Translate this page into:
Role of Magnetic Resonance Enterography in Differentiating between Fibrotic and Active Inflammatory Small Bowel Stenosis in Patients with Crohn's Disease
Address for correspondence: Dr. Francesca Fornasa, Department of Radiology, San Bonifacio Hospital, 1, Via Fontanelle, 37042 San Bonifacio (Verona), Italy. E-mail: francescafornasa@libero.it
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
To assess the diagnostic accuracy of magnetic resonance imaging (MRI) in prospectively differentiating between fibrotic and active inflammatory small bowel stenosis in patients with Crohn's disease (CD).
Materials and Methods:
A total of 111 patients with histologically proven CD presenting with clinical and plain radiographic signs of small bowel obstruction underwent coronal and axial MRI scans after oral administration of polyethylene glycol solution. A stenosis was judged present if a small bowel segment had >80% lumen reduction as compared to an adjacent normal loop and mural thickening of >3 mm. At the level of the stenosis, both T2 signal intensity and post-gadolinium T1 enhancement were quantified using a 5-point scale (0: very low; 1: low; 2: moderate; 3: high; and 4: very high). A stenosis was considered fibrotic if the sum of the two values (activity score: AS) did not exceed 1.
Results:
A small bowel stenosis was identified in 48 out of 111 patients. Fibrosis was confirmed at histology in all of the 23 patients with AS of 0 or 1, who underwent surgery within 3 days of the MRI examination. In the remaining 25 patients (AS: 2–8), an active inflammatory stenosis was suspected and remission of the obstructive symptoms was obtained by means of medical treatment. One of these patients (AS: 2), however, underwent surgery after 14 days, due to recurrence. MRI had 95.8% sensitivity, 100% specificity, and 97.9% accuracy in the diagnosis of fibrotic stenosis.
Conclusion:
MRI is reliable in differentiating fibrotic from inflammatory small bowel stenosis in CD.
Keywords
Crohn's disease
intestinal stenosis
MR enteroclysis
small bowel imaging
INTRODUCTION

This study is aimed at evaluating the diagnostic accuracy of magnetic resonance imaging (MRI) enterography in differentiating between fibrotic and active inflammatory small bowel stenosis in patients with CD.
The clinical concept of different, changing behaviour phenotypes of CD, with many patients progressing overtime to the penetrating or to the small bowel stricture subgroup,[1] has slowly been accepted by radiologists.[2] Identifying the fistulas, abscesses, or strictures, however, is an easier task than distinguishing between an active inflammatory and a fibrotic stenosis, that might direct the therapy toward either medical treatment or surgery.
MRI enterography in the evaluation of the gastrointestinal tract is preferred because of lack of ionizing radiation, high tissue contrast, reasonably safe profile of gadolinium-based contrast media, ability to perform real-time and functional imaging.[3] MRI enterography became more popular in the imaging of small bowel because of the development of fast breath-hold sequences (eliminating motion artifacts) and availability of suitable luminal contrast agents (allowing optimal distension).[4] While several published papers affirm the diagnostic usefulness of MRI enterography in CD,[5–16] most of the recent research has been targeted at the assessment of the degree of inflammatory activity in the affected loops, with the main purpose of monitoring the therapy and detecting the onset of complications.[610121317] However, only a few studies can be found trying to answer the question whether MRI enterography can reliably direct the therapy when a stenosis occurs in CD.[101418]
MATERIALS AND METHODS
Procedure of MRI enterography was fully explained to all patients as part of the informed consent. This study was approved by the institutional review board in accordance with the Helsinki Declaration of 1975 (revised in 2000).
Research plan
All MRI enterography examinations performed including the follow-up examinations were prospectively evaluated by two experienced radiologists independently, before a therapeutic decision was taken by the referring physicians, with the aim of differentiating active inflammatory from fibrotic stenosis. Histological findings were used as a reference standard in the patients who underwent surgery and clinical/radiological evaluations as a standard in those who did not undergo surgery.
A stenosis was defined as a reduction of the bowel lumen (diameter at least 80% lower than that measured in a normal adjacent nonprestenotic loop) associated with focal wall thickening (>3 mm). Both the T2 signal intensity and the post-gadolinium T1 enhancement of the bowel wall at the level of the stenosis were subjectively determined by the two observers using a semiquantitative 5-point scale (Activity score: AS – 0 : very low; 1: low; 2: moderate; 3: high; and 4: very high); when the evaluations of the two observers were different, a final decision was reached by consensus. A stenosis was judged fibrotic if the sum of the two AS values did not exceed 1.
In the patients who underwent surgery, histology was adopted as the gold standard. Based on histopathology a stenosis was classified as fibrotic or as inflammatory. The ambiguous cases in which coexistence of fibrosis and inflammation did not allow ascertaining the actual cause of the stricture were excluded from the study.
To patients in whom surgery was not performed, medical therapy was administered. Systemic treatment with 6-methyl-prednisolone, 1 mg/kg/day, was administered to all patients except those intolerant to steroids. The intolerant patients received infliximab 5 mg/kg/day; in some cases azathioprine 1.5–2.5 mg/kg/day, or methotrexate 25 mg/day, were added. Besides pharmacological treatment, 10 patients received nasogastric suction. Resolution of symptoms and normalization of plain abdominal radiographs within 3 days were used as criteria of active inflammation.
Subjects
Between January 2007 and July 2010, 111 patients with a previous histological diagnosis of CD were referred to our institution with clinical and plain radiographic signs of mild-to-severe intestinal obstruction. Most of them had already suffered similar episodes previously but none had undergone small bowel surgery, with the exception of appendectomy, performed in nine patients at least three years before CD was diagnosed. The majority of these patients were not receiving any treatment at the onset of the symptoms, although in seven of them either azathioprine (five patients) or infliximab (two patients) was administered as maintenance therapy.
Sixteen patients were excluded from this study because CD involved mainly their large bowel. Out of the 95 patients with small bowel CD localization, MRI enterography was not performed in 11 patients. In five patients MRI enterography was not performed because of contraindications (claustrophobia in three cases, pace-maker in one case, and a metallic device from recent aortic surgery in one patient). The other five patients had to undergo surgery without imaging due to the severity of the intestinal obstruction, and one patient refused to drink the required amount of contrast agent.
Among the remaining 84 patients with small bowel localization of CD, 33 cases did not meet the inclusion criteria (>80% lumen reduction and >3 mm wall thickness); another patient was excluded because of poor MRI image quality due to motion artifacts. Additionally, two patients who underwent surgery after MRI enterography were not considered in this study because the histological examination of the resected specimen did not allow an unequivocal differential diagnosis between fibrotic or active inflammatory stenosis.
The final study group included 48 patients (32 females and 16 males; age range 18–64 years, with a mean value of 35 years) with proven CD, in whom a good-quality MRI enterography examination demonstrated the presence of a small bowel stenosis.
Methods
Prior to MRI, all patients were given 1500 ml of a polyethylene glycol (PEG) solution, which was freshly prepared by dissolving a granular powder containing PEG (58.32 g), sodium sulfate (5.69 g), sodium bicarbonate (1.69 g), sodium chloride (1.46 g), and potassium chloride (0.74 g) (“Selg 1000”, Promefarm, Milan, Italy) in water to drink. Twenty minutes after oral contrast adminstration, the adequacy of the bowel distension was assessed by a coronal T2 scan, according to the technical parameters reported below. If the minimum diameter of the small bowel loops was 1.5 cm or larger, the bowel distension was judged satisfactory and MRI enterography was continued after intravenous administration of 20 mg of Hyoscine N-butilbromide (“Buscopan”, Boehringer Ingelheim, Ingelheim am Rhein, Germany). In subjects with small bowel diameter less than 1.5 cm, the sample T2 scan was repeated at 10-minutes intervals until the abovementioned criterion was fulfilled.
All MRI examinations were performed in the supine position with a 1.5 T magnet (“Signa Excite”, General Electric Medical Systems, Milwaukee, Wisconsin, USA) equipped with a phased-array 12-element coil. All patients underwent breath-hold 2D FRFSE (fast-recovery fast spin-echo) sequences (slice thickness 5 mm, gap 1 mm, matrix 256 × 192, NEX 1, phase FOV 1, FOV 400 × 400 mm, bandwidth 50, echo train 21), both in the axial (TR/TE 2400/90 ms; duration 39 s) and in the coronal (TR/TE 2075/90 ms; duration 50 s) planes. Subsequently, a breath-hold fat-suppressed gradient-echo 3D “Lava” T1 sequence (TR/TE 3.8/1.8 ms, slice thickness 4.4 mm, overlap 2.2 mm, matrix 256 × 224, NEX 0.7, FOV 440 × 440 mm, inversion time 7 ms, duration 19 s) was acquired in the axial plane, and was repeated 60 s after intravenous administration of 0.2 mL/kg body weight of gadoteric acid (“Dotarem”, Guerbet, Roissy CdG Cedex, France). The same sequence, in the coronal plane, was acquired 120 s after the administration of the intravenous contrast medium.
In addition, we also performed breath-hold fat-suppressed steady-state 2-D “Fiesta” sequences (slice thickness 5 mm, gap 1 mm, matrix 224 × 320, flip angle 75°, NEX 1, phase FOV 1, bandwidth 83.33, inversion time 200-ms, duration 21–23 s), both in the axial (TR/TE 3.7/1.6 ms, FOV 400 × 400 mm) and in the coronal (TR/TE 4.1/1.8 ms, FOV 460 × 460 mm) planes, with oblique coronal scans added as needed. The results of this Fiesta sequence are not included in this study.
RESULTS
In 41 patients, the bowel distension was considered adequate (minimum loop diameter > 1.5 cm) at the sample coronal T2 scan acquired 20 min after administration of PEG; in the remaining seven patients this cut off value was achieved in the subsequent scan (30 min after PEG).
There were no discrepancies in the evaluation of wall enhancement by the two observers, while in four patients the judgment of the T2 signal intensity differed by one point (0 vs. 1 in 3 cases; 2 vs. 3 in one).
In all patients included in this study one single stenosis was identified in the terminal ileum, within 40 cm from the ileocaecal valve. Out of 48 patients 38 had already experienced intestinal obstructions since the onset of CD, while in 10 cases no major previous episodes were reported.
Among the 48 patients who had a small bowel stenosis, surgery was performed in 23 within 3 days of imaging (10 with AS 0; 13 with AS 1). Histopathology revealed fibrosis in all these patients without significant active inflammatory changes [Figures 1 and 2]. Of these 23 patients, two had no previous episodes of intestinal obstruction.
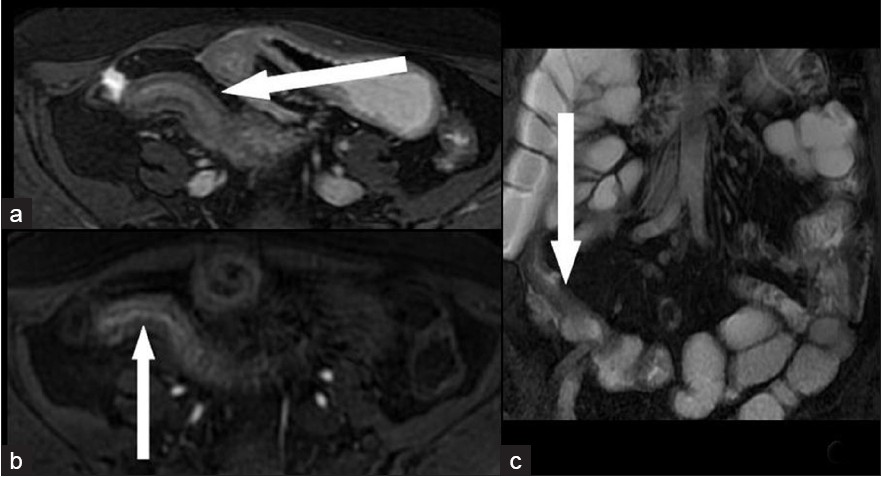
- A 32-year-old woman with a fibrotic stenosis of the terminal ileum. Low signal intensity in T2-weighted FRFSE fat-suppressed sequences seen in (a) axial scan (arrow) and (c) coronal scan: (arrow). (b) Very low enhancement 60 s after i.v. administration of paramagnetic contrast medium is seen on axial “Lava” T1-weighted scan (arrow).
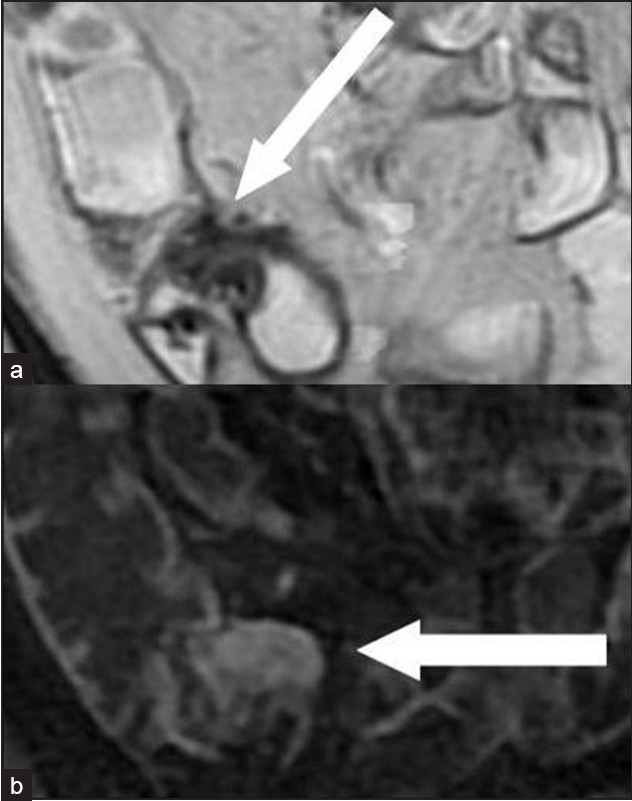
- A 41-year-old man with a fibrotic stenosis of the terminal ileum: Coronal scans show (a) very low signal intensity in a T2-weighted FRFSE sequence (arrow); (b) Low enhancement 120 s after i.v. administration of paramagnetic contrast medium in a “Lava” T1-weighted sequence (arrow).
In the remaining 25 patients with AS varying from 2 to 8, active inflammatory stenosis was suspected [Figures 3–5]. All these patients received anti-inflammatory medical therapy that resulted in remission of the obstructive symptoms. However, one of these patients, whose AS score was 2, underwent surgery 14 days after MRI due to recurrence of the obstruction despite medical treatment. Histopathology revealed mild inflammation superimposed over severe fibrosis.
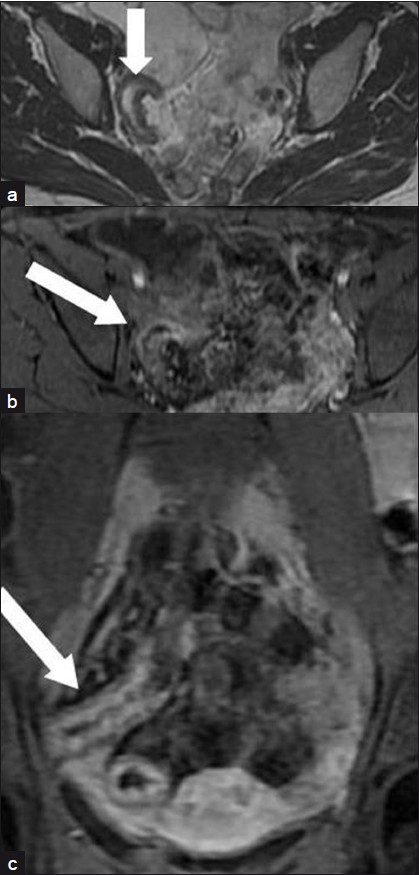
- A 31-year-old woman with an active inflammatory stenosis of the terminal ileum: (a) Moderate signal intensity in an axial T2-weighted FRFSE sequence (arrow). (b) High enhancement in “Lava” T1-weighted sequences axial scan 60 s after i.v. administration of paramagnetic contrast medium (arrow). (c) Coronal scan 120 s after injection (arrow).
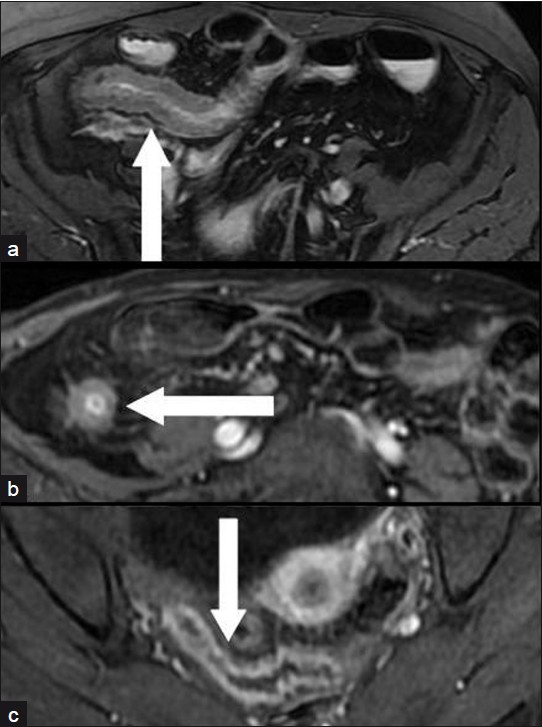
- Three different patients: (a) a 25-year-old man, (b) a 34-year-old man, (c) a 45-year-old woman with an active inflammatory stenosis of a distal ileal loop (axial scans). Moderate signal intensity in a T2-weighted FRFSE fat-suppressed sequence (arrow in a); High enhancement in “Lava” T1-weighted sequences 60 s after i.v. administration of paramagnetic contrast medium (arrows in b and in c).
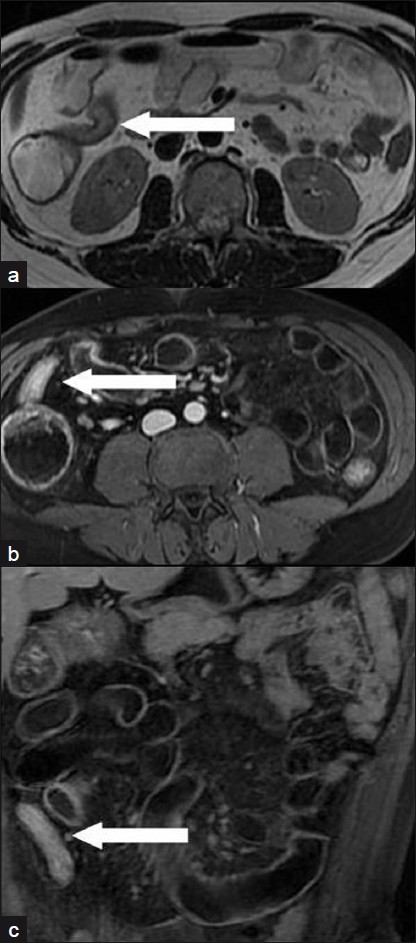
- A 28-year-old man with an active inflammatory stenosis of the terminal ileum. (a) Moderate signal intensity in an axial T2-weighted FRFSE sequence (arrow); (b) Very high enhancement in “Lava” T1-weighted sequences (axial scan 60 s after intravenous administration of paramagnetic contrast medium (arrow); (c) coronal scan 120 s after injection (arrow).
Because of this single false-negative case, MRI had 95.8% sensitivity, 100% specificity, and 97.9% accuracy in the diagnosis of fibrotic stenosis.
DISCUSSION
Both inflammatory changes and fibrosis can cause a clinically relevant reduction of the bowel lumen in CD, and differentiation between these two by MRI may prevent unnecessary surgery and help triage patients for appropriate management.[19]
As for the assessment of CD disease activity, two major methodological issues have to be addressed. What fluid, and through what mode of administration, best allows achieving the bowel distension necessary for a correct diagnosis, and what MRI findings are to be considered reliable indicators of active inflammation.
Adequate small bowel distension is necessary as collapsed loops may hide lesions or mimic disease by suggesting a thickened bowel wall.[19–22] Also, intraluminal air produces susceptibility artifacts[23] and degrades image quality. The fluid necessary to obtain adequate bowel distension can be introduced either orally (enterography) or through a naso-jejunal tube (enterography). Enterography has several limitations including cost, technical difficulty in placing the tube, need for fluoroscopy, exposure to ionizing radiation, and patient intolerance.[316] According to most authors, no significant differences exist between the two methods as to the diagnostic accuracy,[91524–26] especially when stenosis is present.[327] The main diagnostic advantage of enterography over enterography seems to be a better depiction of mucosal abnormalities,[27] which is not the aim of our work. On the basis of this evidence, we decided to fill the bowel orally in all patients and a preliminary MRI is always obtained to ascertain the adequacy of luminal distension.
As for the contrast agent, we like many other institutions[412252829] use an iso-osmotic PEG solution, which rapidly progresses along the bowel (no patients in our series required a waiting time longer than 30 min), has acceptable taste, and is neither fermentable nor degraded by the bacterial flora.[412252829] PEG may, however, cause motion artifacts and diarrhea, sometimes impairing image quality (this is what led to the exclusion of one patient from our series) or interfering with the completion of the examination. PEG binds water molecules preventing their rapid absorption and thus allowing them to act as a biphasic contrast agent, with a low signal intensity in T1 sequences—which adequately demonstrates wall enhancement against a dark lumen[3630] - and high signal in T2 sequences. The use of super paramagnetic particles as negative oral contrast provides low signal intensity lumen in both T1- and T2-weighted sequences,[22] facilitating easy depiction of wall enhancement on T1 sequences[3] and the high T2 signal intensity mural edema caused by inflammation in the bowel wall and in the surrounding fat.[318] However, their availability is limited and they are expensive for regular use.[22] On the other hand, positive contrast agents (paramagnetic substances; milk, vegetable oil, blueberry juice, and other food substances) may mask the enhancement of the inflamed bowel wall.[22] PEG used in our study achieved good bowel distension at a low cost and is well suited for identifying fibrotic stricture as fibrotic wall with low T2 signal intensity is better depicted against the T2 hyperintense lumen [Figure 1].
The degree of bowel wall enhancement on T1 sequences after intravenous administration of gadolinium is the single most trusted MRI criterion of disease activity in CD[4–68–131820222631–34] since it reflects wall vascularity and vessel permeability. Both these increase in active inflammation. In active disease, a layered pattern of wall enhancement is expected, more intense in the mucosa and in the serosa and less intense, because of edema, in the submucosa.[63235] However, some enhancement after gadolinium has been reported within normal[736] and fibrotic bowel.[2237] Enhancement without wall thickening may lead to false-positive results.[311] High T2 signal intensity, caused by wall edema, also adequately correlates with active inflammation.[58121318333437] A normal signal intensity in a thickened wall indicates chronic quiescent CD.[37] A lower value obtained when assessing inflammatory activity has been attributed to several other features,[22] such as the wall thickening alone,[61126] caused in CD by both reversible inflammation and irreversible fibrosis, ulcerations, increased mesenteric vascularity,[36] fibro-fatty proliferation,[6818] mesenteric lymphadenopathy,[624] and complications resulting from penetrating disease. To overcome the limitations in diagnostic accuracy of the above criteria, some authors[173839] propose to evaluate disease activity through scoring systems based on the association of more than one MRI feature.
We agree with the opinion that more than one imaging parameter should be used to assess disease activity. We believe that the degree of mural enhancement and T2 signal intensity are the most reliable combination. Aiming for the most accurate definition of disease activity, we chose a 5-point scale for each parameter, which in turn might have increased the level of subjectivity. However, only four cases in our study had one point difference between the observers’ grading of the T2 signal intensity.[40] Having initially chosen the abovementioned MRI criteria for differentiation between active inflammation and fibrosis, we addressed the issue of CD activity in stenotic segments, which to our knowledge had unsatisfactory accuracy in previously published papers. In two of these works,[1014] the degree of wall enhancement played the most important diagnostic role. Wall hypervascularization and lymphoadenopathy were also taken into account in one case.[14] In one study, both the degree of enhancement and T2 signal intensity were assessed to detect disease activity within strictures.[18] In this study, the former criterion proved less sensitive (66% vs. 83%) and less accurate (81% vs. 91%) than the latter. This was explained as being due to ill-defined bowel profiles on post-gadolinium T1 images being a result of the poor wall enhancement in patients with fibrostenosing disease, while T2 sequences offer excellent anatomic detail in both forms of strictures.[18] Nevertheless, our results are significantly better than those reported in these studies. This leads us to believe that the application of our protocol to the patients with CD-induced small bowel stenosis is feasible and useful.
This study has some limitations. Administration of fluid, either orally or through enterography, might not be safe in patients with a severe bowel stenosis. This led us to exclude five patients from this study. Although the agreement between the two observers was very high, the assessment of both enhancement and T2 signal intensity was made subjectively, and the adoption of a 5-point scale limits the reproducibility of our observations. Some subjectivity might be also found in the criteria leading to diagnosis of small bowel stenosis. Finally, the presence of some fibrotic changes cannot be ruled out in those patients, whose obstructive symptoms resolved with medical therapy and who therefore did not undergo surgery. Two patients who underwent surgery were excluded because the pathologist could not clearly classify their stenosis as fibrotic or as inflammatory. Additional limitation of our study is small cohort and a larger radomized prospective trial is required to validate our results.
In conclusion, in patients with CD, MRI with oral administration of the biphasic contrast agent allows for a reliable differentiation between fibrotic and active inflammatory small bowel stenosis, based on the combined evaluation of the degree of wall enhancement and the T2 signal intensity.
Source of Support: Nil
Conflict of Interest: None declared.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2011/1/1/35/82339
REFERENCES
- The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut. 2006;55:749-53.
- [Google Scholar]
- Classification of small bowel Crohn's subtypes based on multimodality imaging. Radiol Clin North Am. 2003;41:285-303.
- [Google Scholar]
- Breath-hold fast spin-echo MR imaging of Crohn's disease. AJR Am J Roentgenol. 1998;179:127-8.
- [Google Scholar]
- MR imaging evaluation of the activity of Crohn's disease. AJR Am J Roentgenol. 2001;177:1325-32.
- [Google Scholar]
- Crohn's disease evaluation: Comparison of contrast-enhanced MR imaging and single-phase helical CT scanning. J Magn Reson Imaging. 2000;11:127-35.
- [Google Scholar]
- Evaluation of Crohn disease activity with magnetic resonance imaging. Abdom Imaging. 2000;25:219-28.
- [Google Scholar]
- Magnetic resonance imaging of Crohn disease: Early recognition of treatment response and relapse. Abdom Imaging. 1997;22:164-6.
- [Google Scholar]
- Hydro-MRI in Crohn's disease: Appraisal of disease activity. Invest Radiol. 2000;35:431-7.
- [Google Scholar]
- Crohn disease with endoscopic correlation: Single shot fast spin-echo and gadolinium-enhanced fat-suppressed spoiled gradient-echo MR imaging. Radiology. 2002;222:652-60.
- [Google Scholar]
- Contrast enhanced magnetic resonance imaging of the terminal ileum in children with Crohn's disease. Gut. 2003;52:393-7.
- [Google Scholar]
- Small bowel magnetic resonance imaging for inflammatory bowel disease. Top Magn Reson Imaging. 2002;13:409-25.
- [Google Scholar]
- A prospective comparison of MRI versus small bowel follow through in recurrent Crohn's disease. Am J Gastroenterol. 2005;100:2493-502.
- [Google Scholar]
- Abdominal MRI after enteroclysis or with oral contrast in patients with suspected or proven Crohn's disease. Clin Gastroenterol Hepatol. 2004;2:491-7.
- [Google Scholar]
- Small-bowel disease: Comparison of MR enteroclysis images with conventional enteroclysis and surgical findings. Radiology. 2000;215:717-25.
- [Google Scholar]
- Utility of magnetic resonance imaging in small bowel Crohn's disease. Gastroenterology. 2007;133:385-90.
- [Google Scholar]
- MR imaging in patients with Crohn disease: Value of T2- versus T1-weighted gadolinium-enhanced sequences with use of an oral superparamagnetic contrast agent. Radiology. 2006;238:517-30.
- [Google Scholar]
- MR enterography in the management of patients with Crohn disease. Radiographics. 2009;29:1827-46.
- [Google Scholar]
- MR enteroclysis: Technical considerations and clinical applications. Eur Radiol. 2002;12:2651-8.
- [Google Scholar]
- Developing role of magnetic resonance imaging in Crohn's disease. Curr Opin Gastroenterol. 2008;24:135-40.
- [Google Scholar]
- Assessment of Crohn's disease activity in the small bowel with MR and conventional enteroclysis: Preliminary results. Eur Radiol. 2004;14:1017-24.
- [Google Scholar]
- A prospective randomized comparison between two MRI studies of the small bowel in Crohn's disease, the oral contrast method and MR enteroclysis. Eur Radiol. 2007;17:2294-301.
- [Google Scholar]
- MRI evaluation of inflammatory activity in Crohn's disease. AJR Am J Roentgenol. 2005;184:1829-35.
- [Google Scholar]
- Comparison of MR enteroclysis with MR enterography and conventional enteroclysis in patients with Crohn's disease. Eur Radiol. 2008;18:438-47.
- [Google Scholar]
- MR imaging of the small bowel with a true-FISP sequence after enteroclysis with water solution. Invest Radiol. 2000;35:707-11.
- [Google Scholar]
- Small bowel MRI: Comparison of a polyethylene glycol preparation and water as oral contrast media. J Magn Reson Imaging. 2002;15:401-8.
- [Google Scholar]
- MR enteroclysis protocol optimization: Comparison between 3D FLASH with fat saturation after intravenous gadolinium injection and true FISP sequences. Eur Radiol. 2001;11:908-13.
- [Google Scholar]
- Dynamic contrast-enhanced MRI of the bowel wall for assessment of disease activity in Crohn's disease. AJR Am J Roentgenol. 2006;186:1384-92.
- [Google Scholar]
- CT features of ulcerative colitis and Crohn's disease. AJR Am J Roentgenol. 1996;167:3-15.
- [Google Scholar]
- MRI evaluation of Crohn's disease of the small and large bowel with the use of negative super-paramagnetic oral contrast agents. Abdom Imaging. 2002;27:384-93.
- [Google Scholar]
- Evaluation of treatment response in active Crohn's disease by low-field magnetic resonance imaging. Abdom Imaging. 1999;24:232-9.
- [Google Scholar]
- Bowel wall thickening in patients with Crohn's disease: CT patterns and correlation with inflammatory activity. Clin Radiol. 2003;58:68-74.
- [Google Scholar]
- MR imaging of anorectal Crohn disease: A pictorial assay. Radiographics. 1997;17:101-7.
- [Google Scholar]
- Role of spectral presaturation attenuated inversion-recovery fat-suppressed T2-weighted MR imaging in active inflammatory bowel disease. J Magn Reson Imaging. 2008;28:1133-40.
- [Google Scholar]
- Magnetic resonance colonography for the detection of inflammatory disease of the large bowel: Quantifying the inflammatory activity. Gut. 2005;54:257-63.
- [Google Scholar]
- Crohn's disease: Pilot study comparing MRI of the abdomen with clinical evaluation. J Clin Gastroenterol. 1995;21:249-53.
- [Google Scholar]
- Magnetic resonance enteroclysis in the diagnosis of small-intestinal Crohn's disease: Diagnostic accuracy and inter- and intra-observer agreement. Acta Radiol. 2006;47:1008-16.
- [Google Scholar]






