Translate this page into:
A Case of Carotid Body Tumor with Perineural Tumor Spread along the Superior Laryngeal Nerve

*Corresponding author: Ashley Renay Way, Department of Radiology, University of Florida College of Medicine, Jacksonville, Florida, United States. ashleyway@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Way AR, Fiester P, Holtzman A, Rao D. A case of carotid body tumor with perineural tumor spread along the superior laryngeal nerve. J Clin Imaging Sci 2021;11:6.
Abstract
We report a case of a patient with a carotid body tumor with perineural tumor spread along the right superior laryngeal nerve. Perineural spread is most commonly associated with squamous cell, adenoid cystic, and mucoepidermoid carcinoma. To the best of our knowledge, this has not been reported previously with carotid body tumor.
Keywords
Superior laryngeal nerve
Perineural tumor spread
Familial paraganglioma
Carotid body tumor
INTRODUCTION
Paragangliomas (PGs) of the head and neck (HN) are found in characteristic locations, the most common of which is in the carotid body. Carotid body tumors are highly vascular tumors of neuroendocrine origin. Although typically benign, patients can present with symptoms such as sensation related to a growing mass and cranial nerve palsies. Carotid body tumors are typically treated by, or with a combination of, surgical resection, and/or radiotherapy (RT).
CASE REPORT
A 58-year-old female with a history of PG familial form presented to our institution. Approximately 30 years ago, the patient had been diagnosed with left-sided glomus jugulare and carotid body tumors, treated with surgical resection. Postoperatively, she developed a left true vocal cord (TVC) paralysis. A decade later, an asymptomatic right-sided carotid body tumor was discovered on routine surveillance imaging [Figure 1]. This lesion was followed with annual imaging for 6 years [Figure 2], when a CT scan demonstrated interval growth with extension into the right paraglottic soft tissues through the right superior laryngeal nerve (SLN). The patient presented to our institution the following year reporting new dysphagia, hoarseness, and contralateral first bite syndrome [Figure 3].

- A 58-year old woman, axial enhanced computed tomography image of the neck demonstrating a carotid body tumor (yellow arrow) with characteristic splaying the internal (blue) and external (green) carotid arteries.
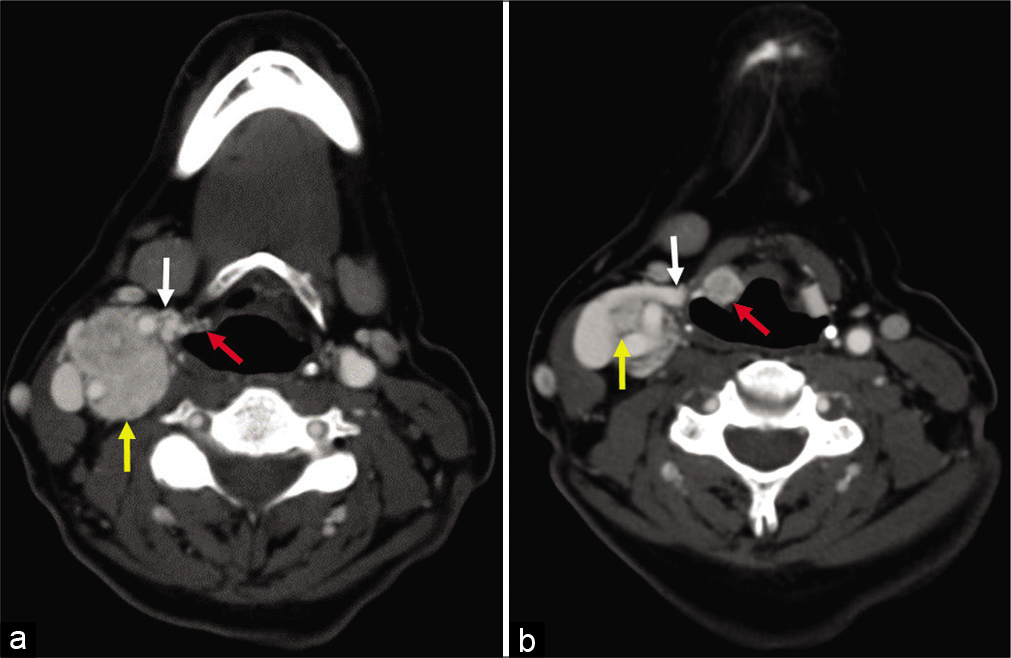
- (a and b) A 58-year-old woman, axial enhanced computed tomography images obtained 5 years after Figure 1, demonstrating interval growth of the paraganglioma (yellow arrow) along the expected course of the superior laryngeal nerve (white arrow) into the paraglottic space (red arrow).
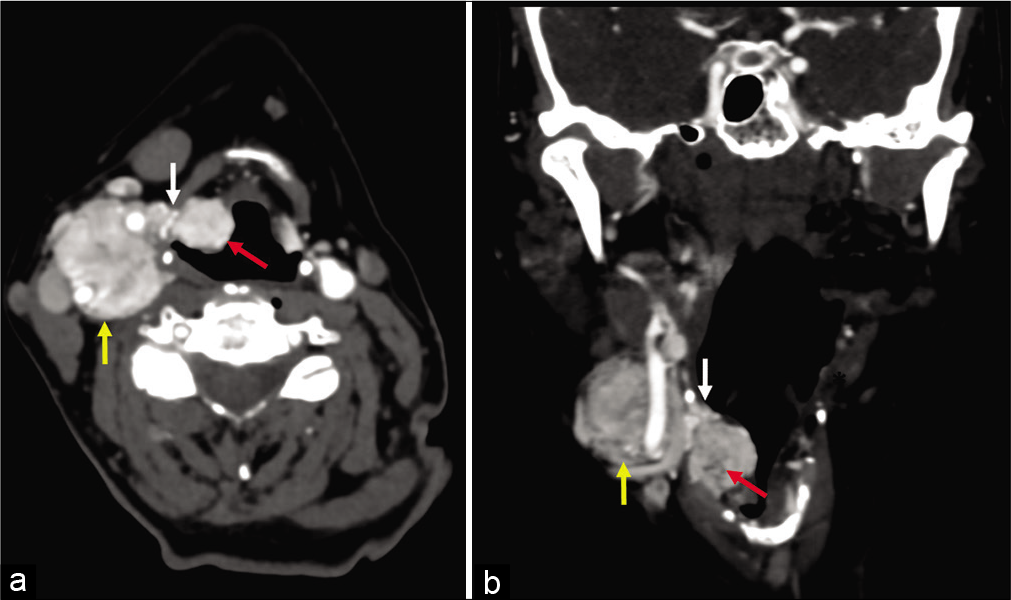
- (a and b) 58-year-old woman, axial and coronal enhanced computed tomography images obtained 5 years after images in Figure 2. There has been interval enlargement of both the primary paraganglioma (yellow arrow) and the lesions within the paraglottic space (red arrow). The pedicle of tumor extending along the superior laryngeal nerve (white arrow) through the thyrohyoid membrane is also larger.
Flexible scope nasopharyngoscopy demonstrated endophytic submucosal volume expansion of the right supraglottic larynx with its epicenter within the right aryepiglottic fold. The mobility of the TVC was noted to be normal on the right, but completely immobile on the left. Positron emission tomography-computed tomography (PET-CT) and CT of the neck with contrast revealed an fluorodeoxyglucose (FDG) avid mass in the right supraglottic larynx and demonstrated enlargement of enhancing masses lateral to the right aryepiglottic fold as well as a PG in the right carotid space. Due to the involvement of the lesion along the right SLN and high risk for surgical injury, and with existing left TVC paralysis, RT was recommended over surgery and observation. The patient chose observation over RT.
DISCUSSION
HN PGs comprise 0.5% of all HN tumors and 3% of all PGs.[1] Up to 50% of PGs are familial, inherited in an autosomal dominant pattern affecting men to women with a 1:1 ratio. They have a peak incidence between the second to fifth decades of life. PGs of the HN carry a natural history 5-year survival rate of 95–99%.[2] The majority of HN PGs are benign, but tumor location and size influence symptomatology. Lesions are commonly located in the carotid body (branchiomeric) followed by the glomus jugulare or glomus vagale (intravagal) and then aorticopulmonary tumors (aortosympathetic).
Mass effect contributes to presenting symptoms at patient evaluation including cranial nerve palsies (e.g., dysphagia, facial weakness, and/or vocal fold paresis), palpable neck mass, tinnitus, vertigo, and/or are incidental findings on imaging studies.[3] Neuronal injury is often explained by nerve compression, vascular steal, and rarely perineural tumor spread. Within the HN, 47% of PGs are located in the carotid body (branchiomeric), 29% in the glomus jugulare or glomus vagale (intravagal), and 4% in aorticopulmonary, mediastinal, intracardiac, or pericaval areas (aortosympathetic).[4] Presenting symptoms are commonly due to mass effect, including 55% palpable neck mass (carotid body tumor), 18% tinnitus (glomus jugulare), 16% cranial nerve palsies, and 10% are observed as incidental findings on imaging studies.[4] Malignant HN PGs are uncommon, with an incidence of 5%.[5]
Duplex ultrasound (US) [Table 1] is an inexpensive initial-evaluation tool for a painless neck mass. Named for their anatomic location and relationship to adjacent structures, PGs will appear similarly on US, which tend to showcase patterns of high vascularity of a solid mass. Carotid body tumors are found at the level of the carotid bifurcation. The identification of the common carotid, internal carotid arteries (ICA), and external carotid arteries (ECA) is distinguishable from the carotid body tumor due to its solid hypoechoic mass.[6] This differs from the location of a glomus vagale which results in anteromedial displacement of the ICA and ECA. Glomus jugulare tumors are typically located at the jugular foramen of the skull base, limiting their evaluation by US.[3] For completion, US is not utilized for assessment of aortosympathetic tumors, most commonly arising in the posterior mediastinum, due to their deep location.[3,7]
| Imaging features of paraganglioma | Duplex ultrasound | Computed tomography | Magnetic resonance | Nuclear medicine |
|---|---|---|---|---|
| General description (all) | Solid, Heterogeneously-hypoechoic, and internal hyper-vascularity Adjacent structure displacement |
Avid mass enhancement with delayed contrast washout relative to adjacent structures | T1-weighted hypointense signal relative to adjacent structures T2-weighted isointense to hyperintense signal |
I-131 and I-123 metaiodobenzylguanidine 111 octreotide, and F-18 PET/CT commonly used Lesion demonstrates focally increased uptake |
| Carotid body (most common head and neck paraganglioma) | Splaying of ICA and ECA Must rule out vagal PG |
Enhanced soft-tissue mass attenuation; splaying of proximal ECA and ICA | T1-post contrast enhanced soft-tissue mass; splaying of proximal ECA and ICA T2-weighted *flow voids (multiple low-signal punctate foci); rule out schwannomas and/or neurofibromas in Carotid Space Angiography reveals “Lyre sign” (see US) |
FDG/PET focal mass uptake area of carotid bifurcation of ECA and ICA |
| Glomus vagale (least common head and neck paraganglioma) | Anteromedial displacement of ICA and ECA | Enhanced soft tissue mass attenuation; anteromedial displacement of ECA and ICA. Osseous erosion of anterior skull base (Proximal vagal) |
T1-post contrast enhanced soft-tissue mass; anteromedial displacement of ECA and ICA T2-weighted *flow voids (multiple low-signal punctate foci) along vagus nerve near jugular foramen |
FDG/PET focal mass uptake in area of proximal ECA and ICA to adjacent jugular foramen |
| Glomus jugulare (second most common head and neck paraganglioma) | Limited by skull base; only detectable by US when tumor extends inferiorly from jugular foramen | Erosion of jugular bulb Bony erosion of jugular foramen walls (temporal bone “moth-eaten appearance”) |
T1-post contrast well defined enhanced soft-tissue mass ; centered in jugular foramen T2-FSE well-defined, hyperintense mass centered in jugular foramen T2-weighted *flow voids (multiple low-signal punctate foci) |
FDG/PET focal mass uptake in jugular bulb within jugular fossa |
| Notes | Evaluation of Palpable HN tumors; location drives naming (i.e., carotid body, vagale, jugulare) | Soft-tissue attenuation, bony erosion, and axon thickening; suspicious for perineural tumor spread | Most sensitive Radiologist should describe degree of vascular encasement of ICA and ECA |
Evaluation for metastatic and or multicentric disease |
ICA: Internal carotid artery, ECA: External carotid artery, *a.k.a. “salt and pepper” appearance
CT [Table 1] is often used during initial diagnosis of HN PG.[8] PGs demonstrate avoid enhancement with delayed contrast washout in relation to the adjacent structures they compromise and is useful for distinguishing glomus vagale from jugulare tumors. Glomus jugulare should be considered in areas of axon thickening, widening jugular foramina, and/or in the presence of bony erosion at the skull base. Carotid body tumors are soft-tissue masses with enhanced attenuation, causing splaying of the ICA and ECA. Whereas, glomus vagale tumor induced anteromedial displacement of the ICA and ECA, and may demonstrate carotid sheath continuity with the vagus nerve.[6]
MRI [Table 1] is superior regarding soft-tissue imaging and used for further assessment of carotid body tumors. On T1-weighted images, lesions have a low to intermediate signal intensity, similar to muscle. On T2-weighted images, carotid body tumors demonstrate hyperintensity with areas of serpentine signal voids which are caused by intratumoral vessels. The classical “salt and pepper” appearance is caused by slow flow or hemorrhage alternating within the tumor matrix. Like evaluation with US or CT, mass location relative to adjacent structures helps in distinguishing carotid body from vagale, or jugulare, and tumors. Catheter angiography is not used for diagnosis, but is reserved for preoperative planning and embolization.[6,8]
FDG-PET/CT [Table 1] is utilized for the detection, staging, treatment planning, and surveillance. Dotatate-PET/ CT can be used in carotid body tumors that are proven somatostatin positive. However, PET alone is limited by its lack of spatial resolution, variability of physiologic uptake in adjacent normal structures, and the lack of supporting literature regarding specificity/sensitivity in paraneoplastic neurological syndromes (PNS) diagnosis.[9] I-123 MIBG scintigraphy is particularly useful for the detection of catecholamine secreting tumors.[10]
To the best of our knowledge, carotid body tumor with PNS along the SLN has not been previously reported in the radiology literature. One case of a carotid body tumor growing along the SLN was reported during surgical resection of a right carotid body tumor.[11]
The SLN is a branch of the vagus nerve (CN X) which supplies sensory innervation to the supraglottic larynx, epiglottis and tongue base, and motor innervation to the cricothyroid muscle and interarytenoid muscle.[12] The SLN arises from the middle portion of the nodose ganglion of the vagus nerve and travels inferomedially toward the thyrohyoid membrane. It, then, branches into the internal and external superior laryngeal nerves (iSLN and eSLN, respectively). The iSLN provides sensory innervation to the anterior wall of the vallecula, epiglottis, and aryepiglottic folds, as well as the supraglottic larynx. The eSLN, the smaller of the two, supplies motor innervation to the cricothyroid muscle and the superior portion of the inferior pharyngeal constrictor. It also gives branches to the pharyngeal plexus and superior cardiac nerve.[3]
Surgical resection is the most common treatment for carotid body tumors (1). Surgery carries a 10% morbidity rate with a recurrence rate of 7%.[13] The major complications of surgical intervention are hemorrhage and nerve injury.[1]
RT is the preferred treatment modality for jugulotympanic PGs, with local control rate of 80% as opposed to 25% with surgery alone.[13] Contraindications to surgery include advanced age, bilateral cranial nerve palsies, extensive skull base involvement, intracranial involvement, and medical comorbidities.[8]
Patients who are poor surgical candidates, or have multi-focal carotid body tumors or debilitating symptoms due to tumor burden may be treated with RT. Fractionated conventional RT doses ranges from 44 to 72 Gy depending on tumor size and symptoms. Symptom improvement with RT varies from 0% to 83%. RT cure rate ranges from 65% to 100%, with a mean of 90%, and a recurrence rate of 8%.[13] A 2017 study using proton therapy reported a median fractionated dose of 50.4 Gy that demonstrated a 100% local control rate and 64.3% improvement of symptoms.[14]
While surgical resection is the primary treatment for carotid body tumors, RT is the preferred primary treatment for jugular or carotid body tumors presenting without lower cranial nerve palsies or in the setting of multiple tumors. Patients undergoing RT may experience acute side effects including alopecia, xerostomia, epilation, mucositis, and otitis externa or media.[13]
The most common imaging differential diagnosis for HN carotid body tumors are schwannomas.[2] A few key features for distinguishing schwannomas from carotid body tumors include avid enhancement post-gadolinium with absent flow voids on MR and fusiform shape. Unlike carotid body tumors, schwannomas are contained within the epineurium and are typically surgically resected sparing the involved nerve fibers.
CONCLUSION
We report a case of a carotid body tumor with perineural spread of tumor along the SLN into the paraglottic space of a 58-year-old woman. To the best of our knowledge, this is the first case of its kind reported in the literature. Carotid body tumors are commonly treated with surgical resection, RT, or a combination thereof. Despite interval growth, our patient refused treatment and decided to be followed with routine imaging surveillance.
Acknowledgments
We would like to thank Dr. Mauricio Hernandez for his assistance with edits through several versions of this manuscript.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Head and neck paragangliomas: Clinical and molecular genetic classification. Clinics (Sao Paulo). 2012;67(Suppl 1):19-28.
- [CrossRef] [Google Scholar]
- Clinicopathological analysis of paraganglioma with literature review. World J Gastroenterol. 2009;15:3003-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pheochromocytoma and Paraganglioma: Introduction 2018. 2020 Available from: https://www.cancer.net/cancer-types/pheochromocytomaand-paraganglioma/introduction#:~:text=about%20paraganglioma,paragangliomas%20form%20in%20the%20abdomen [Last accessed on 2020 Oct 15]
- [Google Scholar]
- Benign paragangliomas: Clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab. 2001;86:5210-6.
- [CrossRef] [PubMed] [Google Scholar]
- Normal life expectancy for paraganglioma patients: A 50-year-old cohort revisited. Skull Base. 2011;21:385-8.
- [CrossRef] [PubMed] [Google Scholar]
- Multimodality imaging of paragangliomas of the head and neck. Insights Imaging. 2019;10:29.
- [CrossRef] [PubMed] [Google Scholar]
- Case report of a rare glomus jugulare tumor extended within the extracranial internal jugular vein: Emphasis on initial ultrasound findings and literature review. Med Ultrason. 2018;1:111-3.
- [CrossRef] [PubMed] [Google Scholar]
- Paragangliomas of the head and neck: An overview from diagnosis to genetics. Head Neck Pathol. 2017;11:278-87.
- [CrossRef] [PubMed] [Google Scholar]
- Perineural spread in head and neck malignancies: Clinical significance and evaluation with 18F-FDG PET/CT. Radiographics. 2013;33:1717-36.
- [CrossRef] [PubMed] [Google Scholar]
- I-123 MIBG imaging of pheochromocytoma-paraganglioma syndrome with succinate dehydrogenase deficiency. J Nucl Med. 2014;55(Suppl 1):1361.
- [Google Scholar]
- Paraganglioma of superior laryngeal nerve mimicking as carotid body tumor: A rare case report. J Surg Allied Sci. 2019;1:5.
- [Google Scholar]
- Surgical anatomy of the internal branch of the superior laryngeal nerve. Eur Spine J. 2006;15:1320-5.
- [CrossRef] [PubMed] [Google Scholar]
- The multidisciplinary management of paragangliomas of the head and neck, Part 2. Oncology (Williston Park). 2003;17:1143-53. discussion 1154, 1158, 1161
- [Google Scholar]
- Proton radiation therapy for head and neck paragangliomas: A single institution experience. Int J Radiat Oncol Biol Phys. 2017;99:E346.
- [CrossRef] [Google Scholar]






