Translate this page into:
Mesenteric Amyloidosis: Radiologic Imaging with Pathologic Correlation

*Corresponding author: Margarita V. Revzin, Department of Radiology and Biomedical Imaging, Yale School of Medicine, 333 Cedar Street, New Haven 06510, Connecticut, United States. margarita.revzin@yale.edu
-
Received: ,
Accepted: ,
How to cite this article: Alkukhun A, Rezek I, Ghiassi S, Zhang X, Revzin MV. Mesenteric amyloidosis: Radiologic imaging with pathologic correlation. J Clin Imaging Sci 2020;10:24.
Abstract
Amyloidosis is a rare disease that is characterized by abnormal deposition of amyloid proteins in tissues, resulting in local, or systemic disease. When localized, it can present as an amyloidoma. We report a case of mesenteric amyloidosis in an 80-year-old male who was found to have an incidental mesenteric mass that was biopsy-proven to represent non-light chain amyloid tissue.
Keywords
Amyloidoma
Amyloidosis
Intraperitoneal mass
Gastrointestinal amyloidoma
Mesenteric amyloid
INTRODUCTION
Amyloidosis is a rare disease that is characterized by abnormal deposition of amyloid proteins in tissues, resulting in local or systemic disease. Amyloid deposition along the mesentery is very rare. Multiple subtypes and associated syndromes of amyloidosis are recognized based on the composition of the fibrillar protein and its precursors. To the best of our knowledge, mesenteric non-light chain amyloidosis without an associated secondary chronic inflammatory disease process has not yet been reported. Familiarity of radiologists with characteristic imaging appearances of mesenteric amyloidosis and knowledge of differential diagnosis can necessitate early histopathological analysis and prompt diagnosis as well as improve patient management and treatment outcome.
In this article we review a case report of mesenteric non-light chain amyloidosis, describe histo- and pathophysiology of this disease, and provides specific imaging appearances and differential diagnosis of mesenteric amyloidosis.
CASE REPORT
An 80-year-old male was referred to general surgery for evaluation of a mesenteric mass found on a computed tomography (CT) scan. Before CT imaging, the patient reported 3 months of diarrhea and an unintentional 22-pound weight loss. The patient denied any other symptoms. His pre-operative work-up included an evaluation by a gastroenterologist with an upper endoscopy that was normal and a colonoscopy that showed diverticulosis. A random duodenal biopsy showed non-specific mild villous flattening. Laboratory work was unremarkable, including a negative celiac panel. After this, non-diagnostic workup, the patient underwent a CT scan of the abdomen and pelvis that demonstrated an ill-defined mass isoattenuating to skeletal muscle that encased the mesenteric root and extended peripherally along the vascular bundles. No vascular or organ invasion or mass effect on adjacent structures was noted. Several scattered focal areas of similar density were noted along the serosa of the bowel loops without evidence of bowel obstruction, likely representing extension of deposits from the periphery. No associated intralesional calcifications or regional lymphadenopathy was present [Figure 1]. The patient was then referred for surgical evaluation.
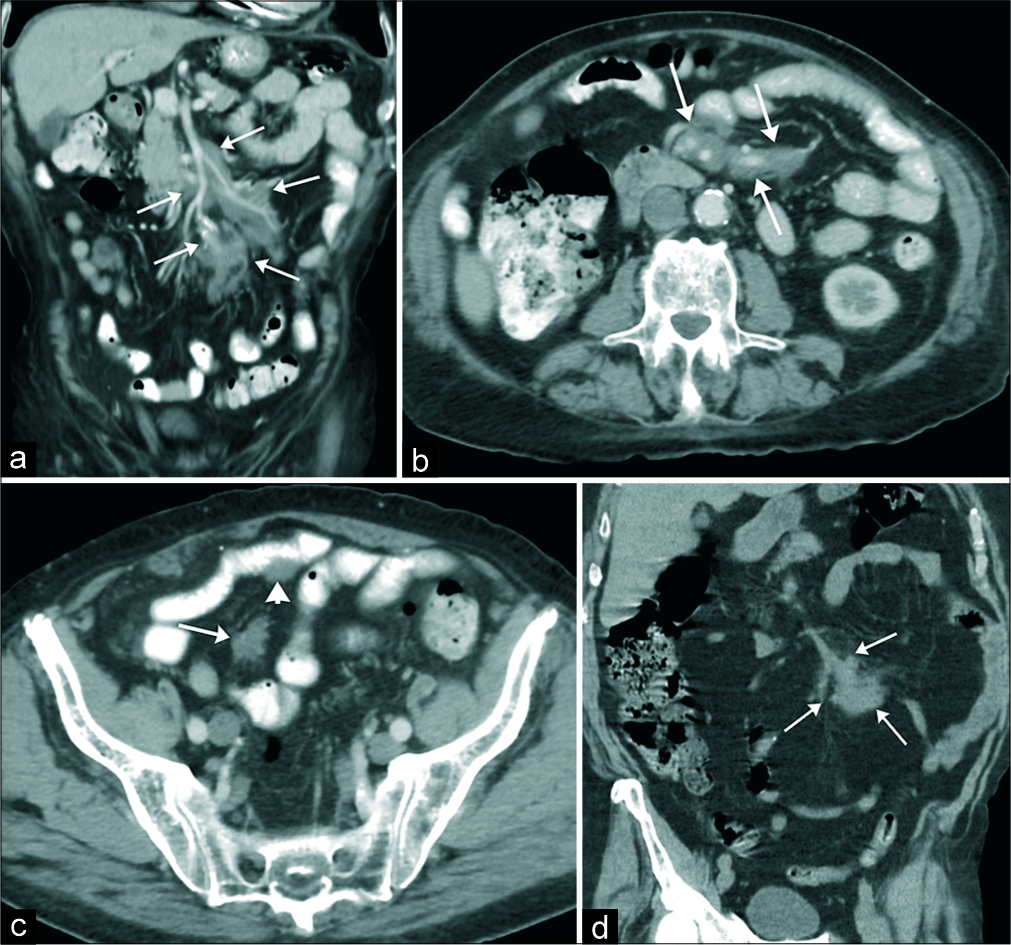
- An 80 year-old male with 3 months history of diarrhea and weight loss presented to the emergency department. (a-c) Contrast-enhanced computed tomography (CT) of the abdomen and pelvis obtained in coronal (a) and axial (b and c) planes demonstrates lobulated isoattenuating to the adjacent muscle mass encasing the mesenteric root and extending to the periphery of the vascular bundles (arrows in a and b). There is no evidence of vascular invasion or obstruction. A few smaller soft-tissue deposits were adherent to the serosa of the bowel without bowel obstruction and/or mass effect (arrowhead in c). There was no calcification and no enlarged lymph nodes were appreciated. (d) Contrast-enhanced CT of the abdomen and pelvis obtained 2 years before initial visit demonstrated a focal area of peripheral mesenteric involvement without central mesenteric root deposits (arrows in d).
Medical history was significant for atrial fibrillation, hypothyroidism, type 2 diabetes mellitus, hypertension, and squamous cell carcinoma of the skin. Past surgical history was significant for an open appendectomy, Mohs surgery (for squamous cell cancer), and laparoscopic right nephrectomy (for hydronephrosis and obstruction leading to recurrent infections). The patient’s medications included amlodipine, apixaban, glipizide, levothyroxine, metoprolol, hydrochlorothiazide, diphenoxylate-atropine, and gemfibrozil.
The patient subsequently underwent a laparoscopic biopsy of the mesenteric mass. The operation was uncomplicated, and the patient was discharged home the same day. The pathology showed pale eosinophilic, amorphous, and homogenous amyloid deposition in the mesentery consistent with the diagnosis of a mesenteric amyloidoma [Figure 2a]. The amyloid was positive on Congo red staining [Figure 2b] and displayed apple-green birefringence under polarized light [Figure 2c], consistent with amyloid tissue. By electron microscopy, the amyloid deposits appeared as randomly oriented, non-branching fibrils measuring 5.62–8.19 nm in diameter [Figure 2d]. The liquid chromatography-tandem mass spectrometry detected peptides commonly deposited with amyloids of all types, including serum amyloid P component, apolipoprotein A IV, and apolipoprotein E, as well as immunoglobulin kappa and lambda light chains and alpha and gamma heavy chains, with no clear predominance of any chain type.
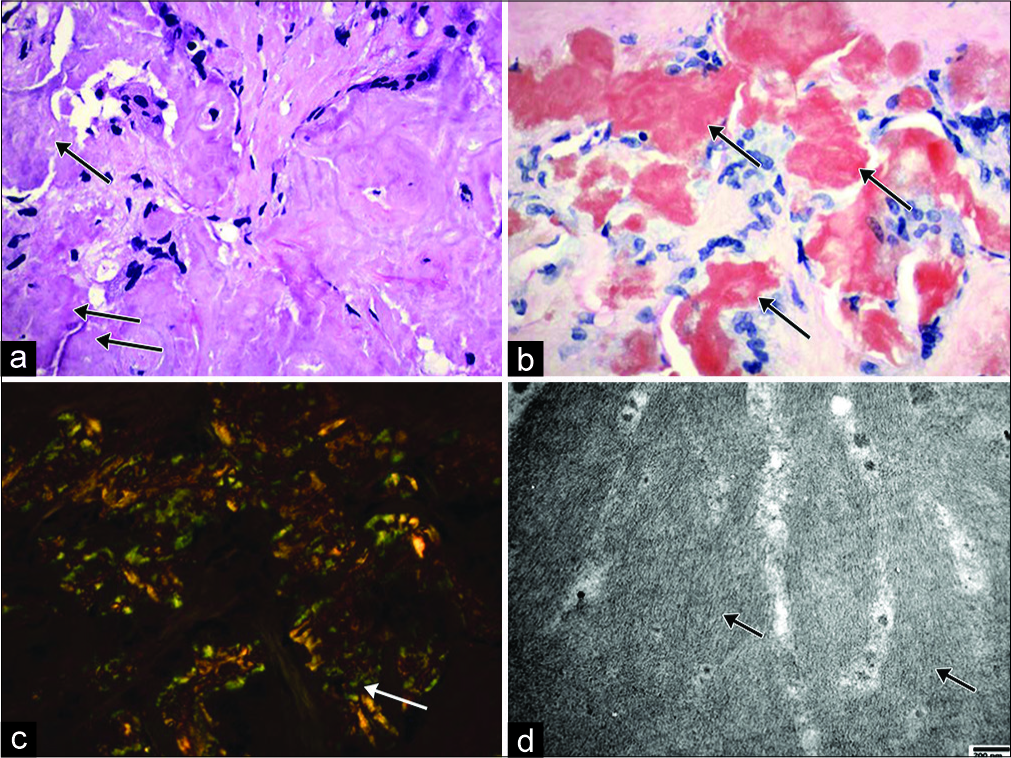
- Illustration of mesentery amyloid deposition. (a) Microscopic view showing eosinophilic amorphous amyloid deposition in the mesentery tissue (arrow) (hematoxylin and eosin stain, original magnification ×400); (b) Microscopic view showing Congo red stained amyloid (arrow) (Congo red stain, original magnification ×400). (c) Microscopic view showing apple- green birefringence under polarized light (arrow) (Congo red stain, original magnification ×400); (d) Electron microscopic view showing randomly arranged non-branching fibrils with diameters ranging from 5.62 to 8.19 nm (arrow) (Uranyl acetate and lead citrate stain, original magnification ×40,000).
DISCUSSION
Amyloidosis is a rare medical condition characterized by extracellular deposition of abnormal fibrillar precursor proteins, termed amyloid, that accumulate in an insoluble form in organs or tissues.[1,2] Amyloidosis can be hereditary or acquired and can range from localized involvement of a single organ to widespread systemic disease.[3] Amyloid deposits cause anatomic disruption of normal tissue function and organ blood supply. The potential to affect almost any organ and associated wide range of clinical presentations often make the diagnosis of amyloidosis a dilemma and challenge.
Based on the variable biochemical composition of the fibrillar protein and its precursors, the World Health Organization’s classification of amyloidosis recognizes multiple subtypes and associated syndromes [Table 1][4,5] summarizes the most common types, the related proteins, and the clinical syndrome that are associated with each type.
| Name | Amyloid protein | Syndrome |
|---|---|---|
| AL | Light chains immunoglobulins (Kappa, Lambda) | Idiopathic primary amyloidosis/Monoclonal gammopathy |
| AA | Serum amyloid A protein (apoSAA) | Reactive/Secondary amyloidosis |
| AB2M | Beta-2 Microglobulin | Dialysis-associated amyloidosis |
| AIAPP | Islet amyloid polypeptide | Insulinoma, type 2 diabetes |
| ATTR | Transthyretin | Familial amyloid neuropathy |
Over 30 different types of proteins have been linked to amyloidosis.[6] Systemic light chain (AL) amyloidosis is the most common type, characterized by deposition of monoclonal immunoglobulins by plasma cells.[7] Reactive amyloid A (AA) amyloidosis is the second most common type, with AA fibrillary protein being formed in the liver as a consequence of interleukin 6 mediated macrophage cleavage of serum AA. Elevation of AA can be seen in a subset of patients with chronic inflammatory conditions such as rheumatoid arthritis, chronic bronchitis, osteomyelitis, or tuberculosis (TB).[3] The incidence of amyloidosis is not well reported, but a recent study showed that there are 12,000 adults in the United States that were living with AL amyloidosis in 2015, with estimated incidence rate of 9.7– 14.0 cases per million person-year.[7]
An amyloidoma is defined as a localized deposit of amyloid tissue without systematic spread, typically presenting as a focal tumor-like deposition.[8] Typically, amyloidomas can be found in the central nervous system, respiratory system, cardiac system, gastrointestinal tract, and soft tissue extremities.[3,9-11] Amyloid deposition along the mesentery is very rare.[12]
Similarly to pulmonary amyloidosis, variable forms of the gastrointestinal amyloidosis can be recognized.[13] On CT amyloidosis has been radiologically described as soft- tissue attenuation lesions isodense to the skeletal muscle that may contain calcifications or focal areas of necrosis.[9] The contour of amyloid lesions may be either smooth, lobular, or ill-defined, and rarely they can present with cavitation.[9] On occasion, amyloidosis may be seen as an infiltrative mass predominately encasing the mesentery.[14] Since its appearance can mimic various abdominal and pelvic pathologies including malignant neoplasms, it is vital to accurately diagnose and work-up a mass when discovered to offer the patient appropriate therapy.
On magnetic resonance imaging, generally amyloid involvement of the gastrointestinal system appears as a low signal intensity on T2-weighted images.[15,16] However, variations of radiological presentation may be seen depending on the abdominal organ involvement. For example, infiltration of the liver by amyloid deposition may demonstrate relatively increased T1 signal intensity and not significantly changed signal on T2-weighted images.[17]
The role of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT in assisting with diagnosing amyloidosis has been described.[18,19] Significant high 18F-FDG uptake of multiple abdominal organs including liver and small bowel was founded; however, the authors noted that the sensitivity was not high and measured at 68.2%.[18] In another study, 18F FDG-PET showed uptake in all patients with localized amyloidosis and no uptake in systemic amyloidosis.[19] It has been suggested that the uptake of FDG in patients with pulmonary amyloidosis could be related to the abnormal production of immunoglobulins by plasma cells.[20]
Radiologically, the imaging features of amyloid deposits are non-specific and differentiation from other benign conditions, primary neoplasms, and metastatic disease may not be feasible. Accurate diagnosis can only be made on biopsy or after surgical excision.
The primary tumors arising in the mesentery are relatively rare.[21,22] On the other hand, the mesentery is a frequent avenue of spread for malignant neoplasms through the peritoneal cavity between the peritoneal spaces and the retroperitoneum.[22,23]
Desmoid tumors are one of the primary mesenteric neoplasms that should be differentiated from amyloidosis. Although these tumors display similar features on CT, they may be differentiated based on the MR imaging characteristics due to their fibrotic nature, demonstrating hypointense signal intensity on T1- and T2-weighted images and hypo or no enhancement on post-contrast imaging. Most commonly these tumors are homogenous and well-circumscribed; however, on occasion can be ill-defined. The masses tend to splay the mesenteric vessels without their encasement. These aggressive tumors are associated with familial adenomatous polyposis (Gardner’s syndrome) and also seen in patients with a history of prior abdominal surgery.[24] Desmoid tumors can result in bowel and ureteral encasement and obstruction, and central necrosis can be seen when they reach a large size. Other primary neoplasms of the mesentery are much rarer and have distinct radiologic appearances when compared to amyloid, either with predominantly lipid content as seen in lipomas and liposarcomas, T2 hyperintense signal as seen in schwannomas, or hypervascularity as seen in cases of leiomyosarcoma, myxofibrosarcoma, and paraganglioma.[25]
Sclerosing mesenteritis (SM) is another disease that should be considered in the differential diagnosis. SM is a rare inflammatory condition that can affect the mesentery, and the acute form is characterized by a low attenuation focal area within the mesenteric fat that is surrounded by a pseudocapsule, commonly referred to as a “misty mesentery.”[24] In contrast, the chronic form of SM (aka retractile mesenteritis), including its rare associated autoimmune condition immunoglobulin G4- related disease (IgG4-RD), has different radiologic appearance. It manifests itself as a soft-tissue mass which may contain coarse calcifications and significant fibrosis and may extend as fibrotic bands along the mesenteric root. In the case of IgG4- RD, there is lymphoplasmacytic infiltration with eosinophils and development of fibrosis in the mesentery. Mesenteric varices, vein occlusion, and encasement of the arteries can help to differentiate this condition from amyloidosis.
Several infectious and inflammatory conditions, associated with characteristic mesenteric nodal enlargement, may also mimic amyloidosis. Diseases such as TB, Whipple disease, sarcoidosis, and mastocytosis may have similar findings to amyloidosis and can be differentiated on the basis of laboratory results and clinical history.[26]
Lymphoma is the most common malignant neoplasm affecting the mesentery and should be differentiated from amyloidosi.[26] It has been reported that 30–50% of patients with non-Hodgkin’s lymphoma and a significant number of patients with chronic lymphocytic leukemia have mesenteric involvement,[26] which may manifest as confluent lymphadenopathy encasing the retroperitoneum and the mesenteric root without significant displacement of the intervening vessels [Figure 3]. Patterns of the presentation of mesenteric lymphoma include homogenous masses encasing mesenteric vessels, large lobulated masses with central necrosis, and ill-defined infiltrative masses displacing bowel loops. One potential differentiating clue is the presence of concomitant bulky retroperitoneal lymphadenopathy. A biopsy is required in those cases in which imaging cannot differentiate amyloidosis from lymphoma.[27,28]
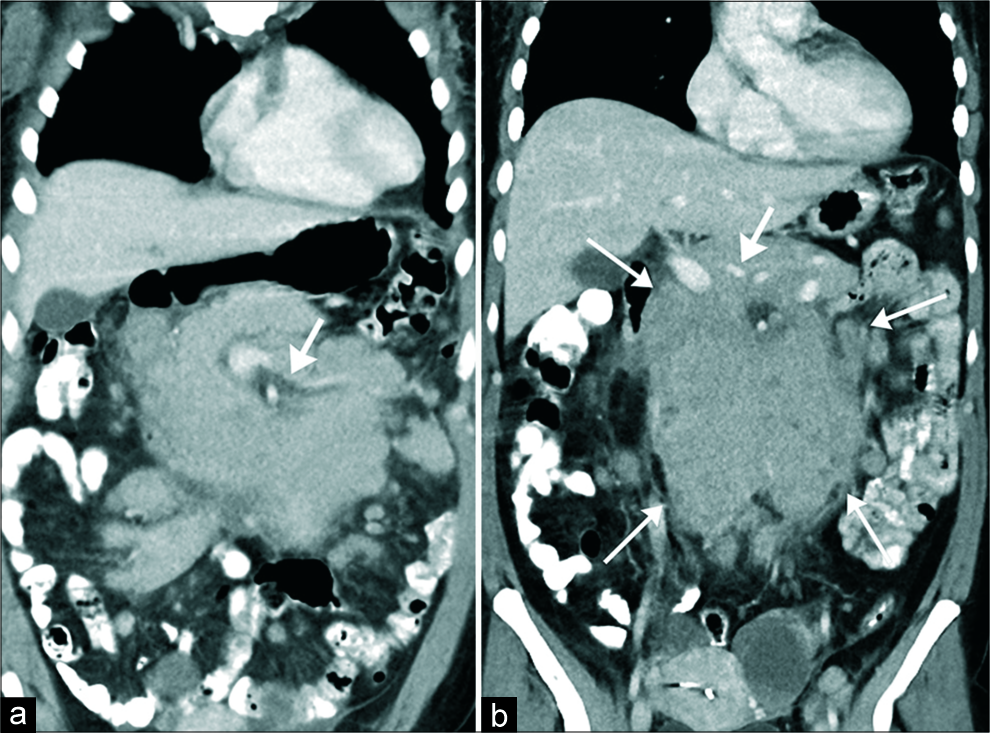
- Confluent follicular lymphoma in a 54 year-old women presenting with weight loss. (a and b) Contrast-enhanced computed tomography of the abdomen and pelvis, obtained in coronal (a and b) plane demonstrates lobulated isoattenuating to the adjacent muscle mass encasing the mesenteric root compatible with confluent lymphadenopathy (arrows in a). No vascular compression is noted (thick arrows in a and b). The mass is encasing the central mesenteric root. No adjacent organ invasion is seen.
Metastatic cancers from breast, gastrointestinal primaries, pancreas, lung, germ cell testicular tumors, carcinoid, and melanoma can also have mesenteric involvement and should be included in the differential diagnosis.[26,29]
In our case, amyloid deposits were predominately extending along the perivascular sheaths of the mesenteric root with a few areas of amyloid deposits extending to the very periphery where they manifested as serosal deposits along the bowel loops [Figure 1a-c]. Serosal bowel deposits did not result in bowel obstruction [Figure 1c]. A prior CT obtained several years earlier demonstrated a focal area of peripheral mesenteric involvement, without central mesenteric root involvement [Figure 1d]. Progression of the disease, from the periphery to the center of the mesenteric root, was observed on the subsequent CT scan. No vascular invasion was identified on the pathological assessment.
In the literature, there have been only eight cases of mesenteric amyloidoma reported.[8,12,30,31] All eight cases were associated with AL amyloidosis.[12] The mean age was 66 (with a range of 48-29), with equal gender occurrence. Three of the patients had an underlying lymphoproliferative disorder, two had multiple myeloma, and two patients had plasmacytoma. One patient had no data available. Five out of eight patients were managed with chemotherapy, radiation therapy, or partial excision. Of note, the average survival of the eight patients was 11.8 months, with a range of 5–17 months, suggesting an overall poor prognosis of mesenteric amyloidoma.
In our case, the work-up for a primary cause for the amyloidosis was unrevealing. The patient underwent an extensive post-operative work-up that included a bone marrow biopsy that showed no findings suggestive of amyloidosis, urinalysis that revealed elevated protein levels however with normal levels of alpha and beta globulins, and a nuclear medicine 99mTechnetium-Pyrophosphate examination with inconclusive results for cardiac amyloidosis.
CONCLUSION
To the best of our knowledge, this is the first report of a mesenteric non-light chain amyloidosis without an associated secondary chronic inflammatory disease process. Given that the radiological characteristics of the disease mimic more common pathological peritoneal mesenteric processes (including malignancies); we recommend early histopathological analysis of an identified mass and appropriate follow-up/work-up to establish a prompt diagnosis. In addition, recognizing the radiological findings of amyloidosis can improve management and treatment outcomes of this highly morbid disease.
Acknowledgments
The authors thank Henry Douglas for his help with images.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Amyloidosis: Review and CT manifestations. Radiographics. 2004;24:405-16.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic AA amyloidosis: Epidemiology, diagnosis, and management. Clin Epidemiol. 2014;6:369-77.
- [CrossRef] [PubMed] [Google Scholar]
- Nomenclature 2014: Amyloid Fibril Proteins and Clinical Classification of the Amyloidosis United Kingdom: Taylor & Francis; 2014.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of AL amyloidosis: A real-world study using US claims data. Blood Adv. 2018;2:1046-53.
- [CrossRef] [PubMed] [Google Scholar]
- Tumoral presentation of amyloidosis (amyloidomas) in soft tissues: A report of 14 cases. Am J Clin Pathol. 1993;100:135-44.
- [CrossRef] [PubMed] [Google Scholar]
- Amyloidosis: Modern cross-sectional imaging. Radiographics. 2015;35:1381-92.
- [CrossRef] [PubMed] [Google Scholar]
- Differentiated plasma cell myeloma presenting as a solitary spinal amyloidoma: A case report, possible pitfall and review to the literature. Clin Neurol Neurosurg. 2015;137:1-4.
- [CrossRef] [PubMed] [Google Scholar]
- Gastric amyloidoma in patient after remission of non-hodgkin's lymphoma. World J Gastrointest Oncol. 2009;1:93-6.
- [CrossRef] [PubMed] [Google Scholar]
- A case of abdominal aortic retroperitoneal and mesenteric amyloid light chain amyloidoma. Case Rep Rheumatol. 2016;2016:4146030.
- [CrossRef] [PubMed] [Google Scholar]
- IgG4-related sclerosing disease: Autoimmune pancreatitis and extrapancreatic manifestations. Radiographics. 2011;31:1379-402.
- [CrossRef] [PubMed] [Google Scholar]
- Abdominal amyloidosis: Spectrum of radiological findings. Clin Radiol. 2003;58:610-20.
- [CrossRef] [Google Scholar]
- MRI of primary amyloidosis. Gastrointest Radiol. 1990;15:199-201.
- [CrossRef] [PubMed] [Google Scholar]
- Primary hepatic amyloidosis: Report of an unusual case presenting as a mass. Korean J Radiol. 2011;12:382-5.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging findings and literature review of (18)F-FDG PET/CT in primary systemic AL amyloidosis. Nucl Med Mol Imaging. 2015;49:182-90.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of 18F-FDG PET(/CT) in patients with systemic and localized amyloidosis. Eur J Nucl Med Mol Imaging. 2013;40:1095-101.
- [CrossRef] [PubMed] [Google Scholar]
- Intense fluorodeoxyglucose activity in pulmonary amyloid lesions on positron emission tomography. Clin Nucl Med. 2003;28:975-6.
- [CrossRef] [PubMed] [Google Scholar]
- Computed tomography diagnosis of mesenteric masses. AJR Am J Roentgenol. 1979;132:33-6.
- [CrossRef] [PubMed] [Google Scholar]
- CT patterns of mesenteric disease. J Comput Assist Tomogr. 1982;6:490-6.
- [CrossRef] [PubMed] [Google Scholar]
- The misty mesentery on CT: Differential diagnosis. AJR Am J Roentgenol. 1996;167:61-5.
- [CrossRef] [PubMed] [Google Scholar]
- IgG4-related sclerosing disease with mesenteric and retroperitoneal involvement. Egypt J Radiol Nucl Med. 2017;48:767-9.
- [CrossRef] [Google Scholar]
- Mesenteric neoplasms: CT appearances of primary and secondary tumors and differential diagnosis. Radiographics. 2003;23:457-73.
- [CrossRef] [PubMed] [Google Scholar]
- CT of lymphoma: Spectrum of disease. Radiographics. 1991;11:647-69.
- [CrossRef] [PubMed] [Google Scholar]
- Soft-tissue sarcomas of the abdomen and pelvis: Radiologic-pathologic features, Part 1-common sarcomas: From the radiologic pathology archives. Radiographics. 2017;37:462-83.
- [CrossRef] [Google Scholar]
- Calcification and fibrosis in mesenteric carcinoid tumor: CT findings and pathologic correlation. AJR Am J Roentgenol. 1995;164:387-91.
- [CrossRef] [PubMed] [Google Scholar]
- Mesenteric amyloidoma: A clinical case report. Intern Med Serv Hosp Dist Faro. 2008;16:17-20.
- [Google Scholar]
- Soft tissue amyloidoma with features of plasmacytoma: A case report and review of the literature. Arch Pathol Lab Med. 2002;126:969-71.
- [Google Scholar]






