Translate this page into:
A Case of Secondary Angiosarcoma of the Breast after Breast-conserving Surgery and Radiation: Review of Radiologic and Pathologic Findings
Address for correspondence: Dr. Christine N Eppelheimer, Department of Radiology, Mount Sinai Beth Israel, 10 Nathan D Perlman Place, New York - 10003, USA. E-mail: christine.eppelheimer@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Angiosarcoma of the breast is a rare and potentially life-threatening disease. It can present as a palpable mass or subtle erythematous lesion, depending on the predisposing clinical factors. Erythematous skin lesions may be confused for a benign process, which may lead to a delay in diagnosis. We present a case of an 80-year-old woman who developed secondary angiosarcoma after undergoing breast-conserving therapy for Stage IA breast cancer. In this article, we review our experience with a case of secondary angiosarcoma of the breast and discuss the presentation, evaluation, and treatment of this disease. This case demonstrates the importance of vigilance regarding erythematous or papular breast lesions in the setting of prior local radiation.
Keywords
Breast-conserving surgery
cutaneous angiosarcoma
radiation
secondary angiosarcoma

INTRODUCTION
Angiosarcoma of the breast is a rare disease accounting for 0.04% of malignant neoplasms of the breast.[1] Angiosarcomas are a subtype of soft-tissue sarcomas with vascular or endothelial origin. The tumor is composed of complex anastomosing vascular channels which lack a true epithelial component, with the most common histologic appearance described as a vasoformative pattern of growth.[2]
There are two forms of this disease: Primary angiosarcoma and secondary angiosarcoma. Primary angiosarcoma tends to occur in younger patients and is more likely to present as a palpable mass.[3] Secondary angiosarcoma may occur after local radiation or in the setting of chronic post-operative lymphedema (Stewart–Treves syndrome).[4] With the advent of breast-conserving therapy (lumpectomy plus radiation), the incidence of Stewart–Treves syndrome has decreased while that of post-irradiation angiosarcoma has increased.[2] In women with a history of prior breast radiation, the adjusted odds ratio for the development of angiosarcoma was estimated to be 11.6 (95% CI = 4.3–26.1).[5]
Secondary angiosarcoma is also commonly referred to as cutaneous angiosarcoma as it tends to involve the subcutaneous tissues of the breast, while sparing the underlying breast parenchyma. As a result, secondary angiosarcoma most commonly presents as a violaceous or erythematous lesion. However, a small percentage (7%) of patients with secondary angiosarcoma will present with the chief complaint of a palpable mass.[3]
RADIOLOGIC FEATURES
Our patient was an 80-year-old woman with a history of Stage IA invasive ductal carcinoma of the right breast diagnosed in 2008, which was estrogen receptor positive, progesterone receptor negative, and Her2neu negative. She underwent right partial mastectomy and sentinel lymph node biopsy, and received adjuvant whole breast external beam radiation with a standard dose of 5040 cGy and hormonal therapy. Four years after surgery, she presented with a small erythematous lesion on the right breast. The mammogram at that time showed stable post-surgical changes and smooth skin thickening, consistent with prior radiation. A punch biopsy was performed by a dermatologist at another institution and reported it as eczematous dermatitis. The erythematous skin lesion enlarged over the next 2 years to 14 cm [Figure 1]. A repeat punch biopsy then revealed high-grade angiosarcoma.
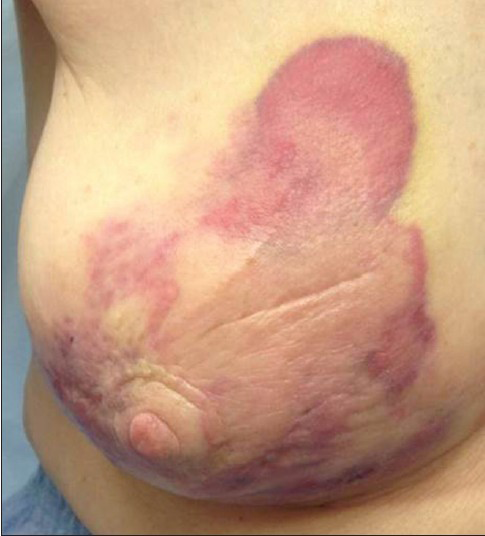
- 80-year-old woman with an erythematous lesion with irregular borders diagnosed with secondary angiosarcoma.
Concurrent mammogram showed increased skin thickening with new irregularity and dimpling of the skin surface [Figure 2]. Skin thickening alone is expected in patients with a history of prior local radiation, but it should be smooth and uniform. Breast sonography demonstrated irregular skin thickening without a discrete underlying breast mass [Figure 3]. Breast magnetic resonance imaging (MRI) also revealed skin thickening with nonspecific foci of enhancement predominantly in the medial and inferior breast [Figure 4]. There was no suspicious enhancing mass or abnormal parenchymal enhancement appreciated on MRI. Our patient then underwent an extended right total mastectomy, with wide resection (5 cm gross margins), and transverse rectus abdominus myocutaneous (TRAM) flap reconstruction [Figure 5]. She has been closely followed with physical exam and has shown no evidence of recurrence at 8 months.
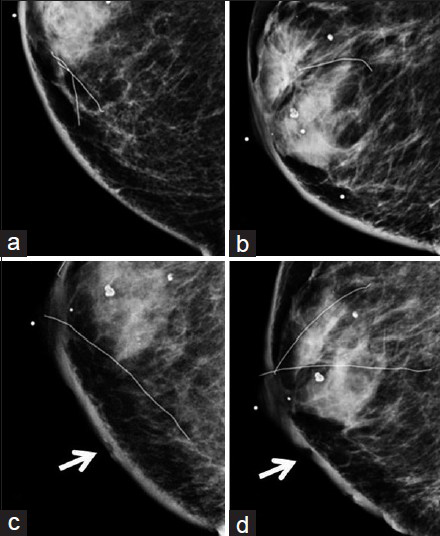
- 80-year-old woman with an erythematous lesion with irregular borders diagnosed with secondary angiosarcoma. (a) Craniocaudal (CC) and (b) mediolateral oblique (MLO) views of the right breast from a mammogram taken 2 years prior to diagnosis of angiosarcoma show smooth skin thickening and post-surgical change consistent with prior lumpectomy and radiation. (c) CC and (d) MLO views of the right breast at the time of diagnosis of angiosarcoma demonstrate interval increased skin thickening, as well as dimpling and irregularity of the skin (arrows).
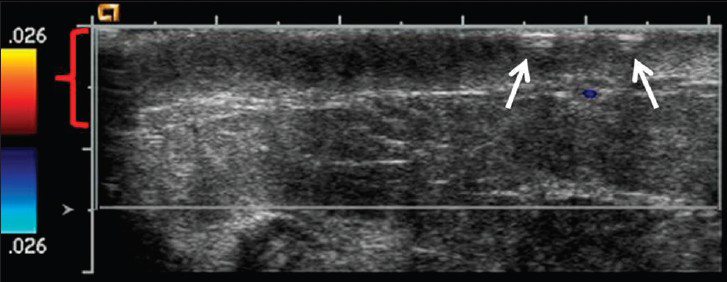
- 80-year-old woman with an erythematous lesion with irregular borders diagnosed with secondary angiosarcoma. Ultrasound image with color Doppler of the right breast reveals skin thickening of the inner central right breast (red bracket) as well as the dimpling and irregularity (arrows). No discrete mass or nodule was identified on ultrasound.
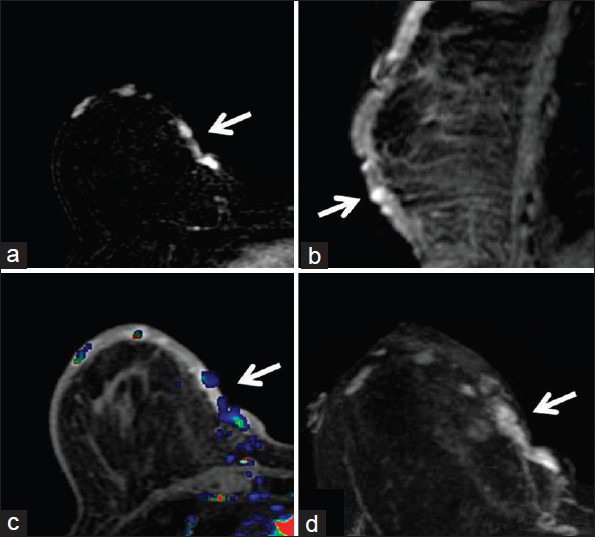
- 80-year-old woman with an erythematous lesion with irregular borders diagnosed with secondary angiosarcoma. (a) MRI subtraction axial image of the right breast demonstrates foci of enhancement medially (arrow). (b) Delayed contrast-enhanced T1-weighted sagittal image of the right breast shows the foci of enhancement (arrow) within the thickened skin of the inferior breast. (c) Early contrast-enhanced T1-weighted axial image with color-coded map of the right breast demonstrates the abnormal enhancement in the medial breast (arrow). (d) Early contrast-enhanced T1-weighted axial image of the right breast shows the foci of enhancement in the medial breast (arrow).
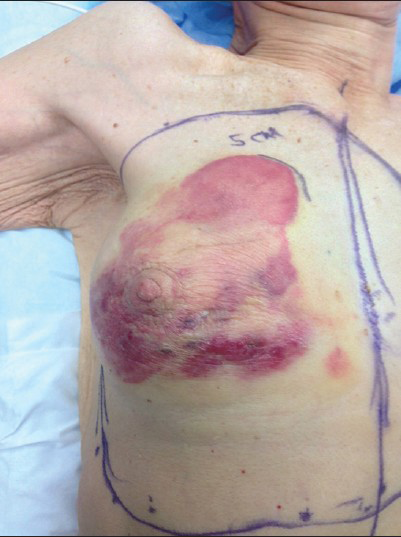
- 80-year-old woman with an erythematous lesion with irregular borders diagnosed with secondary angiosarcoma. Intraoperative photograph shows the extent of angiosarcoma and the outline of planned gross resection margins.
PATHOLOGIC FEATURES
The whole right breast was excised and sent for pathologic examination. A 14.5 × 11.4 × 0.5 cm, irregular, thickened, mildly elevated purple skin lesion was identified surrounding the nipple [Figure 6]. The lesion was located 3.6 cm from the closest resection margin. On sectioning, the discolored lesion was focally hemorrhagic and thickened (up to 0.5 cm in thickness compared to the normal-looking tan skin which measured 0.2 cm in thickness). The remaining breast parenchyma was composed of predominantly soft, yellow tissue admixed with soft, pink tissue.
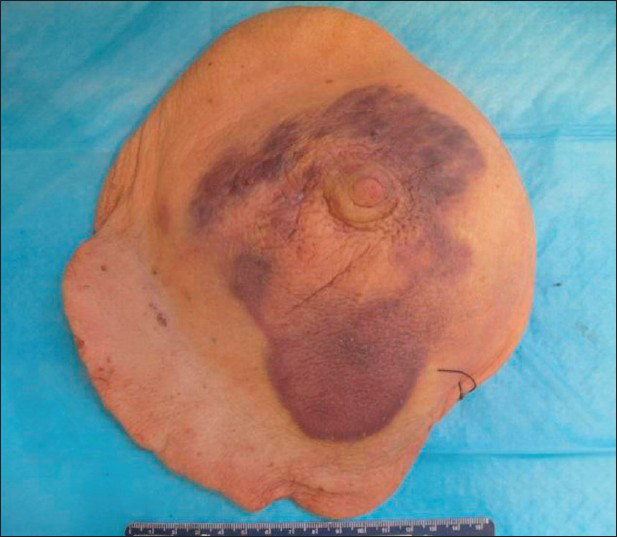
- 80-year-old woman with an erythematous lesion with irregular borders diagnosed with secondary angiosarcoma. Gross pathology image shows a 14.5 × 11.4 × 0.5 cm, irregular, blue/purple skin lesion surrounding the nipple.
Microscopically, examination of the lesion with hematoxylin and eosin (H and E) staining revealed subcutaneous lesion consisting of aggregates of solid spindle cell areas admixed with irregular, variably sized interconnecting vascular channels [Figure 7]. The irregular, interconnecting vascular channels were lined by similar pleomorphic cells with hyperchromic nuclei and a high nuclear to cytoplasm ratio [Figure 8]. The solid, nodular areas were composed of pleomorphic spindle cells with frequent mitotic figures.
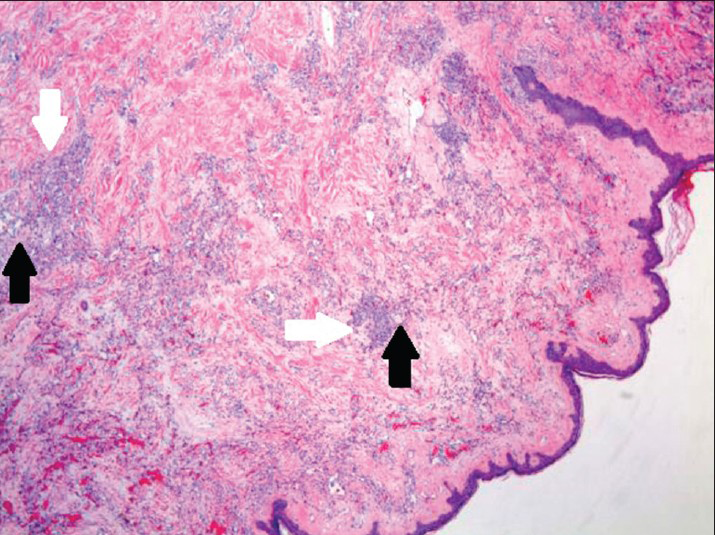
- 80-year-old woman with an erythematous lesion with irregular borders diagnosed with secondary angiosarcoma. Hematoxylin and eosin stained biopsy tissue at low magnification shows subcutaneous aggregates of solid spindle cell areas (white arrows) admixed with proliferation of irregular, variably sized interconnecting vascular channels (black arrows).
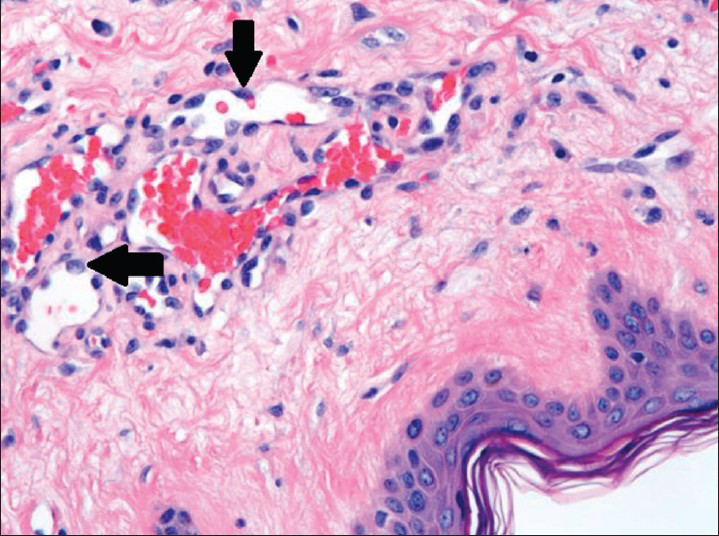
- 80-year-old woman with an erythematous lesion with irregular borders diagnosed with secondary angiosarcoma. Hematoxylin and eosin stained biopsy tissue at higher magnification shows irregular, variably sized interconnecting vascular channels lined by highly pleomorphic neoplastic cells (arrows), which have hyperchromatic nuclei and high nuclear to cytoplasmic ratio.
DISCUSSION
The average age of presentation for patients with secondary angiosarcoma of the breast is 73 years versus 43 years of age for patients with primary angiosarcoma. In addition, secondary angiosarcoma of the breast is more likely to be high grade, compared to primary angiosarcoma. In a series of patients published by Scow et al., only 33% of patients with primary angiosarcoma of the breast had high-grade features compared to 82% of patients with secondary angiosarcoma.[3] The latency period from radiation to development of angiosarcoma is a median of 7 years (range 3–19 years).[5] Our patient initially presented 4 years after breast-conserving surgery and local radiation, with a small erythematous lesion. She was misdiagnosed at that time with eczematous dermatitis. The clinical presentation may vary significantly, with erythematous or violaceous skin lesions being the most common. As a result, a delay in diagnosis is, unfortunately, common.[6]
The imaging features of secondary angiosarcoma can also be variable. Often, the mammogram will show only post-surgical changes and nonspecific skin thickening. This may be mistaken for the usual skin changes related to previous radiation. However, radiation changes would not be expected to increase at a later date, but rather decrease in prominence over time.[7] Comparison with sequential mammograms, not just the patient's most recent study, may reveal subtle developing changes in skin irregularity or dimpling. Also, if skin thickening is irregular, further investigation and a query regarding an associated dermal lesion would be warranted. In the subset of patients who have an associated parenchymal mass, the mammogram may show an ill-defined, asymmetric mass.[4]
Sonography may also demonstrate nonspecific findings such as skin thickening. If an associated mass is seen on ultrasound, it may be circumscribed or ill-defined with associated hypervascularity. Ultrasound may also show a mixed hyperechoic and hypoechoic region without a discrete mass.[1] Breast MRI with gadolinium, although not necessary prior to treatment, may reveal foci of enhancement within the thickened skin and may show either plateau or washout kinetic curve.[8] These foci may be small (less than 1 cm) in size.
We stress that the diagnosis of secondary angiosarcoma of the breast is made with clinical exam and punch biopsy, and imaging plays a complementary role in diagnosis. However, in rare instances, where the patient has been lost to follow-up with interval breast exams by a surgeon or oncologists, irregular, worsening skin thickening noted on routine mammogram and sonogram may prompt a clinician to evaluate the patient more thoroughly. Once the diagnosis is made, imaging plays an important role preoperatively to rule out the rare occurrence of an underlying mass or a co-existing disease prior to surgery.
Overall, secondary angiosarcoma of the breast has a poor prognosis, with a median disease-free survival of 2.41 years.[9] The 5-year disease-specific survival is 47%.[5] At the time of recurrence, 41% of patients will have distant metastases, most commonly to lymph nodes, lungs/pleura, and bone.[5]
Treatment of secondary angiosarcoma involves wide resection with mastectomy. The addition of adjuvant chemotherapy with doxorubicin or paclitaxel-based agents may be considered in patients with advanced-stage or metastatic disease.[10] Prospective studies are needed to determine if adjuvant chemotherapy has an impact on local recurrence or disease-specific survival.
CONCLUSION
Secondary angiosarcoma of the breast is rare; however, its incidence is increasing as the use of breast-conserving therapy has increased. Clinically, patients will commonly present with a papular, erythematous skin lesion. Imaging features, such as progressive skin changes presenting many years after radiation, are important diagnostic clues. Careful attention must be given to new skin lesions in patients with a history of prior breast irradiation, and diagnosis with a punch biopsy or excisional biopsy must be performed urgently. Early recognition can assure that treatment is instituted in order to, hopefully, improve patient outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2015/5/1/45/163989
REFERENCES
- Mammary angiosarcomas: Imaging findings in 24 patients. Radiology. 2007;242:725-34.
- [Google Scholar]
- Cutaneous angiosarcoma following breast-conserving surgery and radiation: An analysis of 27 cases. Am J Surg Pathol. 2004;28:781-8.
- [Google Scholar]
- Primary and secondary angiosarcoma of the breast: The mayo clinic experience. J Surg Oncol. 2010;101:401-7.
- [Google Scholar]
- High-risk features in radiation-associated breast angiosarcomas. Br J Cancer. 2013;109:2340-6.
- [Google Scholar]
- Secondary angiosarcoma of the breast: Can imaging findings aid in the diagnosis? Breast J. 2008;14:293-8.
- [Google Scholar]
- Challenges in the treatment of angiosarcoma: A single institution experience. Am J Surg. 2014;208:254-9.
- [Google Scholar]






