Cerebral Blood and CSF Flow Patterns in Patients Diagnosed for Cerebral Venous Thrombosis - An Observational Study
Address for correspondence: Dr. Souraya ElSankari, Department of Imaging and Biophysics, Centre Hospitalier Universitaire Nord, Place Victor Pauchet, 80054 Amiens cedex, France. E-mail: sorayaelsankari560@hotmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background and Purpose:
Recent studies of the organization of the cerebral venous system in healthy subjects using phase contrast magnetic resonance imaging (PC-MRI) show its structural complexity and inter-individual variations. Our objective was to study the venous blood and CSF flows in cerebral venous thrombosis (CVT).
Materials and Methods:
PC-MRI sequences were added to brain MRI conventional protocol in 19 patients suspected of CVT, among whom 6 patients had CVT diagnosis confirmed by MR venography. Results were compared with 18 healthy age-matched volunteers (HV).
Results:
In patients without CVT (NoCVT) confirmed by venography, we found heterogeneous individual venous flows, and variable side dominance in paired veins and sinuses, comparable to those in healthy volunteers. In CVT patients, PC-MRI detected no venous flow in the veins and/or sinuses with thrombosis. Arterial flows were preserved. CSF aqueductal and cervical stroke volumes were increased in a patient with secondary cerebral infarction, and decreased in 4 patients with extended thrombosis in the superior sagittal and transverse sinuses. These results suggest the main role of the venous system in the regulation of the dynamic intracranial equilibrium.
Conclusions:
CVT produces highly individualized pattern of disturbance in venous blood drainage. Complementary to MRI venography, PC-MRI provides non-invasive data about venous blockage consequences on CSF flow disturbances.
Keywords
Cerebral venous thrombosis
CSF
hydrodynamics
PC-MRI
veins
INTRODUCTION

In adults, regulation of intracranial pressure (ICP) results from the dynamic interaction between the arterial, venous, cerebrospinal fluid (CSF), and brain parenchyma compartments.[1–3]
The role of the arterial system has been assessed through human studies, and confirmed using mathematical models.[45] The arterial intracerebral systolic inflow is commonly considered now as the “driving force,”[2] which initiates a mechanical coupling between intracerebral mobile compartments, resulting in aqueductal, subarachnoid CSF, and venous venting during the systole. The aqueductal and cervical subarachnoid CSF stroke volumes represent the mobile compensatory reserve of respective compartments, and their modulation depends on the “driving current,” in order to preserve ICP throughout the cardiac cycle (CC).
The role played by the venous system in the volume regulation remains controversial. For a long time, veins have been considered as passive conduits, modulating their cross-sectional area according to the blood flow and transmural pressure (cerebral venous pressure minus ICP). Anatomic configuration in veins is different from in arteries, because venous walls do not contain smooth muscle layers and therefore they cannot change their lumen actively.
Although the pressure waveforms are highly attenuated from the arteries when passing in the capillary bed and reaching the venous system, pulsation of ICP is reflected by pulsation of intracranial venous pressure. Specific modifications of venous flow pattern have been suggested to be associated with abnormalities of CSF dynamic in patients with hydrocephalus,[6] and the authors hypothesized that the venous system may originate numerous neurological disorders, such as Idiopathic Intracranial Hypertension or Normal Pressure Hydrocephalus.[67]
Although veins are supposed to play a crucial role in regulating intracranial dynamics, only a few studies have been devoted to the cerebral venous system physiology and pathology.[89] Veins functioning remained difficult to study non-invasively in vivo until the advent of phase contrast magnetic resonance imaging (PC-MRI).
In a recent study,[10] it has been shown that PC-MRI enables reliable, reproducible, and rapid measurement of venous flows (in both dural sinuses and cervical veins), and of their interactions with arterial and CSF flows. The heterogeneity of venous drainage and the variability of side dominance in paired veins and sinuses in healthy young adults were concordant with previous anatomic, angiographic, and PC-MRI studies.[1112] The role of accessory venous pathways, mainly epidural, in drainage of venous blood from the cranium, was also emphasized, independently of commonly described postural[1314] or respiratory factors.[15]
Cerebral venous thrombosis (CVT) can be considered as a model of dynamic disorder in the intracerebral venous system. Reports on the evaluation of venous dynamics in CVT are anecdotal,[16] and quantitative evaluations of intracerebral venous flows have never been published.
Our purpose was to study the variations and the dynamic coupling between vascular and CSF compartments in patients with CVT.
MATERIALS AND METHODS
1 – Participants
We studied successive patients admitted via the emergency department, presenting with neurological symptoms suggesting CVT, and who consequently underwent a brain MRI. Phase contrast sequences were added to the conventional imaging protocol used by the neuroradiologists with the intention to visualize the region of “no flow” in thrombosis section of venous bed. According to the results of the MRI protocol, this population was then divided into 2 groups: patients with confirmed thrombosis (CVT) or those without (NoCVT).
The NoCVT group consisted of 13 patients (9 women and 4 men) with a mean age of 34 ± 13 years, in whom diagnosis different from CVT were finally confirmed (migraine, multiple sclerosis, meningitis, etc.)
The CVT group consisted of 6 female patients (mean age: 32 ± 11 years) who were admitted for unusual headaches or/and seizures, and in whom cranial MRI showed CVT of dural sinuses or of a cortical vein.
Flow results were compared in CVT, NoCVT, and an age-matched control population, who underwent the same PC-MRI examination protocol. The control population, hereby called HV, consisted of 18 healthy young volunteers (9 women and 9 men), who underwent cine PC-MRI. The mean age was 31 ± 7 years. The exclusion criteria were any neurological, psychiatric or severe general disease, alcoholism, or abnormalities detected with clinical MRI exams.
All of the participants (patients and controls) gave their informed consent to participate in the study.
2 – Data acquisition
All MRI exams were performed using a 3-Tesla machine (Signa; General Electric Medical System, Milwaukee, WI). Subjects were supine.
Conventional morphologic sequences were acquired in each patient with CVT suspicion according to the protocol routinely used in our clinic. This protocol includes the following sequences: Sagittal T1 Flair, Axial FSE (Fast Spin Echo) T2, T2*, Flair, diffusion-weighted, SE (Spin Echo) T1 before and after contrast-enhancing agent injection, 3D TOF (Time-of-Flight), coronal 2D TOF, and cerebral MR venography.In all subjects, flow images were acquired with a 2D fast cine PC-MRI pulse sequence with retrospective peripheral gating, so that the 32 frames analyzed covered the entire CC. The MRI parameters were as follows: echo-time TE = 11-17 ms; repetition time TR = 29-43 ms; 4 views per segment, flip angle: 25° for vascular flows, 20° for CSF flows; field of view (FOV) = 14 × 14 mm2; matrix 256 × 128; slice thickness 5 mm. Velocity (encoding) sensitization was set at 80 cm/s for the vessels, 15 cm/s for the aqueduct and 5 cm/s for the cervical subarachnoid space. Sagittal scout view sequences were used to localize the anatomical levels for flow quantification. The acquisition planes were selected perpendicular to the presumed direction of the flow, and are represented on Figure 1. Sections through the C2-C3 subarachnoid spaces level (a1), and Sylvius aqueduct (b) were used for CSF flow measurement. By varying the velocity encoding, the same cervical section level (a2) was used to measure vascular flows in left and right internal carotids (ICA), vertebral arteries (VA), and internal jugular veins (IJV). By using 3D TOF sequence as a localizer, and with adjusted velocity encoding, the intracerebral vascular plane (c) was used to measure arterial flows in the basilar artery (BA) and in the intrapetrous ICA, and venous flows in the superior sagittal sinus (SSS) and the straight sinus (SRS). Finally, a coronal section (d) enabled measurement of the venous flows in the SSS, and in the left and right lateral transverse sinuses (TS).
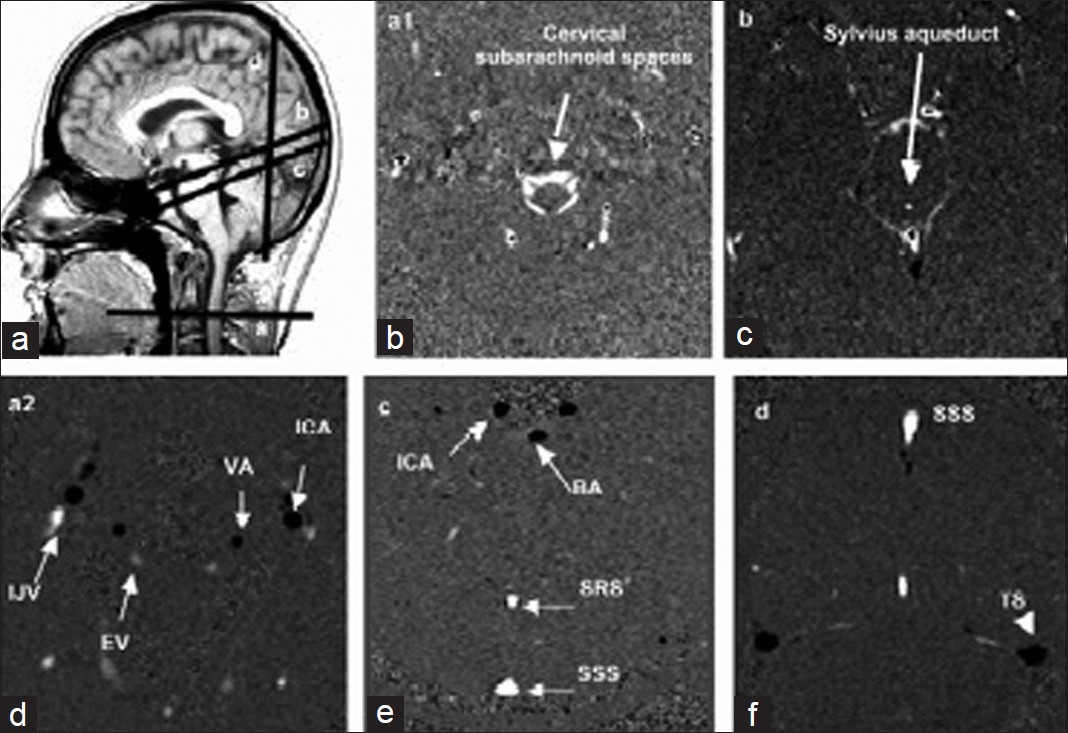
- Data acquisition. Sagittal scout view sequences were used to localize the anatomical levels for flow quantification. The acquisition planes were selected perpendicular to the presumed direction of the flow. Sections through these different levels, for each flow series are represented.
Black pixels represent the flows entering in the section plane, white pixels represent flows directed out of the section plane, and gray pixels correspond to immobile tissues.
The acquisition time for each flow series was about 40 seconds, with a slight fluctuation that depended on the participant's heart rate, resulting in a total additional examination time of 5 minutes.
3 - Data analysis
Data were analyzed using validated image processing software[3] with an optimized CSF and blood flows segmentation algorithm, which automatically extracts the region of interest (ROI) at each level, and calculates its flow curves over the 32 segments of the CC.
Then, the venous, arterial, and CSF flow curves were generated within one CC.
For the arterial flows, we calculated the mean cerebral arterial total blood flow (CBF) in milliliters per minute, at cervical and cerebral levels. The cervical CBF was evaluated as the sum of arterial flows in left and right ICA and VA, and the cerebral CBF as the sum of arterial flows in left and right intrapetrous ICA and in the BA. Similarly, the cervical cerebral venous flow was calculated by summing the venous flows in left and right Internal jugular vein (IJV) and epidural veins (EV). The cerebral venous blood flow was calculated as the sum of mean venous flows in the SSS and SRS.
Similarly, CSF flow curves at aqueductal and cervical levels were reconstructed, and CSF stroke volumes, which represent the CSF volumes displaced in both directions through the considered ROI at each level.[1718]
4 - Statistical analysis
Comparison of vascular flows and CSF stroke volumes between the 3 populations (HV, CVT, and NoCVT) was performed using multivariate analysis. The level for statistical significance (P value) was set at 0.05.
RESULTS
1 - General
Table 1 compares mean flow values between the 3 studied groups (volunteers – HV, NoCVT patients confirmed with MRI venogram, and CVT patients).
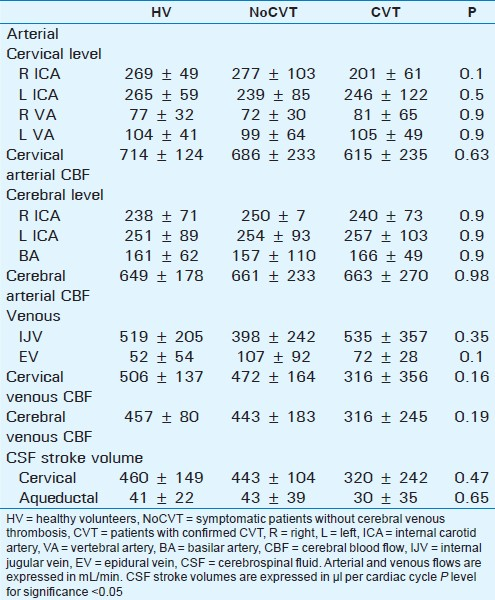
The mean arterial blood flows in each arterial vessel are shown, expressed in mL/min. Comparison showed no side difference for even arteries.
Mean CBF comparison between the 3 groups showed no statistical difference, at both cervical and cerebral levels.
Similarly there were no significant differences between cerebral and cervical venous CBF. Although mean value in CVT patients was lower than in HV and NoCVT groups, standard deviation was greater, which makes testing insignificant. CVT group seemed to have much greater heterogeneity in patterns of venous CBF than remaining groups.
2 - Symptomatic patients without confirmed thrombosis (NoCVT)
All of the patients were admitted to the emergency room for unusual headaches, and cerebral MRI venography excluded the diagnosis of CVT. Nevertheless, they were hospitalized for etiological exploration (lumbar puncture, biological exams, etc.).
Table 2 shows the individual flow values in each vein and sinus in NoCVT patients. The averaged venous flows are summarized in the bottom rows, with large standard deviations, indicating aforementioned heterogeneity of venous flows.
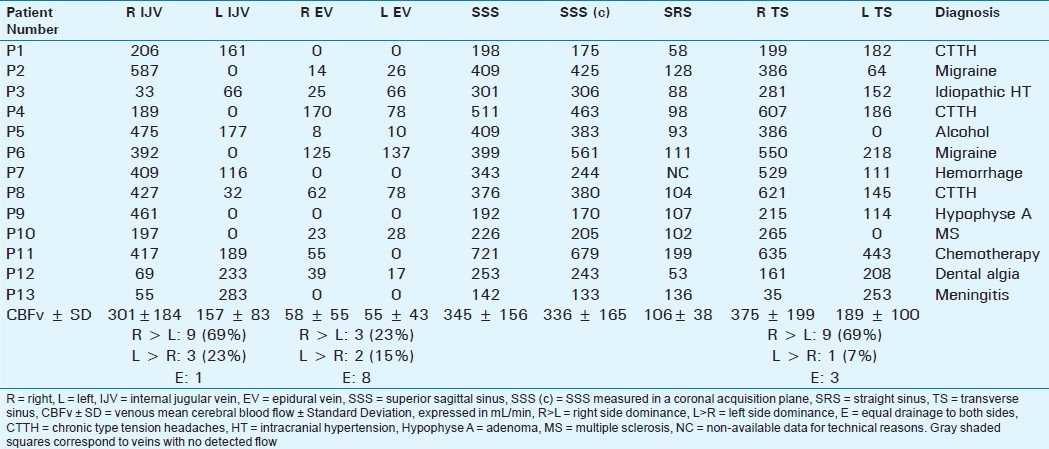
In agreement with previous results published in healthy young controls,[19] we found:
-
Significant right-sided predominance of venous flows in the IJV and in the TS
-
However, a high heterogeneity of venous drainage was detected [Table 2], in both quantitative evaluations (represented with value dispersion and high standard deviations), and in lateralization. In addition, 5 subjects had a strictly unilateral right IJV collecting the cerebral venous blood flow, with contralateral IJV flow equal to 0, and in 4 of them, the homolateral TS flow was significantly reduced (<20%) or null (patients P2, P4, P5, and P10).
-
Significant preponderance of the jugular venous drainage (left + right IJV mean flow: 398 ± 242 mL/min) over the epidural drainage (107 ± 92 mL/min) in a significant way (P = 0.001). Cervical drainage was exclusively jugular in 4 patients, with absent flows in both epidural veins.
CSF flow curves analysis provided the CSF stroke volumes at the aqueductal (43 ± 39 μL/min) and cervical (443 ± 104 μL/min) levels [Table 1], which were not statistically different from those obtained in HV (P: 0.65 and 0.47, respectively).
PC-MRI analysis found unusual results in 1 patient (P13), who had a dramatic reduction of CBF (total arterial flow: 387 mL/min; total venous flow: 278 mL/min), with a significant increase of aqueductal CSF stroke volume (146 μL/CC) and a blockage of cervical CSF circulation. This 32-year-old young female had serious neurological dysfunction (coma, severe headaches) related to bacterial meningitis.
3 - Patients with confirmed thrombosis (CVT group)
Six female patients were diagnosed with CVT, with different single or multiple thrombosis on MR venography.
Arterial flows were rather normal in these patients, with a mean cerebral mean flow of 663 ± 270 mL/min, and a mean cervical cerebral flow at 615 ± 235 ml/min, not statistically different from those obtained in the other populations (P: 0.98 and 0.63, respectively).
CSF stroke volumes were more heterogeneous, and are represented in Figure 2 for cervical and aqueductal CSF stroke volumes. We found CSF flow abnormalities in 5 of these patients.
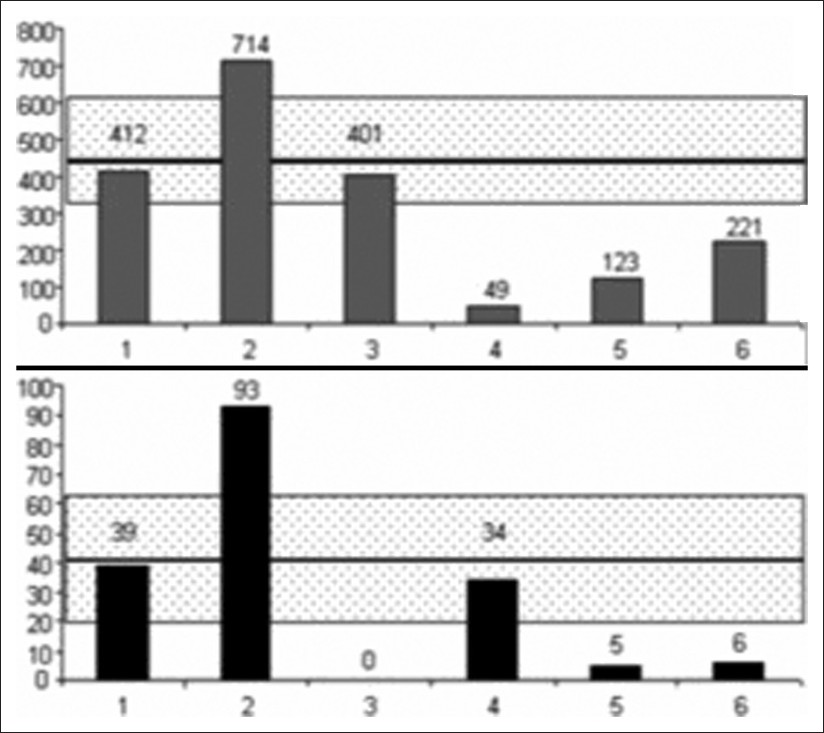
- Cervical (upper) and aqueductal (lower) CSF represented in patients with CVT. CSF stroke volumes are represented in μL per cardiac cycle in each 1 of the 6 patients. The dashed rectangle represents the average ± 1 standard deviation, observed in the control HV's population.
DISCUSSION
Veins
Our observational study confirms the heterogeneity in venous flows [Tables 1 and 2] in NoCVT group, similar to the results previously published.[10] This heterogeneity shows the difficulty in distinguishing between the physical side dominance and partial venous thrombosis, as shown in Table 3. In patients T1 and T2, flow is not null in the IJV but highly asymmetric as compared to contralateral vein. The blockage of these IJV was confirmed on venography. On the contrary, in patients T4, T5, and T6, the venous flow was bilaterally absent in the IJV although venography did not show thrombosis at this level. In patients T5 and T6, the absence of flow can be functional, related to the stenosis in the upstream flow in the ipsilateral TS.
Our results showed higher levels of lateralized venous flows in both IJV and TS, in the NoCVT population. This side dominance in paired venous pathways has been described in previous studies with PC-MRI technique[910] or with angiography[19] with different percentages probably related to the used technique.
PC-MRI enables quantification of venous flows in SSS, SRS, TS, as well as in cervical veins, with a good detection rate, as previously shown in HV.[101520]
Anatomical, and physiological publications have stressed the preponderant role played by the jugular system in cephalic venous drainage in physiological states. Although the vertebral and cervical epidural venous drainage have been considered as accessory until recently, it is now admitted that epidural veins can enlarge in some pathological states as intracranial hypotension,[21] and that they can play a crucial role in the venous drainage of the cephalic extremity in the upright position.[13]
Arteries
The small individual variability of arterial bed helps reproducibility of these studies in large populations.[8] Recent studies have used MRI techniques, with comparable and reproducible results.[2223] Buijs et al.,[24] studied CBF values in a large population, and found a constant contribution of the right, left ICA, and BA (41%, 40% and 19% of total CBF, respectively), which was stable through ageing, although associated with a proportional decrease in CBF. In our study, total CBF measured at the cervical and intracerebral levels in HV and NoCVT were comparable to those obtained in healthy adults in a previous study,[12] and contribution of each intracranial (right ICA: 36% / left ICA: 40%, BA: 24%) vessel was comparable to published results.
Observations in patients with confirmed CVT
CVT offers the opportunity to investigate intracerebral dynamic modifications initiated with venous dysfunction. The limit of this study is the small size of CVT patients, especially with different sites of sinus and / or vein occlusion, which makes it difficult to generalize.
Furthermore, all CVT patients were female, but cerebral blood and CSF published results with PC-MRI in healthy young or elderly subjects have failed to show sex-related differences.[1025] Nevertheless, the study has rather an observational nature. Our aim was not to prove PC-MRIs’ superiority to MR venogram or standard MR protocols in CVT diagnosis, but to suggest that these techniques could be used complementarily to understand intracranial dynamics.
Firstly, the absence of flow in SSS (T1, T5, and T6) was correlated to MR venography results. On the contrary, technical problems in TS flows acquisition were encountered in T1 and T2, which makes it difficult to reach a conclusion.
Interestingly, although very weak, a venous flow was measured in IJV that were considered as blocked on MR venography for 2 patients (T1 and T2). In these 2 patients, the cervical level of IJV flow analysis was caudal to the thrombosis level, which suggests the development of a collateral drainage pathway.
Jugular drainage was absent in 3 patients (T4, T5, and T6), without visualized jugular blockage on MR venography. This is probably related to the blockage of cerebral venous structures above. More surprisingly, epidural drainage was not increased in these patients, which suggests the implication of other accessory venous drainage pathways (as pterygoid plexus and ophthalmic veins). Furthermore, in CVT patients, arterial to venous blood flows ratios were elevated [Table 1], which suggests that when venous blockage occurs in principal venous pathways, drainage in the accessory veins becomes preponderant.
Mean CSF flows were comparable to those in healthy adults, but individual analysis showed normal results in only 1 patient (T1) and major alterations in 5 patients.
Aqueductal and Cervical CSF stroke volumes were increased in 1 patient (T2), with obstruction of left venous drainage pathways (TS and IJV) and hemorrhagic infarct. In this patient, although venous outflow was exclusively drained by the right IJV, cervical venous flow was significantly elevated. These results suggest a hyper-dynamic functioning in the 3 outflow systems (subarachnoid, ventricular, and jugular), which according to the Monro-Kellie doctrine, must be related to a raise of ICP, provoked by the hemorrhage.
In patient T3, cervical CSF stroke volume was within normal values, while the aqueductal flow was null. The re-examination of conventional MRI images visualized blockage at the aqueductal level [Figure 3], probably related to secondary intraventricular hemorrhage.
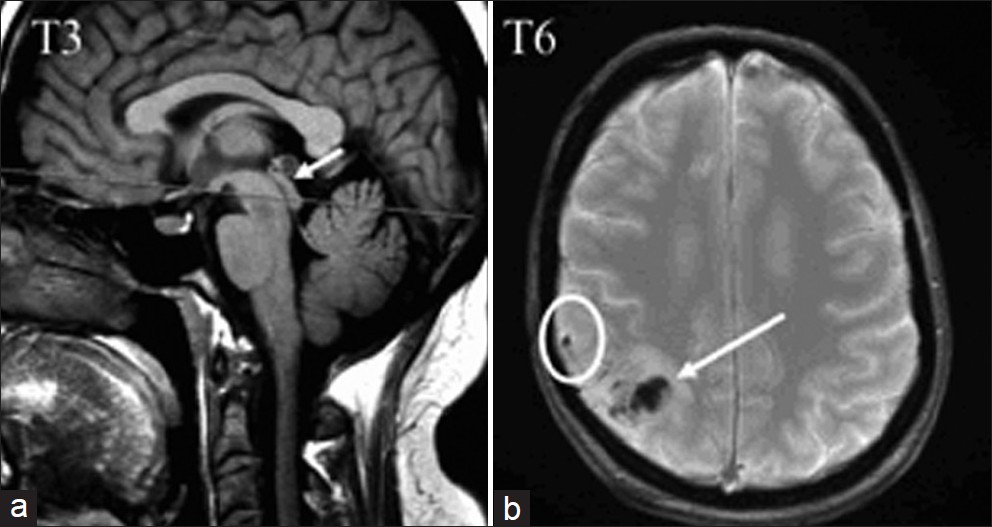
- In patient T3, a sagittal T1 view shows the aqueductal stenosis (arrow), probably secondary to the diffusion of hemorrhage in the ventricular space. In patient T6, an axial T2* image shows the parietal cortical vein thrombosis (in a circle), and cortical venous infarct (arrow).
In the other 3 patients, we observed a decrease in CSF stroke volume either in the cervical (T4) or in both cervical and aqueductal (T5 and T6) compartments. We did not find any clinical, chronological, or radiological specificity in these patients, and correlations to occlusion site were not possible. These results were surprising, as it is logically expected to observe hyper-dynamic flows, related to elevated ICP. In these patients, attenuation of CSF oscillations was probably related to hemorrhage extension to the cerebral SAS [Figure 3], which would increase resistance to CSF flows in the SAS, and thus reduce the compliance of the subarachnoid CSF compartment.
CONCLUSION
CVT produces highly individualized patterns of disturbances in the venous blood drainage.
The results obtained in 5 out of 6 patients with CVT stress the importance of dynamic interaction between brain compartmental components, especially the role played by the venous system in the volume regulation, and thus in CSF flow dynamics.
Due to the small size of CVT cohort, these results need to be confirmed in a larger population.
ACKNOWLEDGMENTS
This study has been financially supported by the European FEDER intereg IVA. We thank Dr. Agnès Villette who helped in the selection and inclusion of patients.
Source of Support: European FEDER intereg IVA
Conflict of Interest: None declared.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2012/2/1/41/99158
REFERENCES
- Radiological assessment of hydrocephalus: New theories and implications for therapy. Neurosurg Rev. 2004;27:145-65. discussion 166-7
- [Google Scholar]
- Pulsatile brain movement and associated hydrodynamics studied by magnetic resonance phase imaging. The Monro-Kellie doctrine revisited. Neuroradiology. 1992;34:370-80.
- [Google Scholar]
- Cerebrospinal fluid dynamics and relation with blood flow: A magnetic resonance study with semiautomated cerebrospinal fluid segmentation. Invest Radiol. 2001;36:368-77.
- [Google Scholar]
- Hemodynamically independent analysis of cerebrospinal fluid and brain motion observed with dynamic phase contrast MRI. Magn Reson Med. 1996;35:741-54.
- [Google Scholar]
- Relationship between cerebrospinal fluid and blood dynamics in healthy volunteers and patients with communicating hydrocephalus. Invest Radiol. 2004;39:45-55.
- [Google Scholar]
- Idiopathic hydrocephalus in children and idiopathic intracranial hypertension in adults: Two manifestations of the same pathophysiological process? J Neurosurg. 2007;107:439-44.
- [Google Scholar]
- The pathophysiology of idiopathic normal pressure hydrocephalus: Cerebral ischemia or altered venous hemodynamics? AJNR Am J Neuroradiol. 2008;29:198-203.
- [Google Scholar]
- Physiology of cerebral venous blood flow: From experimental data in animals to normal function in humans. Brain Res Brain Res Rev. 2004;46:243-60.
- [Google Scholar]
- Cerebral MR venography: Normal anatomy and potential diagnostic pitfalls. AJNR Am J Neuroradiol. 2000;21:74-8.
- [Google Scholar]
- A phase-contrast MRI study of physiologic cerebral venous flow. J Cereb Blood Flow Metab. 2009;29:1208-15.
- [Google Scholar]
- The use of gated cine phase contrast and MR venography in achondroplasia. Childs Nerv Syst. 2000;16:569-75. discussion 575-67
- [Google Scholar]
- Brain hydrodynamics study by phase-contrast magnetic resonance imaging and transcranial color doppler. J Magn Reson Imaging. 2006;24:995-1004.
- [Google Scholar]
- Quantifying the effect of posture on intracranial physiology in humans by MRI flow studies. J Magn Reson Imaging. 2005;22:591-6.
- [Google Scholar]
- Human cerebral venous outflow pathway depends on posture and central venous pressure. J Physiol. 2004;560:317-27.
- [Google Scholar]
- Physiologic variations in dural venous sinus flow on phase-contrast MR imaging. AJR Am J Roentgenol. 2000;175:221-5.
- [Google Scholar]
- Assessment of intracranial venous hemodynamics in normal individuals and patients with cerebral venous thrombosis. Stroke. 1999;30:70-5.
- [Google Scholar]
- Flow dynamics of cerebrospinal fluid: Assessment with phase-contrast velocity MR imaging performed with retrospective cardiac gating. Radiology. 1992;183:395-405.
- [Google Scholar]
- Cerebrospinal fluid flow measured by phase-contrast cine MR. AJNR Am J Neuroradiol. 1993;14:1301-7. discussion 1309-10
- [Google Scholar]
- Evaluation by angiography of the lateral dominance of the drainage of the dural venous sinuses. Surg Radiol Anat. 1993;15:125-30.
- [Google Scholar]
- Flow quantification in the superior sagittal sinus using magnetic resonance. Neurology. 1990;40:813-5.
- [Google Scholar]
- Giant cervical epidural veins after lumbar puncture in a case of intracranial hypotension. AJNR Am J Neuroradiol. 2000;21:787-9.
- [Google Scholar]
- Measurement of time-averaged flow in the middle cerebral artery by magnetic resonance imaging. Br J Radiol. 1991;64:178-81.
- [Google Scholar]
- Comparison of cerebral artery blood flow measurements with gated cine and ungated phase-contrast techniques. J Magn Reson Imaging. 1993;3:705-12.
- [Google Scholar]
- Effect of age on cerebral blood flow: Measurement with ungated two-dimensional phase-contrast MR angiography in 250 adults. Radiology. 1998;209:667-74.
- [Google Scholar]
- Aging effects on cerebral blood and cerebrospinal fluid flows. J Cereb Blood Flow Metab. 2007;27:1563-72.
- [Google Scholar]






