Translate this page into:
Parotid sebaceous lymphadenoma associated with Sjogren’s syndrome: Review of pathologic and radiologic characteristics and clinical presentation

*Corresponding author: Alok A. Bhatt, Department of Radiology, Mayo Clinic, Jacksonville, Florida, United States. bhatt.alok@mayo.edu
-
Received: ,
Accepted: ,
How to cite this article: Janus JR, Mzaik O, Bhatt AA. Parotid sebaceous lymphadenoma associated with Sjogren’s syndrome: Review of pathologic and radiologic characteristics and clinical presentation. J Clin Imaging Sci 2022;12:14.
Abstract
Salivary lymphadenoma is an extremely rare neoplasm that is usually seen in the older patient population, arising sporadically on one side and almost exclusive to the parotid gland. Imaging and pathology findings can mimic both benign and malignant tumors, and therefore, this lesion may be misdiagnosed if not on the differential diagnosis. This article reviews the clinical presentation, as well as the pathology and imaging findings of salivary lymphadenoma in the setting of Sjogren’s syndrome.
Keywords
Parotid
Sialadenitis
Tumor
CASE REPORT
A 66-year-old female with a past medical history of Sjogren’s syndrome presented with bilateral facial swelling for 18 months. She denied facial numbness or pain. Physical exam demonstrated bilateral nodular parotid swelling and a dominant lesion at the angle of the right mandible. Laboratory tests showed elevated antinuclear antibodies ANA >12.0 U (normal <1.0), SS-A/RO Ab IgG >2.8 U (normal <1.0), and Rheumatoid factor (RF) 43 IU/mL (normal <15 IU/mL), compatible with history of Sjogren’s syndrome.
Imaging evaluation was performed with computed tomography (CT) of the neck with intravenous contrast, which demonstrated bilaterally enlarged, heterogeneous parotid glands with multiple scattered calcifications and small cysts [Figure 1]. The tail of the right parotid gland had a dominant well-defined, peripherally enhancing lesion with internal septations separating areas of low attenuation [Figure 2].
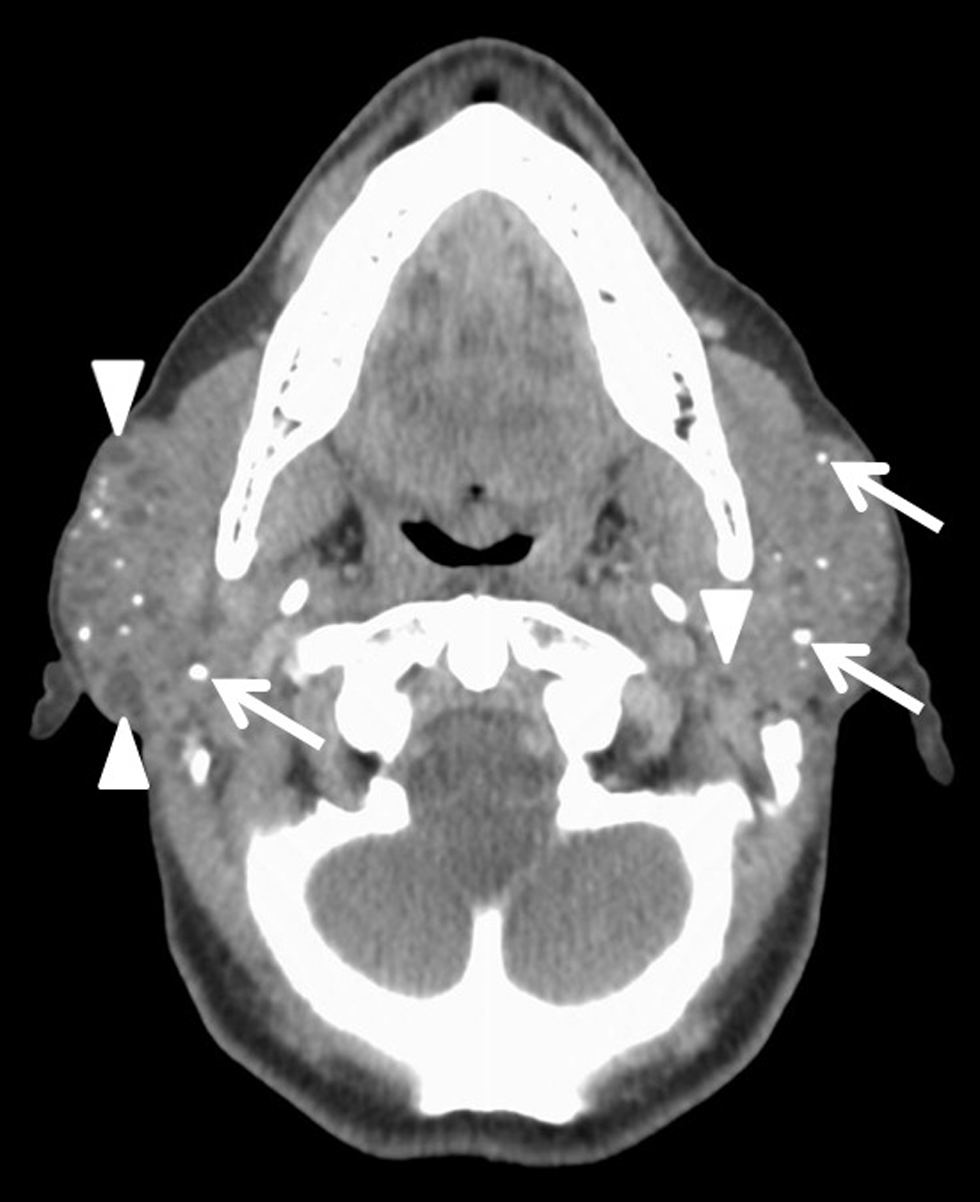
- A 66-year-old female with a history of Sjogren’s syndrome presents with bilateral facial swelling and palpable mass on the right. Axial contrast-enhanced CT image demonstrates enlarged, heterogeneous bilateral parotid glands with multiple calcifications (white arrows) and small cysts (arrowheads), consistent with chronic inflammation
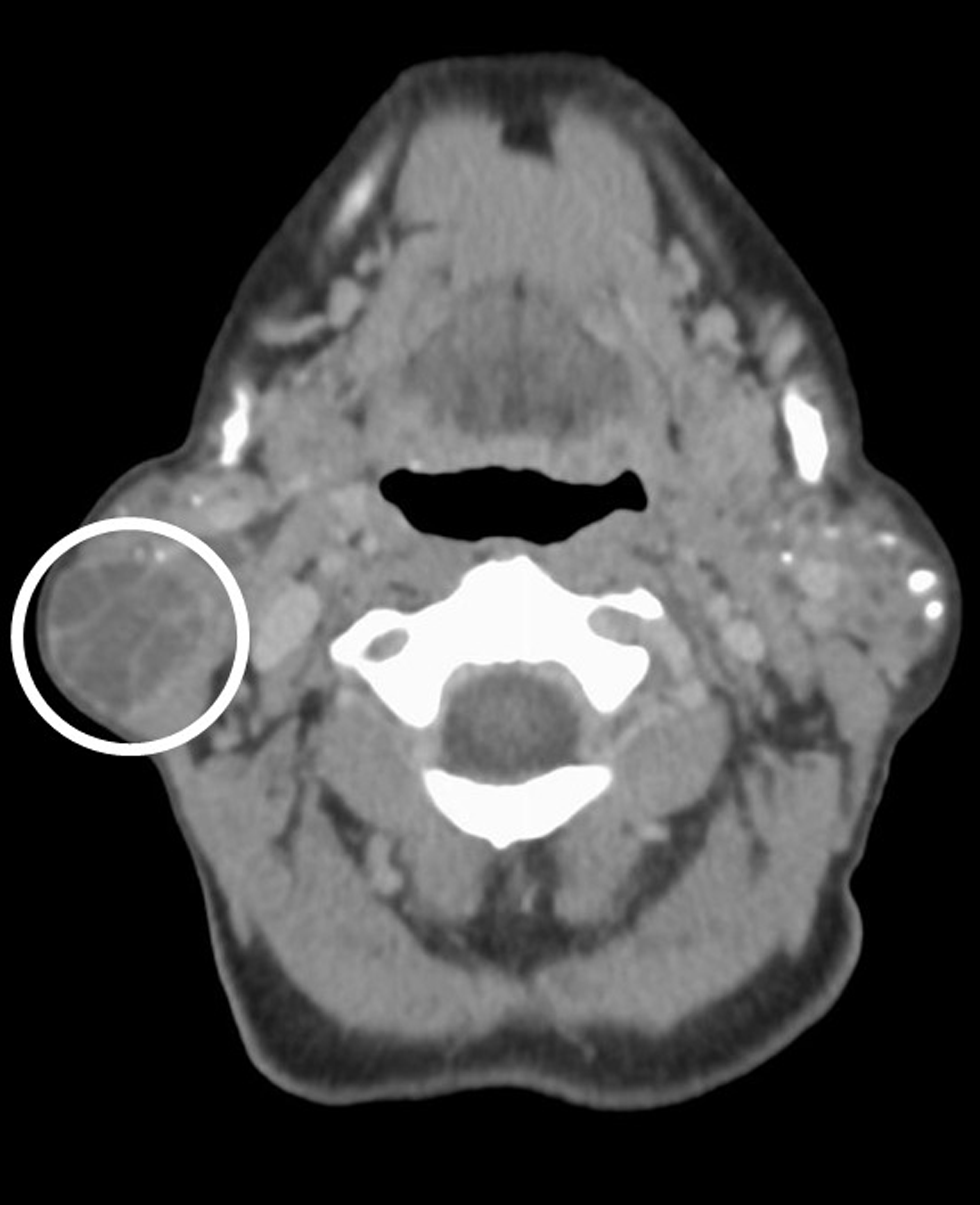
- A 66-year-old female with a history of Sjogren’s syndrome presents with bilateral facial swelling and palpable mass on the right. Axial contrast-enhanced CT image demonstrates a well-circumscribed (smooth, peripherally enhancing capsule), predominantly low attenuating lesion within the tail of the right parotid gland (circled). The lesion has thin, enhancing internal septa. Pathology proven sebaceous lymphadenoma
The patient underwent right superficial parotidectomy, pathology revealed a multiloculated intact cyst within the resected tissue measuring 5.5 × 3.0 × 1.5 cm, which was filled with brownish fluid. Pathology was consistent with a cystic sebaceous lymphadenoma. To achieve volumetric symmetry, a left superficial parotidectomy was also performed 6 weeks later. Both the right and left excised parotid gland tissue showed sialolithiasis with calcification and multifocal ductal dilation with cyst formation, as well as acute on chronic sialadenitis; findings concordant with the patient’s history of Sjogren’s syndrome. The patient did well after surgery.
DISCUSSION
Salivary lymphadenoma is a rare tumor that is predominantly seen in adults over the age of 50 and has also been reported as early as 11 years of age with no clear sex predilection.[1] The parotid gland is the most common location; however, it has also been reported in locations of the minor salivary glands, such as the buccal mucosa. An altered immune system is believed to be a predisposing factor and is suspected to be the reason for occurrence in our patients. These tumors typically present as a unilateral, painless facial swelling.[1,2] Tumors are treated by parotidectomy, local excision, or enucleation. Recurrence is rare.[3]
There are two subtypes: non-sebaceous and sebaceous (more common). Non-sebaceous lymphadenoma is composed of epithelial cell islands in a prominent lymph background. In the sebaceous form, mature sebaceous cells coexist with epithelial cells and salivary ducts. Sebaceous lymphadenoma is an encapsulated or circumscribed tumor that varies from solid to cystic on gross appearance. Lymphoid follicles and/or subcapsular sinuses are seen along with histocytes and foreign body giant cells. The presence of these cells may indicate an inflammatory reaction to the extravasated sebum. Focal necrosis and irregular fibrosis have been reported occasionally. In addition, minimal atypia in salivary lymphadenoma makes local invasion extremely unlikely.[3] Although extremely rare, malignant transformation into sebaceous lymphadenocarcinoma may occur, which presents as an enlarging preexisting mass.[4]
Histologic appearance of salivary lymphadenoma can be like other more common parotid tumors such as a Warthin’s tumor and mucoepidermoid carcinoma, thus knowledge of distinguishing features is crucial for accurate diagnosis and treatment. Bilayered oncocytes and papillary configurations are present in Warthin’s and are lacking in sebaceous lymphadenoma. Parotid lymphadenoma is hard to distinguish from mucoepidermoid carcinoma because mucoepidermoid carcinoma is sometimes associated with tumor-associated lymphoid response. However, the lack of intermediate cells and atypia in salivary lymphadenoma is a distinguishing feature.[5] In addition, mucoepidermoid tumors can be differentiated from salivary lymphadenoma because sebaceous cells stain for fat with oil red O stain and not for mucin.[6]
CT is an important tool in the evaluation of parotid lesions. Salivary lymphadenoma may show sparse foci of fat attenuation within a heterogeneous, homogeneous, or enhancing soft tissue mass; the capsule of the lesion demonstrates enhancement.[7] Unfortunately, imaging appearance may be variable and nonspecific. In our case, contrast-enhanced CT demonstrates a well-circumscribed, predominantly low-attenuating lesion within the tail of the right parotid gland and enlarged, heterogeneous bilateral parotid glands with multiple calcifications and small cysts, consistent with chronic inflammation. Irregular borders or suggestion of perineural spread is worrisome for mucoepidermoid carcinoma. Warthin’s tumor is usually located in the parotid tail, at the angle of the mandible (like our case), and may have a more heterogeneous, nodular area of solid enhancement (rather than the septa in our case).
CONCLUSION
Serous lymphadenoma is a rare lesion and can be seen in the setting of an altered immune state such as Sjogren’s syndrome. Pathologic and imaging features may be confused with other primary salivary gland neoplasms such as Warthin’s tumor and mucoepidermoid carcinoma. Management is different, as serous lymphadenomas are treated with complete resection, after which no further intervention is necessary. For this reason, serous lymphadenoma must be on the differential diagnosis when a mass lesion is seen on imaging in the setting of Sjogren’s disease.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Lymphadenoma of the salivary gland: Clinicopathological and immunohistochemical analysis of 33 tumors. Mod Pathol. 2012;25:26-35.
- [CrossRef] [PubMed] [Google Scholar]
- Unilocular cystic sebaceous lymphadenoma of the parotid gland. Arch Otolaryngol. 1985;111:273-5.
- [CrossRef] [PubMed] [Google Scholar]
- Sebaceous neoplasms of salivary gland origin. Report of 21 cases. Cancer. 1984;53:2155-70.
- [CrossRef] [PubMed] [Google Scholar]
- Sebaceous lymphadenocarincoma of paroid gland. Eur Arch Oto-Rhino-L. 2006;263:940-2.
- [CrossRef] [Google Scholar]
- lymphadenoma arising in the parotid gland: A case report. Yonsei Med J. 2002;43:536-8.
- [CrossRef] [PubMed] [Google Scholar]
- Salivary gland pathology In: In: Granick M.S, ed. Hamma III, D.C. Management of salivary gland lesions. Vol 1002. Baltimore: Williams & Wilkins; p. :66-111.
- [Google Scholar]
- Sebaceous lymphadenoma demonstrated by CT and MRI. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e59-62.
- [CrossRef] [PubMed] [Google Scholar]






