categories and risk stratification
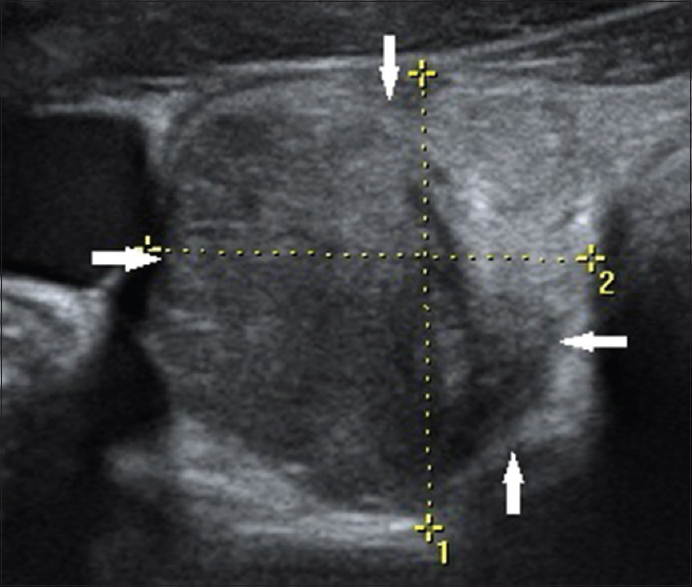
Of the 365 cases studied, there were a total of 340 cases (93.16%) that were proven to be benign and 25 cases (6.84%) that were proven to be malignant cyto-histopatholgically. There was no significant statistical relation between sex of a patient and malignancy. The one clinically suspected lesion that was categorized under TIRADS 1 was a normal anatomical variant where the right lobe and isthmus of the thyroid gland showed smooth, macrolobulated margins along the anterior surface [Figure 1]. In our study, we found the risk of malignancy to significantly rise as the TIRADS category increases. The risk of malignancy in TIRADS categories 1 and 2 was found to be 0%, 0.64% in category 3, 4.76% in category 4A, 66.67% in category 4B, 83.33% in category 4C, and 100% in category 5 [Table 1].
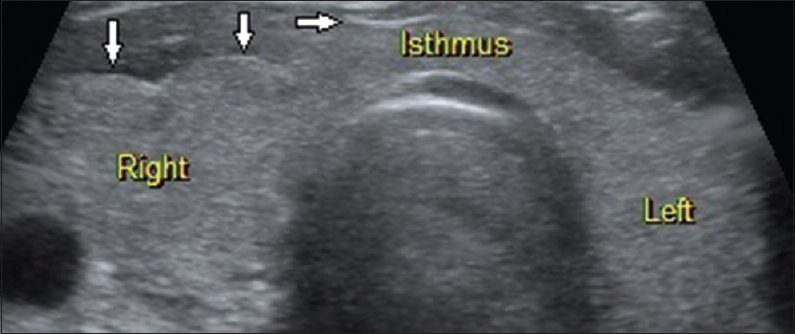
- 38-year-old female presented with dysphagia, breathlessness and lower cervical pain, gray-scale ultrasound image of the thyroid gland in a transverse plane shows essentially normal thyroid gland with smooth, macrolobulations (arrows) along the anterior surface of the right lobe and isthmus (normal anatomical variant; thyroid imaging reporting and data system category 1). Further follow-up attributed the clinical symptoms to a case of systemic sclerosis.
Correlation between thyroid imaging reporting and data system sonological features, benignity, and malignancy
We found that these five sonological features showed a significant association with malignancy: Solid composition, marked hypoechogenicity, irregular margins, microcalcifications, and taller than wide shape. The odds ratio for a lesion with solid composition to be malignant was found to be 25.22 (95% confidence interval [CI]: 9.75–65.20) [Table 2]. The odds ratio for a lesion with marked hypoechogenicity to be malignant was found to be 30.46 (95% CI: 11.14–83.27). The odds ratio for a lesion with irregular margins to be malignant was found to be 1779.75 (95% CI: 190.37–16638.27). The odds ratio for a lesion with microcalcifications to be malignant was found to be 44.66 (95% CI: 13.56–147.06). The odds ratio for a lesion that was taller than wide to be malignant was found to be 367.25 (95% CI: 44.35–3041). Out of the five suspicious sonological features, irregular margins showed the highest positive predictive value (PPV) (95.45%) for malignancy followed by taller than wide shape (92.86%), microcalcifications (66.67%), marked hypoechogenicity (54.55%) and solid composition (48.15%) [Table 3]. Likewise, there were three features which showed a 100% PPV and 100% sensitivity for benignity in our study: Completely cystic composition, hyperechogenicity, and macrocalcifications. There were totally 90 lesions which were completely cystic, 111 lesions which were hyperechoic and 26 lesions with macrocalcifications and all these lesions were confirmed to be benign on cytopathology. The rest of the lesions comprised 248 mixed composition lesions (236 benign and 12 malignant lesions), 139 isoechoic lesions (131 benign and 8 malignant lesions), 93 hypoechoic lesions (88 benign and 5 malignant lesions), 324 lesions with no calcifications, (309 benign and 15 malignant lesions), 343 lesions with regular margins (339 benign and 4 malignant lesions), and 351 wider than tall lesions (339 benign and 12 malignant lesions). Cervical lymphadenopathy was found in 81 out of the 365 cases (70 benign and 11 malignant). The average size of malignant lymph nodes (29.1 mm longitudinally × 25.3 mm transversely) was more than that of benign lymph nodes (24.8 mm longitudinally × 11.0 mm transversely). In 11 malignant nodes, the central fatty echogenic hilum was not visualized in 100% (11 nodes) of these nodes while there was presence of an irregular and indistinct margin in 63.63% (7 nodes), microcalcifications in 27.27% (3 nodes), and necrosis in 9.09% (1 node) of these nodes. We found that non-visualization of the central fatty echogenic hilum, presence of an irregular and indistinct margin, microcalcifications and necrosis showed a specificity of 95.7% (67 out of 70 benign nodes), 98.5% (69 out of 70 benign nodes), 100% (70 out of 70 benign nodes) and 100% (70 out of 70 benign nodes), respectively. No significant differences in the Doppler indices, between a benign and malignant node were noted in our study (Average RI of 0.86 and PI of 1.53 in a malignant node and average RI of 0.80 and PI of 1.40 in a benign node). In 14 of the 25 malignant thyroid nodules in our study, there was no cervical lymphadenopathy.

Inter-observer agreement
In our study, as TIRADS features were interpreted separately by 6 different radiologists, we calculated the Cohen's Kappa value for each of these 5 sonological features suspicious for malignancy. We found that the inter-observer agreement was maximum for taller than wide shape and micro calcifications. The Kappa value for these two features was 1.0 (95% CI: 1–1). The Kappa value for marked hypoechogenicity, solid composition, and irregular margins was 0.90 (95% CI: 0.82–1), 0.88 (95% CI: 0.77–0.92) and 0.82 (95% CI 0.64–1), respectively. We found that there was excellent agreement among the different observers, thus implying that this TIRADS system shows comparable results in the analysis of thyroid nodules by different radiologists in thyroid imaging. We also found that inter-observer agreement among the different observers improved with increasing experience of the observer in thyroid ultrasound.
Estimation of the decrease in unnecessary fine-needle aspiration cytology's using thyroid imaging reporting and data system
The Bethesda system was used to classify cytologic results in our institution. A total of 160 nodules were characterized under category 2 (benign) on TIRADS, all of which were benign on cytopathology. Therefore, the specificity of TIRADS category 2 labeling a nodule benign was 100% [Figures 2 and 3]. A total of 155 nodules were characterized under category 3 (probably benign) on TIRADS, 154 of which were benign on cytopathology. Therefore, the specificity of TIRADS category 3 labeling a nodule benign was 99.35% [Figures 4 and 5]. Just to leave no space for error, even if all the lesions under TIRADS category 2 only were left alone without any further FNAC, the estimated decrease in unnecessary FNACs in our study was found to be 43.83%. If all the cases under TIRADS 2 and 3 were left alone, the estimated decrease in unnecessary FNACs was found to be 86.30% but that carried a malignancy risk of 0.317% (1 in every 317 lesions under this category could be malignant). Cytohistopatholgically, there were 25 proven cases of malignancy, which comprised 17 cases of papillary carcinoma, 4 cases of follicular carcinoma, 3 cases of medullary carcinoma, and 1 case of metastasis to the thyroid gland from a renal cell carcinoma. Except for 1 malignant case (a case of follicular carcinoma) which was classified under TIRADS category 3 (Probably benign), TIRADS classification was able to classify the remaining 24 lesions accurately under category 4A (1 lesion), 4B (8 lesions), 4C (5 lesions), and 5 (10 lesions) [Figures 6–9].
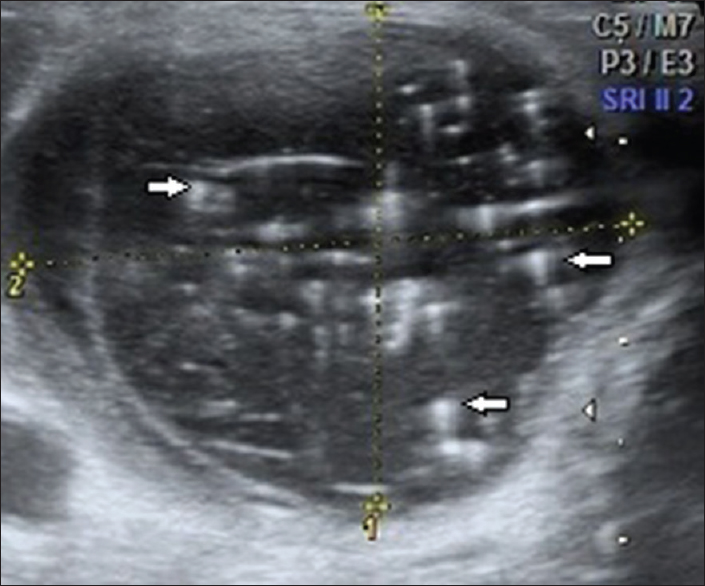
- 23-year-old female presented with painless swelling of the neck, gray-scale ultrasound image of the thyroid gland in a transverse plane shows a wider than tall, well-marginated, anechoic lesion in the right lobe with multiple internal echogenic foci (arrows) casting distal comet-tail artifact (colloid nodule; thyroid imaging reporting and data system category 2).
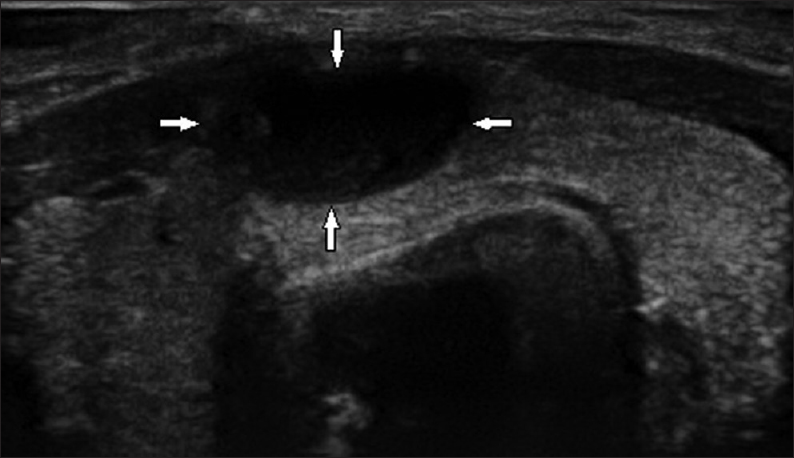
- 19-year-old female presented with a painless swelling of the neck that had been there for 3 years, gray-scale ultrasound image of the thyroid gland in a transverse plane shows a wider than tall, well-marginated, anechoic lesion (arrows) in the isthmus with an imperceptible wall and casting posterior acoustic enhancement (Benign thyroid cyst; thyroid imaging reporting and data system category 2).
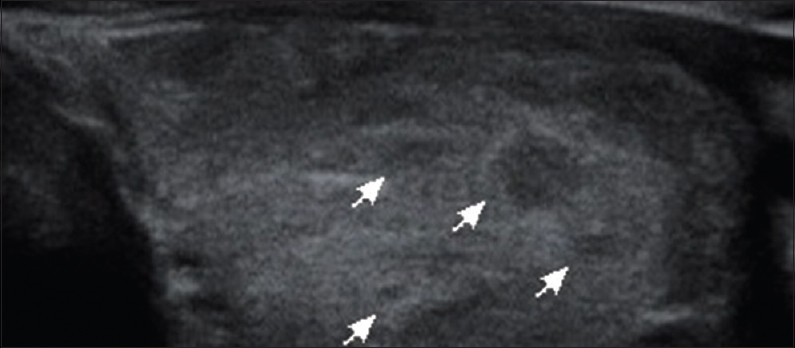
- 42-year-old female presenting with swelling of the neck since 3 months, gray scale ultrasound image of the left lobe of thyroid gland in a transverse plane shows diffuse enlargement with a heterogeneous echotexture, coarse, linear striations and multiple, hypoechoic nodular lesions (arrows) measuring around 1–6 mm each (Hashimoto's thyroiditis/chronic autoimmune lymphocitis; thyroid imaging reporting and data system 3).
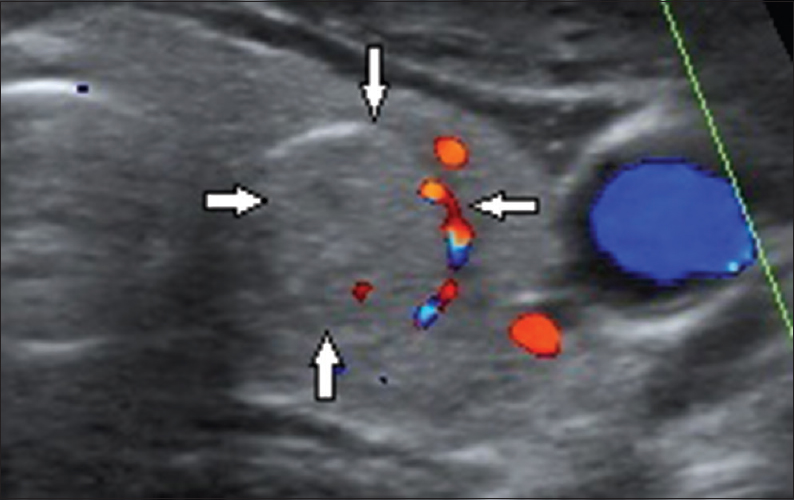
- 35-year-old female presented with swelling of the neck that had been there since 1 month, gray-scale ultrasound image of the thyroid gland in a transverse plane shows a solitary, wider than tall, well-marginated, isoechoic lesion (arrows) in the left lobe with perilesional vascularity (colloid nodule; thyroid imaging reporting and data system category 3).
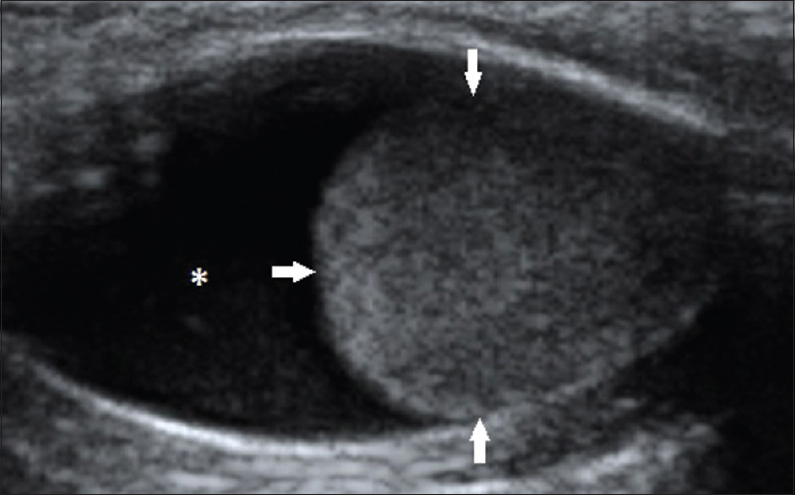
- 37-year-old female presented with swelling of the neck that had been there for 10 months, gray-scale ultrasound image of the left lobe of thyroid gland in a transverse plane shows a solitary, wider than tall, mixed lesion, with a markedly hypoechoic, solid, component (arrows) within a cystic lesion (asterix) (follicular adenoma; thyroid imaging reporting and data system category 4A).
- 59-year-old female presented with swelling of the neck that had been there for 8 months, gray-scale ultrasound image of the right lobe of thyroid gland in a transverse plane shows a solitary, poorly marginated, markedly hypoechoic lesion (arrows) occupying majority of the right lobe (follicular carcinoma thyroid; thyroid imaging reporting and data system category 4B).
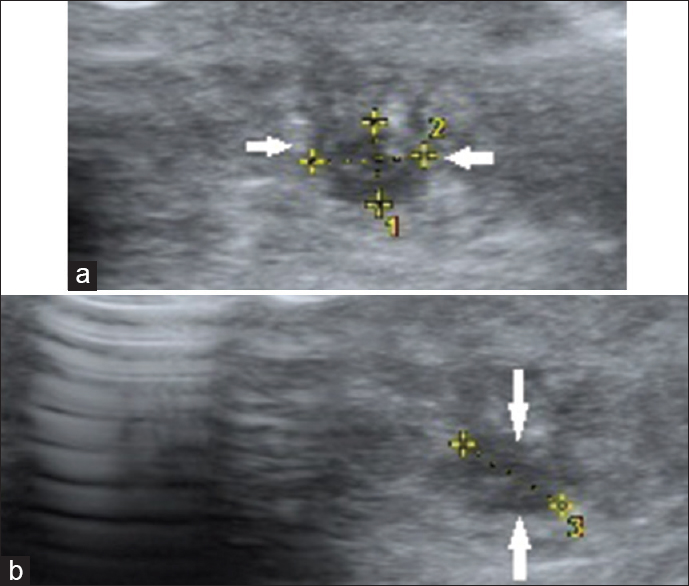
- 35-year-old female presented with a 10-day history of severe neck pain, gray-scale ultrasound images of the thyroid gland in (a) transverse and (b) longitudinal planes show a solitary, irregular, poorly marginated, markedly hypoechoic lesion (arrows) with intralesional punctate, calcific foci in right lobe (medullary carcinoma thyroid; thyroid imaging reporting and data system category 4C).
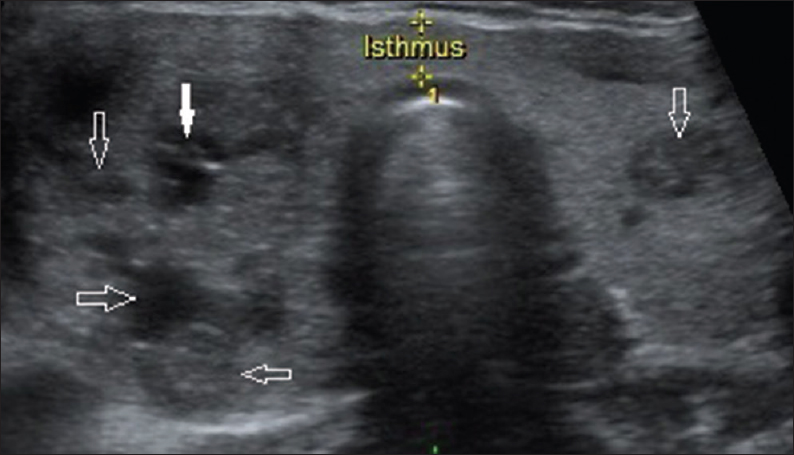
- 38-year-old female presented with a 2-month history of severe neck pain and swelling of the neck, gray scale ultrasound image of the thyroid gland in a transverse plane shows multiple, taller than wide, irregular, poorly marginated, markedly hypoechoic lesions in both lobes, predominantly the right lobe with tiny, scattered, intralesional microcalcifications (solid arrow represents the sampled nodule; hollow arrows represent rest of the nodules) (multifocal papillary carcinoma thyroid; thyroid imaging reporting and data system category 5).
DISCUSSION
Over the past few years, with advances in high resolution ultrasound technology coupled with its widespread availability, there has been a significant increase in the detection of thyroid lesions resulting in a proportional rise in the number of thyroid FNAC procedures and subsequently an increase in number of thyroid carcinomas diagnosed.[78] However, according to the Bethesda system for thyroid cytopathology, approximately just 3–7% of thyroid FNACs have conclusive features of malignancy and a “benign” result is obtained in at least 60–70% of thyroid FNACs.[4] Ultrasound is a safe, noninvasive and easily reproducible imaging modality and suitable sonological criteria could help in precluding these unnecessary invasive FNACs in majority of these cytologically benign thyroid nodules.
Since 2009, many studies have suggested an TIRADS and suitable modifications derived from the widely used and reliable BIRADS and revealed a number of significant parameters for the quantitative analysis of the ultrasound features.[56] However, the significance of these parameters varied in each study. Few studies also suggested that the TIRADS system was difficult to apply in the clinical field due to its complexity.[567]
In the TIRADS sonological lexicon, sonological features for the thyroid lesions are solid composition, marked hypoechogenicity, irregular margins, micro calcifications, and taller than wide shape.[7] Horvath et al., first described 10 sonological patterns of thyroid nodules and related the rate of malignancy according to the pattern.[5] However, these sonological patterns were not applicable to all thyroid nodules and this stereotypic sonological application is difficult to use.[7] Park et al., proposed an equation using a multiple logistic regression analysis for predicting the probability of malignancy in thyroid nodules on the basis of 12 sonological features. Calculation using a regression equation itself is difficult to apply in any circumstance due to the complicated equation and 12 variables of sonological findings. To overcome these limitations of previous studies, Kwak et al., used the number of suspicious sonological features and calculated the fitted probability of malignancy. There apparently appeared to be a limitation to their approach in that each suspicious sonological feature was weighted identically.[9] Thereafter, there have been modifications to TIRADS model by applying a different risk score to each suspicious ultrasound feature which however made it all the more complex for the routine use of TIRADS in the clinical practice. There is a need for simple, uniform guidelines for facilitating sonological reports in order to reduce confusion not just among the referring physicians and patients but among the radiologists too. Therefore, we investigated the basic, simpler TIRADS classification proposed by Kwak et al., for the management of thyroid nodules. Our study differed from the one by Kwak et al., which was a retrospective study in which selection bias could have occurred due to some of their initial population not undergoing follow-up. Furthermore in their study, follow-up management of TIRADS 4A nodules, which have a relatively low risk of malignancy was not evaluated.[7] In addition to our study being a prospective study, we also evaluated the inter-observer agreement of the TIRADS features and estimated the decrease in the number of unnecessary FNACs in this study.
Our results suggested that five independent sonological features are significantly associated with malignant cytology: Solid composition, marked hypoechogenicity, irregular margins, micro calcifications, and taller than wide shape. Among these features, irregular margins were the most sensitive for malignancy followed by taller than wide shape, micro calcification, marked hypoechogenicity, and solid composition in that order. We found that as the number of suspicious TIRADS features increased, the risk of malignancy also increased. Kwak et al., have also observed that irregular margins had the highest odds ratio for malignancy followed by taller than wide shape, marked hypoechogenicity, micro calcification, and solid composition of a thyroid nodule.[7] Unlike their study, we observed that micro calcifications had a higher odds ratio compared to marked hypoechogenicity. Horvath et al., found that hypoechogenicity, irregular shape and margins, multiple peripheral micro calcifications and hypervascularization in combination were all features of malignancy.[5]
Fernandez-Sanchez in his study observed that a modified TIRADS classification where an extra point was added to a TIRADS score when features of malignant cervical lymphadenopathy was found can be applied in daily practice.[10] In our study, we found malignant lymph nodes to be almost rounder in shape due to the little variation between the longitudinal and the transverse lengths of the lymph node. We observed that in the setting of a TIRADS 4 or a TIRADS 5 category lesion, concurrent sonological features of malignancy in a lymph node increased the confidence of the radiologist while labeling a TIRADS lesion as malignant. The most sensitive features for malignant lymph node in our study were found to be non-visualization of the central fatty echogenic hilum, presence of an irregular and indistinct margin, micro calcifications, and necrosis. However, we found no significant differences in the Doppler indices between a benign and malignant node. This might be due to the differences in the cellularity of the lesion. The resistive index can be low in malignant nodes with necrotic changes while it can be high in malignant nodes without any necrotic changes. Kessler et al., showed that 70% of metastatic nodes from papillary thyroid carcinoma had a cystic component.[1112] So, the Doppler values in these cases may be significantly lower than those of malignant nodes which do not have any cystic component.
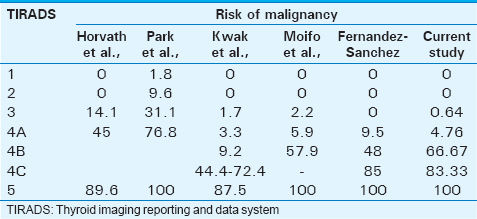
Many authors have stratified the risk of each TIRADS category separately. Though, there were minor differences in these values, they all followed a common pattern, with the risk of malignancy increasing from TIRADS 2 to TIRADS 5 category [Table 4]. The risk of malignancy in our study was 0%, 0.64%, 4.76%, 66.67%, 83.33%, and 100%, respectively for a TIRADS category 2, 3, 4A, 4B, 4C, and 5 lesion. The risk of malignancy/PPV for malignancy of a lesion in TIRADS 2 category was found to be 0% by Horvath et al., Kwak et al., Moifo et al., and Fernandez-Sanchez while it was found to be 1.8% (TIRADS 1 as benign lesion) and 9.6% (TIRADS 2 as probably benign lesion) by Park et al.[5671013] All these studies showed increasing risk and probability of malignancy as the TIRADS category increases with a 89.6%, 100%, 87.5%, 100% and 100% risk of malignancy in TIRADS 5 category by Horvath et al., Park et al., Kwak et al., Moifo et al., and Fernandez-Sanchez, respectively.[5671013]
One common long standing misconception about TIRADS classification is that it may be difficult to apply in routine clinical practice because it is operator dependent. In our study, there were six radiologists who separately performed ultrasound scans on each of these patients and interpreted their findings. We found that there was excellent inter-observer agreement on all the 5 TIRADS features suspicious for malignancy. The maximum Kappa value was observed for taller than wide shape and micro calcification which was 1.0 (95% CI: 1–1) each. The Kappa value for marked hypoechogenicity, solid composition and irregular margins was 0.90 (95% CI: 0.82–1), 0.88 (95% CI: 0.77–0.92) and 0.82 (95% CI: 0.64–1), respectively. Fleiss's guidelines characterize Kappa values over 0.75 as excellent, 0.40–0.75 as fair to good, and below 0.40 as poor. Landis and Koch characterized Kappa <0 as indicating no agreement and 0–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1 as almost perfect agreement. When we applied both these guidelines to the Kappa values in our study, we found that there was “excellent” “almost perfect” agreement among the different observers, thus implying that this TIRADS system shows comparable results in the analysis of these suspicious thyroid nodules by different radiologists in thyroid imaging. Although there was very minimal inter-observer variation, we found that inter-observer agreement amongst the different observers improved with increasing experience of the observer in thyroid ultrasound. Ko et al., observed that both the number of suspicious ultrasound features and the total risk score are applicable and show comparable results in the risk stratification of thyroid nodules even by less experienced radiologists in thyroid imaging.[9] Russ et al., in their prospective study found that the K value for the total TIRADS score was 0.72 (95% CI: 0.62–0.81). When considering only scores 4A, 4B, and 5, which correspond to theoretical indications for biopsy according to the TIRADS system, they observed that the K = 0.74 (95% CI: 0.65–0.84).[14]
Almost all sonological benign characteristics have been studied by many authors previously. However, there is no clear, standard consensus in the present routine clinical practice as to which thyroid nodules be left alone and which be followed up. FNAC is still recommended for most of the sonologically classified “benign” thyroid nodules for confirmation, increasing the health care costs, hospital time and psychological stress to the patient.First, it is a well-documented fact that a “benign” result is obtained in at least 60–70% of thyroid FNACs while approximately only 3–7% of thyroid FNACs have conclusive features of malignancy.[4] Second, nondiagnostic or unsatisfactory samples due to obscuring blood, overly thick smears, air drying of alcohol fixed smears, or an inadequate number of follicular cells occur in 2–20% of cases due to which this invasive procedure has to be repeated all over again.[4] Third, high resolution ultrasound is capable of evaluating an entirely cystic lesion in the thyroid gland while a similar cystic lesion is considered as a clearly identified subset of non-diagnostic/unsatisfactory sample for FNAC.[4] A repeat aspiration is recommended in this group of lesions but some nodules remain persistently nondiagnostic. Fourth, in 3–6% of thyroid FNACs, there is a “atypia of undetermined significance” report while not clearly indicating if a lesion is benign or malignant.[4] Fifth, FNAC does not conclusively and definitively differentiate a benign follicular adenoma from a malignant follicular carcinoma. It requires histopathological examination for confirmation. Sixth, there is a false negative rate of 0–3% in a FNAC classified “benign” lesion.[4] So, FNAC is not completely error-free. Finally, even if FNAC was indeed required, ultrasound is required for guidance in a small nodular lesion which is not palpable or only vaguely palpable as FNAC is a blind approach.
In our study, we tried to establish the sonological features which are highly sensitive for benignity. All the 160 lesions in TIRADS category 2 were found to be benign on FNAC. The sonological features which were found to have 100% sensitivity for benignity in our study were “completely cystic component” of a thyroid nodular lesion, “hyperechogenicity” and presence of “macrocalcification” in the absence of microcalcification. Bonavita et al., in their study also observed that presence of hyperechogenicity or the “white knight” appearance was 100% sensitive for a nodule to be benign.[15] They also observed that a cyst with a colloid clot would not require further biopsy because of its high sensitivity for benignity.[15] Hoang et al., have noted that though a cystic component occurs in 13–26% of all thyroid malignancies, a predominantly cystic appearance is uncommon.[12] Popli et al., in their study observed that all of the 4 cystic nodular lesions and 7 lesions which showed hyperechogenicity in their study were found to be benign on FNAC.[16] Horvath et al., in their study found that anechoic, non-vascularized lesions with hyperechoic spots classified as a colloid type 1 lesion was found to have 0% risk of malignancy.[5] Vinayak and Sande in their study found that all 14 thyroid lesions which had macrocalcifications were found to be benign. They also observed that thyroid ultrasound can accurately characterize benign thyroid nodules using an index scoring system and therefore preclude FNAC in these patients.[17] In our study, we found a 0% risk of malignancy in TIRADS 2 lesions and 0.64% risk of malignancy in TIRADS 3 lesions. So, it can be affirmed confidently that all TIRADS category 2 and 3 lesions can be left alone and regular periodic follow-up for the change in the sonological features of the lesion would suffice and help in reducing unnecessary FNACs by around 43.83–86.30%.
Limitations
The authors recognize few limitations of the study.First, few previous studies have mentioned that combination of elastography with gray-scale ultrasound helps to improve sensitivity and specificity in differentiating benign and malignant lesions and that could have given us slightly lower sensitivity and specificity values as we did not use elastography.[14] However, our primary goal was to focus on the simpler TIRADS ultrasound examination for the routine clinical practice. Second, we used cytologic results as the reference standard for comparison in our study and false negative cytologic results in some thyroid nodules with benign results could have occurred for these nodules without surgical confirmation. However, we point out that suggesting surgery for a lesion that is most likely to be benign is unethical. Third, we did not consider the serum thyroid stimulating hormone levels in this study. A correlation of sonological features with other investigations such as serum thyroid profile and galectin-3 expression analysis could be useful in avoiding the higher costs of thyroid surgical procedures. Finally, the study size in our paper is relatively small (365 lesions) as compared to few other studies on thyroid sonological features such as Cappelli et al., (7455 lesions), Russ et al., (4550 lesions), Kwak et al., in 2011 (3674 lesions), Fernandez-Sanchez (3650 lesions), Kwak et al., in 2013 (2000 lesions), Horvath et al., (1959 lesions), Park et al., (1694 lesions), Kim et al., (1419 lesions), Hong et al., (594 lesions), Bonavita et al., (500 lesions), Cheng et al., (498 lesions) and Moifo et al., (430 lesions).[567101314151819202122] However, we also note that there were numerous other previous studies on thyroid sonological features with a smaller study size like Ardakani et al., (60 lesions), Kim et al., (155 lesions), Lee et al., (191 lesions), Ko et al., (204 lesions), Choi et al., (204 lesions), Vinayak and Sande (284 nodules), and Alexander et al., (330 lesions).[291723242526]
Finally, this paper is unique to the previous studies because this was a prospective study which threw light on all aspects of the very simple, easy to follow TIRADS classification system and its reliability including the role of cervical lymphadenopathy, an estimated decrease in the proportion of unnecessary FNACs and interobserver agreement in interpretation of TIRADS features among different radiologists.
CONCLUSION
The simple Kwak et al., TIRADS classification is a reliable, imaging modality with good interobserver agreement in differentiating benign lesions from the malignant lesions and the presence of sonological features of malignant cervical lymphadenopathy enhances the radiologist's confidence while classifying a lesion as malignant on TIRADS. Furthermore, TIRADS can safely preclude unnecessary FNACs in a significant group of benign thyroid lesions. Currently, there is no standard TIRADS classification system that is widely accepted and followed in the routine clinical practice. We therefore advocate this classification system first proposed by Kwak et al., as a simple to use, reliable, easily reproducible tool toward standardizing and following a sonological protocol and reducing confusion among the referring physicians, radiologists, and the patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2016/6/1/5/177551
REFERENCES
- Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008;22:901-11.
- [Google Scholar]
- Classification of benign and malignant thyroid nodules using wavelet texture analysis of sonograms. J Ultrasound Med. 2015;34:1983-9.
- [Google Scholar]
- Nondiagnostic thyroid fine-needle aspiration cytology: Management dilemmas. Thyroid. 2001;11:1147-51.
- [Google Scholar]
- NCI Thyroid FNA State of the Science Conference. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132:658-65.
- [Google Scholar]
- An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009;94:1748-51.
- [Google Scholar]
- A proposal for a thyroid imaging reporting and data system for ultrasound features of thyroid carcinoma. Thyroid. 2009;19:1257-64.
- [Google Scholar]
- Thyroid imaging reporting and data system for US features of nodules: A step in establishing better stratification of cancer risk. Radiology. 2011;260:892-9.
- [Google Scholar]
- Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164-7.
- [Google Scholar]
- Application of the thyroid imaging reporting and data system in thyroid ultrasonography interpretation by less experienced physicians. Ultrasonography. 2014;33:49-57.
- [Google Scholar]
- TI-RADS classification of thyroid nodules based on a score modified according to ultrasound criteria for malignancy. Rev Argent Radiol. 2014;78:138-48.
- [Google Scholar]
- Cystic appearance of cervical lymph nodes is characteristic of metastatic papillary thyroid carcinoma. J Clin Ultrasound. 2003;31:21-5.
- [Google Scholar]
- US Features of thyroid malignancy: Pearls and pitfalls. Radiographics. 2007;27:847-60.
- [Google Scholar]
- Reliability of Thyroid Imaging Reporting and Data System (TIRADS) classification in differentiating benign from malignant thyroid nodules. Open J Radiol. 2013;3:103-7.
- [Google Scholar]
- Prospective evaluation of thyroid imaging reporting and data system on 4550 nodules with and without elastography. Eur J Endocrinol. 2013;168:649-55.
- [Google Scholar]
- Pattern recognition of benign nodules at ultrasound of the thyroid: Which nodules can be left alone? AJR Am J Roentgenol. 2009;193:207-13.
- [Google Scholar]
- Utility of gray-scale ultrasound to differentiate benign from malignant thyroid nodules. Indian J Radiol Imaging. 2012;22:63-8.
- [Google Scholar]
- Avoiding unnecessary fine-needle aspiration cytology by accuractely predicting the benign nature of thyroid nodules using ultrasound. J Clin Imaging Sci. 2012;2:23.
- [Google Scholar]
- The predictive value of ultrasound findings in the management of thyroid nodules. QJM. 2007;100:29-35.
- [Google Scholar]
- Image reporting and characterization system for ultrasound features of thyroid nodules: Multicentric Korean retrospective study. Korean J Radiol. 2013;14:110-7.
- [Google Scholar]
- Ultrasonographic guideline for thyroid nodules cytology: Single institute experience. J Korean Surg Soc. 2013;84:73-9.
- [Google Scholar]
- Positive predictive values of sonographic features of solid thyroid nodule. Clin Imaging. 2010;34:127-33.
- [Google Scholar]
- Characterization of thyroid nodules using the proposed thyroid imaging reporting and data system (TI-RADS) Head Neck. 2013;35:541-7.
- [Google Scholar]
- New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178:687-91.
- [Google Scholar]
- Differentiation between benign and malignant solid thyroid nodules using an US classification system. Korean J Radiol. 2011;12:559-67.
- [Google Scholar]
- Interobserver and intraobserver variations in ultrasound assessment of thyroid nodules. Thyroid. 2010;20:167-72.
- [Google Scholar]
- Natural history of benign solid and cystic thyroid nodules. Ann Intern Med. 2003;138:315-8.
- [Google Scholar]






