Translate this page into:
Focal Hepatic Fluorodeoxyglucose Uptake Mimics Liver Metastasis Following External Beam Radiation for Gastroesophageal Cancers: A Case and Review of the Literature
Address for correspondence: Dr. Samuel J Klempner, The Angeles Clinic and Research Institute, 11818 Wilshire Blvd, Los Angeles, CA 90025, USA. E-mail: sklempner@theangelesclinic.org
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Patients with locally advanced gastroesophageal cancers frequently undergo concurrent chemotherapy and radiation (CRT). 18-fluorodeoxyglucose-positron emission tomography (18FDG-PET) in combination with computed tomography is used for disease staging and assessing response to therapy. 18FDG-PET interpretation is subject to confounding influences including infectious/inflammatory conditions, serum glucose, and concurrent medications. Radiotherapy induces tissue damage, which may be associated with FDG-avidity; however, few reports have described the focal areas of hepatic uptake following concurrent chemoradiation (CRT). Distinguishing hepatic FDG uptake from disease progression represents an important clinical scenario. Here, we present two cases of unexpected FDG uptake in the liver after CRT and review the literature describing incidental liver uptake on FDG-PET.
Keywords
False-positive
gastroesophageal
liver
metastases
positron emission tomography-computed tomography
radiation

INTRODUCTION
Positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG) has emerged as an increasingly common component of pretreatment cancer staging and response assessment. The use of concurrent computed tomography (CT) and PET (PET-CT) can improve detection sensitivity and is used in target delineation for radiation treatment planning.[123]
The MUNICON Phase II trial included 110 patients with locally advanced gastroesophageal adenocarcinoma and suggested that a metabolic response (decrease ≥35% in standardized uptake value [SUV]) 2 weeks after starting chemotherapy was strongly associated with improved survival.[2] Despite established sensitivity, 18F-FDG PET is not tumor specific, and there can be nontumor-related uptake of FDG in postoperative wound sites, normal physiological FDG uptake, and sites of postradiation inflammatory changes. These “false-positive” findings can be mistaken as residual disease or sites of new disease that may trigger unwarranted biopsies and exploratory surgeries.
We reviewed 64 patients and identified those who received 18F-FDG PET before and after concurrent chemotherapy and radiation. We present two patients with Her2-amplified gastroesophageal adenocarcinoma who were identified to have a focal area of intense 18F-FDG uptake in the left lobe of the liver in posttreatment PET/CT obtained after completion of concurrent CRT. Close radiographic monitoring observed waning FDG-uptake confirming nonmalignant etiology and avoidance of more invasive approaches. This represents the largest series focused on this important clinical scenario.
CASE REPORTS
Case 1
A 60-year-old man was diagnosed with clinical Stage IV, Her2-amplified metastatic gastroesophageal junction (GEJ) adenocarcinoma. Pretreatment PET-CT revealed an intensely FDG-avid GEJ mass extending to fundus with FDG-avid subcarinal and retroperitoneal lymph nodes. The patient underwent laparoscopic staging demonstrating large bulky tumor along lesser curvature extending to and encasing the left gastric artery with adenopathy. The patient received five cycles of biweekly leucovorin, 5-flurouracil, and oxaliplatin (FOLFOX) and trastuzumab 6 mg/kg every 3 weeks. A PET/CT showed decreased FDG avidity in the GEJ mass [Figure 1a and b]. To maximize local control and swallowing function, he was treated with 5-fluorouracil and oxaliplatin with concurrent radiation to 37.5 Gy at 2.5 Gy per fraction. Following radiation, he resumed full dose FOLFOX and trastuzumab. A PET/CT scan was obtained 34 days after completion of radiation [Figure 1c and d]. There was 109 mm × 82 mm focal area of FDG uptake in the left lobe measuring SUV 12.1. Hepatic function was within normal limits, and he tolerated CRT with good clinical response. Due to concerning PET-CT findings, a magnetic resonance imaging (MRI) was performed which did not show any focal hepatic abnormalities. On retrospective imaging review, the FDG avid area of the liver was within the 37.5 Gy isodose line [Figure 1d]. Due to clinical stability, a plan for repeat imaging was favored over more invasive tissue sampling, and a repeat PET/CT scan 3 months [Figure 1e and f] and 6 months [Figure 1g and h] later showed resolution of the FDG avid area in the left hepatic lobe and ongoing systemic response.
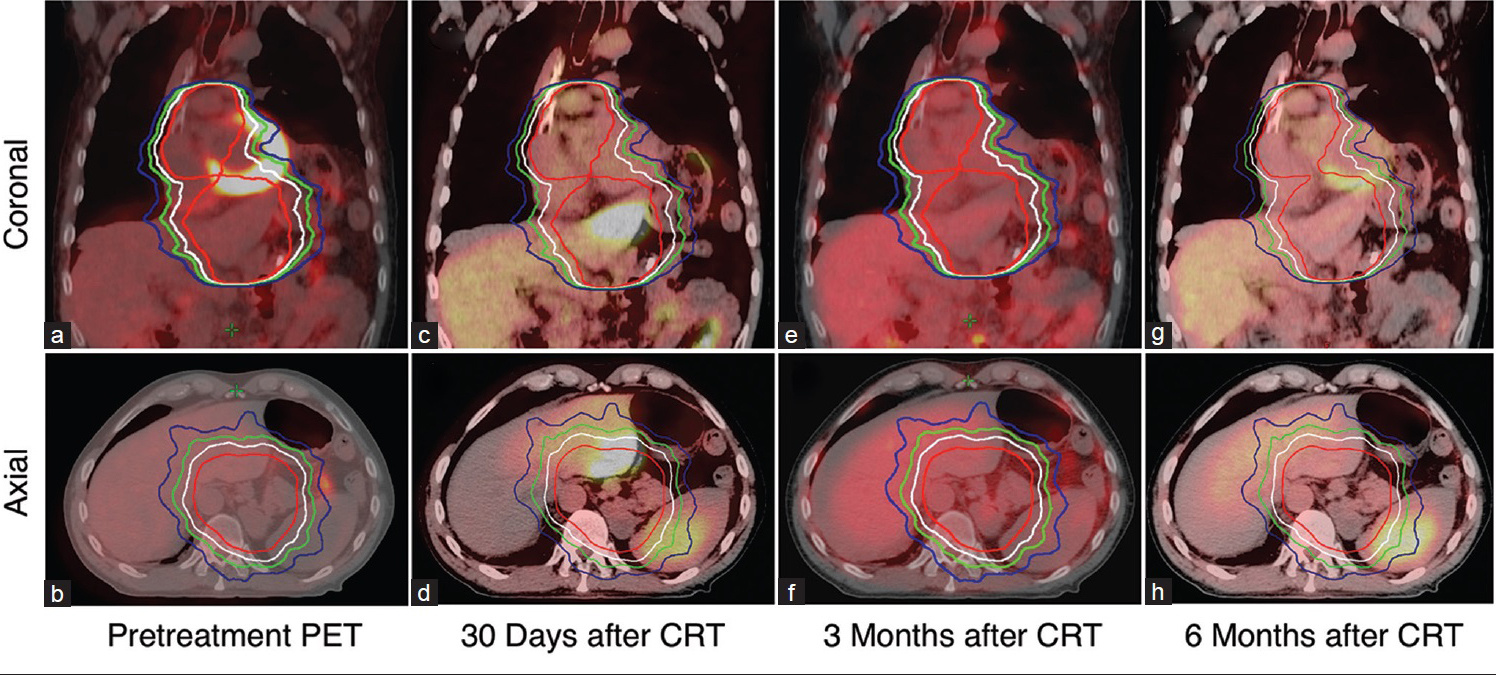
- A 60-year-old man with gastroesophageal junction adenocarcinoma with the radiographic evolution of segment three hepatic uptake in a patient with advanced gastroesophageal adenocarcinoma. (a) Axial images of pretreatment positron emission tomography-computed tomography confirms the absence of fluorodeoxyglucose uptake in the liver (b) positron emission tomography-computed tomography 30, 90, and 180 days after completion of chemoradiation revealed fluorodeoxyglucose uptake in the left lobe of liver (arrow) (d, f, h) positron emission tomography-computed tomography at 3 and 6 months after CRT revealed no fluorodeoxyglucose uptake in the left lobe of the liver. Respective coronal images are shown (a, c, e, g). Area within red, white, green, and blue isodose line encompasses 37.5 Gy, 33.3 Gy, 30 Gy, and 26.3 Gy, respectively.
Case 2
A 55-year-old man diagnosed with clinical T3N0M0 overall Stage IIb Her2-amplified distal esophageal. A pretreatment staging PET/CT scan demonstrated a large 51 mm mass in the lower esophagus extending to the GEJ and to the gastric fundus measuring SUV 8.4 with no suspicious nodal uptake [Figure 2a and b]. He was enrolled in a neoadjuvant CRT trial investigating the addition of trastuzumab to trimodality treatment in Her2-overexpressing esophageal adenocarcinomas. He received 50.4 Gy of radiation with concurrent carboplatin, taxol, and trastuzumab 2 mg/kg weekly. Radiation was delivered using volume modulated arc therapy. A repeat PET-CT was performed 42 days from completion of radiotherapy and demonstrated FDG uptake (SUV 4.5) in the left lobe of the liver corresponding an area of low attenuation in the periphery of the left lobe of the liver measuring 28 mm × 35 mm on CT [Figure 2c and d]. Similar to Case 1, the hepatic function was normal, and retrospective review confirmed the FDG avid area was within the 50.4 Gy isodose line [Figure 2d]. No biopsy was taken from the FDG avid lesion in the liver. A follow-up PET scan 3 months later showed the resolution of FDG uptake [Figure 2e and f].
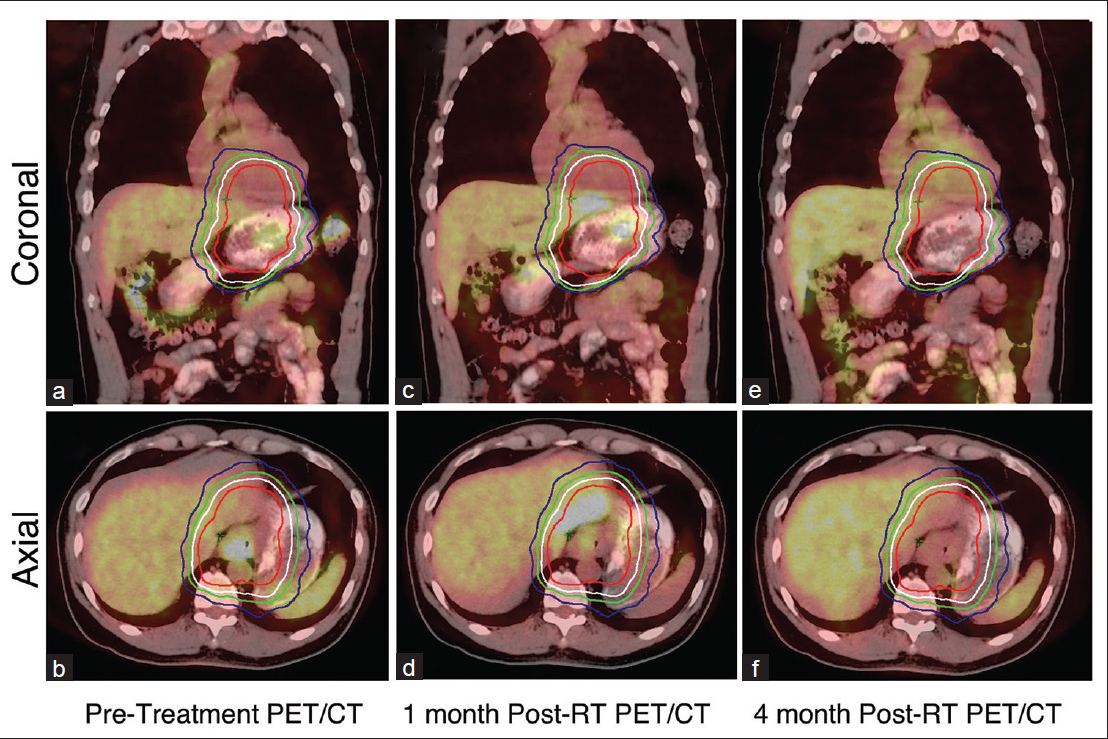
- A 55-year-old man with adenocarcinoma of the distal esophagus. (a and b) Axial images of pretreatment positron emission tomography-computed tomography confirms the absence of fluorodeoxyglucose uptake in the liver, (c and d) positron emission tomography-computed tomography 45 days after completion of chemoradiation revealed fluorodeoxyglucose uptake in the left lobe of liver (arrow), (e and f) positron emission tomography-computed tomography 3 months posttreatment revealed no fluorodeoxyglucose uptake in the left lobe of the liver. Area within red, white, green, and blue isodose line encompasses 50.4 Gy, 45.4 Gy, 40.3 Gy, and 35.3 Gy, respectively.
DISCUSSION
Here, we present the largest series to date highlighting the radiographic phenomenon of focal FDG-uptake in the liver mimicking residual or progressive disease following radiotherapy in gastroesophageal cancers. Our cases suggest that hepatic uptake resolves within 12 weeks of radiotherapy and with careful clinical and radiographic communication invasive procedure may be avoidable.
Patients with GEJ cancers who undergo concurrent chemotherapy and radiation can develop radiation-induced hepatic injury.[4] The symptomatology of radiation-induced livery disease (RILD) typically includes fatigue, right upper quadrant pain, ascites, anicteric hepatomegaly, and elevated alkaline phosphatase. Pathologically, the liver parenchyma displays veno-occlusive disease, marked central venous congestion, sparing of large veins, and atrophy of adjacent hepatocytes. However, elevated liver enzymes are not reliable markers for RILD. Paudel et al. reported two patients with 18F-PET uptake in the left lobe of the liver following chemoradiation. There were no elevated liver enzymes during or after chemoradiation[5] and personal communication with corresponding author. Biopsy from both cases showed dense fibrous tissue without any evidence of tumor. Our findings are consistent Paudel et al. with neither of our patients experiencing liver function abnormalities. Although not well studied, both our patients were treated with intensity-modulated radiotherapy which optimizes dose to target while minimizing dose to surrounding normal organs such as the liver and kidney, possibly reducing RILD risk.
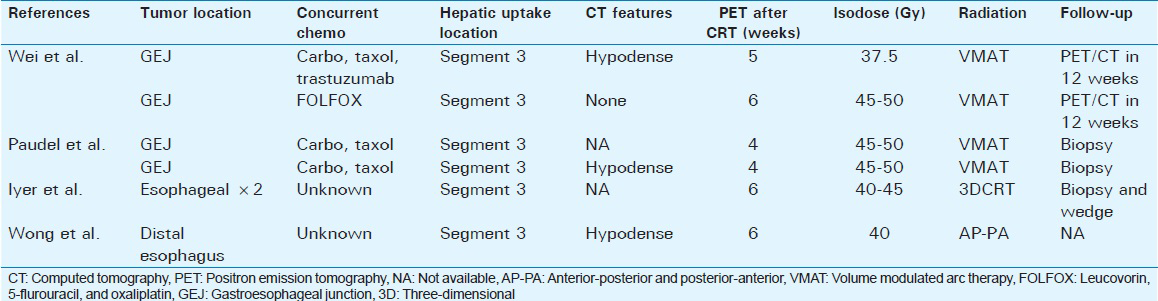
Previous small studies have suggested that hepatic FDG-uptake is seen within 2–6 weeks after radiation therapy in distal esophageal cancers.[6] In a series of 26 patients, abnormal left lobe hepatic FDG-uptake was seen in 8% of patients at 6 weeks, with FDG uptake increasing focally >50% over baseline in two patients (54% and 133%).[6] Further clinic pathologic details were not described in these cases [Table 1]. False-positive FDG-uptake after RT has led to potentially avoidable surgical intervention in some cases.[57] Nakahara et al. reported a case of FDG uptake in the left lobe of liver 28 days after completion of radiation for esophageal cancer, with resolution on follow-up PET scan 3 months later, consistent with our cases.[8] In our series, we found FDG uptake within 34 and 42 days postradiation. Interestingly, among the remaining 62 patients in our database, hepatic FDG-uptake was not observed, suggesting that intrinsic biologic features in addition to treatment and imaging timing may play a role. Neither of our patients was known to have preexisting hepatitis, steatohepatitis, or on concurrent hepatotoxins. We conducted a literature search in the PubMed database to identify similar cases. Using the search terms, false-positive, FDG uptake, liver, and radiation we identified a total of four additional cases. Where available clinic pathologic characteristics are presented in Table 1.
Our case reinforces the importance of a multidisciplinary approach to the care of patients with esophageal and gastric cancers to diagnose and manage complex cases. Radiologists who suspect false-FDG uptake should correlate PET-CT findings with radiation treatment fields. The radiation oncologist can perform image fusion of the CT portion of the PET-CT scan with the CT planning scan used for radiation treatment planning, thus allowing for co-registration of PET images with radiation isodose lines to determine dose to the area of PET uptake. If there is continued concern for malignancy, a follow-up liver MRI with and without gadolinium contrast would be warranted. However, if the multidisciplinary team is still concerned for malignancy, then a CT-guided biopsy is warranted.
Notably, both our patients had Her2-amplified disease, a feature not reported most prior series but shared with Paudel et al.[5] To our knowledge, there is no preclinical or clinical evidence that Her2 amplification is associated with increased radiation risk. However, trastuzumab has been affiliated with radiation recall in breast cancer patients receiving whole breast radiotherapy.[9] Larger studies are needed to confirm the possible associated between Her2-amplified GEJ tumors and hepatic FDG-uptake. The ongoing Radiation Therapy Oncology Group 1010 trial of neoadjuvant chemoradiation with trastuzumab may clarify our findings.
CONCLUSIONS
Determination of whether radiographic findings represent true disease progression or not depends importantly on clinical considerations. Although metastatic disease to the liver is common in GEJ cancers, the clinical features supported false-positive hepatic FDG-uptake in both our patients. With other imaging modalities such as MRI, the confidence in short interval imaging over invasive procedure can be further increased. The follow-up MRI in our patient failed to demonstrate any focal increased T2-signal intensity as may be expected from a liver metastasis.[10] If clinical suspicion remains high, our data will support repeat PET-CT to provide a functional assessment 3 months after the incident imaging. With the rising incidence of distal esophageal adenocarcinomas and increasing use of PET-CT and neoadjuvant therapy, an appreciation of false-positive hepatic uptake is important to both radiologists, radiation oncologists, and medical oncologists.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2016/6/1/30/188089
REFERENCES
- Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: Results of a prospective trial. J Clin Oncol. 2003;21:428-32.
- [Google Scholar]
- PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: The MUNICON phase II trial. Lancet Oncol. 2007;8:797-805.
- [Google Scholar]
- Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol. 2004;22:3805-12.
- [Google Scholar]
- Evaluating the role of fluorodeoxyglucose positron emission tomography-computed tomography in multi-disciplinary team recommendations for oesophago-gastric cancer. Br J Cancer. 2013;109:1445-50.
- [Google Scholar]
- False positive 18F-fluorodeoxyglucose positron emission tomography/computed tomography liver lesion mimicking metastasis in 2 patients with gastroesophageal cancer. Pract Radiat Oncol. 2014;4:368-71.
- [Google Scholar]
- PET/CT and hepatic radiation injury in esophageal cancer patients. Cancer Imaging. 2007;7:189-94.
- [Google Scholar]
- Hepatic radiation injury mimicking a metastasis on positron-emission tomography/computed tomography in a patient with esophageal carcinoma. J Thorac Oncol. 2009;4:1442-4.
- [Google Scholar]
- Dose-related fluorodeoxyglucose uptake in acute radiation-induced hepatitis. Eur J Gastroenterol Hepatol. 2008;20:1040-4.
- [Google Scholar]
- Radiation recall reaction induced by adjuvant trastuzumab (herceptin) Case Rep Med 2009 2009 307894
- [Google Scholar]
- Identifying and distinguishing treatment effects and complications from malignancy at FDG PET/CT. Radiographics. 2013;33:1817-34.
- [Google Scholar]






