Translate this page into:
Effects of Neoadjuvant Chemotherapy on Benign Breast Lesions Compared to Cancers: Should an Additional Lesion on Magnetic Resonance Imaging Responding Similar to Cancer after Neoadjuvant Chemotherapy be Viewed with Suspicion?
Address for correspondence: Dr. Rebecca Leddy, Department of Radiology, Medical University of South Carolina, 96 Jonathan Lucas Street, Charleston, SC 29425, USA. E-mail: leddyr@musc.edu
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Purpose:
Determining the effects of neoadjuvant chemotherapy (NAC) on benign breast lesions and to evaluate their response in comparison to breast cancers.
Methods:
A retrospective analysis performed on breast cancer patients between 2008 and 2014 to identify patients who had a pre- and post-NAC magnetic resonance imaging (MRI) and biopsy-proven benign lesions. Pre- and post-NAC size and intensity of enhancement of benign lesions and cancers were measured. Breast glandularity and background enhancement were graded. A 2 × 2 repeated measures ANOVAs and Sidak post hoc tests were conducted for multiple comparisons. Paired t-tests were conducted to examine changes over time, and two-tailed P values were reported.
Results:
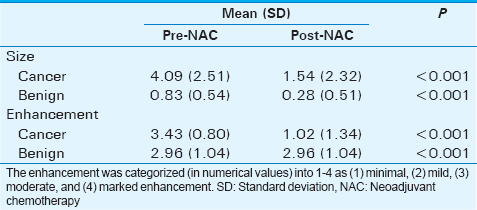
The effects of NAC in 38 cancers were compared to the effects of NAC in 47 benign lesions in these patients. From pre- to post-NAC, the mean size (cm) of malignant lesions on MRI decreased from 4.09 (±standard deviation [SD] 2.51) to 1.54 (±SD 2.32), (P < 0.001); the mean size (cm) of benign lesions decreased from 0.83 (±SD 0.54 cm) to 0.28 (±SD 0.51), (P < 0.001). Both benign and malignant lesions decreased in size after NAC, the size reduction in malignant lesions was significantly greater than benign lesions. From pre- to post-NAC, the mean lesion enhancement of the malignant lesions (scale 1–4) decreased from 3.43 (±SD 0.80) to 1.02 (±SD 1.34); the mean lesion enhancement of benign lesions decreased from 2.96 (±SD 1.04) to 0.98 (±SD 1.51). For both benign and malignant lesions, there was a significant overall reduction in enhancement after NAC from moderate at pre-NAC to minimal at post-NAC, P < 0.001. There was no overall difference in the enhancement of cancers (mean = 2.22, SD = 0.79) versus benign lesions (mean = 1.97, SD = 1.08), (P = 0.23). There was no significant change in glandularity from pretherapy (mean = 3.11, SD = 0.84) to posttherapy (mean = 3.13, SD = 0.82), P < 0.001.
Conclusion:
Similar to cancers, benign breast lesions also show a significant decrease in size and enhancement after NAC; however, the decrease in size is less compared to cancers.
Keywords
Benign breast lesions on magnetic resonance imaging
breast cancer
breast magnetic resonance imaging
neoadjuvant chemotherapy

INTRODUCTION
Neoadjuvant chemotherapy (NAC) has evolved in its role in therapy of both inoperable and operable breast cancer.[123] Potential advantages of NAC include improved outcomes in breast conservation therapy, reduction in size of inoperable cancers rendering them operable, decrease in mastectomy rates in patients with large cancers, decrease in the size of involved axillary nodes, and early treatment of micrometastatic disease.[1345678] The early assessment of tumor response to NAC also allows for a potential modification in the treatment plan and may affect long-term outcome.[6]
NAC has been reported to significantly change tumor morphology, grade, and receptor expression.[9] Reported findings include the loss of support in the tumor vasculature due to direct effects on endothelial cell function; lobular morphologic change due to decreased cellularity and increased fibrosis;[10] increased nuclear grade; changes in tubule formation and mitotic count; tumor cell enlargement; and altered inflammatory reaction.[51011] In addition to its effects on the pathologic tumor, NAC also leads to morphologic changes in the surrounding normal breast tissue as evidenced on histologic analysis.[5] The histologic changes induced by NAC in nonneoplastic breast tissue include fibrosis, diffuse lobular atrophy, intralobular fibrosis, and drug-induced mild epithelial atypia.[58]
Contrast-enhanced breast magnetic resonance imaging (MRI) has shown to accurately assess pathologic response to NAC.[1213141516171819] Contrast-enhanced breast MRI is valuable before, during, and/or after NAC to assess treatment response and the extent of residual disease before surgery.[20] Pre- and post-NAC contrast-enhanced MRI not only provides morphologic details of the tumor but also helps in assessing the enhancement characteristics based on the uptake of contrast agent into the tumor. It has been reported in some studies that tumors with a higher degree of pretreatment enhancement tend to have a better response to treatment.[192122] It is hypothesized that increased angiogenesis in these cases may favor accessibility of the chemotherapy within the tumor leading to an improved response.[19]
Background parenchymal enhancement (BPE) on MRI is a recognized phenomenon in which the breast parenchyma physiologically enhances after intravenous contrast administration. The degree of BPE can be variable and depends on the patient age, menopausal status,[232425] lactational status,[262728] phase of the menstrual cycle,[232930] and use of hormone replacement therapy.[303132] Other exogenous/technical factors that can affect apparent BPE include the type of MRI sequences and type of contrast agent used.[33] BPE can also be affected by treatment with NAC, radiation therapy, and endocrine therapies.
Although the effects of NAC (as seen on MRI) on breast cancers and BPE have been well studied in the previous literature, the effects of NAC on benign breast lesions have not been evaluated to the best of our knowledge. The lack of such data may lead to management dilemmas in certain clinical situations. In routine clinical practice, many times the patients with newly diagnosed breast cancer may show on MRI, additional benign appearing (Breast Imaging Reporting and Data System [BI-RADS] 2 or 3) lesions in the ipsilateral or contralateral breast that are not biopsied.[34] One may face a management dilemma when such a lesion responds to NAC in a similar fashion as known cancer. Since there is no data available, some radiologists or surgeons may prefer the excision of those lesions as well due to the suspicion of cancer-based on their similar response. Our study may be considered as a baseline study in this regard that will show how benign lesions respond to NAC treatment, and this may help in making appropriate management decisions.
METHODS
Study population
The study was approved by the Institutional Review Board for human investigation. The mammography database was reviewed for patients diagnosed with breast cancer who underwent NAC from 2008 to 2014, and a total of 195 patients were found. From these, 82 patients were excluded because they did not have a complete set of pre- and post-NAC breast MRI imaging available. The charts of the remaining 113 patients who have had both pre- and post-NAC MRI were evaluated for a history of any additional previous benign biopsy performed before the start of NAC treatment. Among these 113 newly diagnosed breast cancer patients, 38 patients were found to have undergone additional benign biopsies for 47 ipsilateral or contralateral lesions either before their breast cancer diagnosis or before receiving NAC. The biopsy-proven benign lesions were identified on the pre-NAC MRI by their enhancement and by the location of the postbiopsy clip markers in place. These 47 biopsy-proven benign lesions formed the cohort for our study. The demographic information including age, race, and gender was noted for each patient.
Imaging and image interpretation
All imaging was performed at an American College of Radiology (ACR) certified “Breast center of excellence” under the ACR guidelines.[35]
Breast MRI with a dedicated phase array breast coil (Siemens Healthcare, Erlangen, Germany) was performed with patients in the prone position. The standard sequences were obtained using the ACR breast MR accreditation guidelines.[36] Axial T1- and T2-weighted precontrast MRI followed by five postcontrast T1-weighted dynamic sequences at 90 s intervals were obtained. Reconstructed sagittal T1-weighted postcontrast images were generated from the axial T1-weighted postcontrast images. The breast glandularity was noted based on the amount of fibroglandular tissue using the noncontrast axial fat-suppressed T1-weighted images on both the pre- and post-NAC MRI. Using the ACR BI-RADS MRI lexicon, the amount of background glandularity was visually assessed by 2 fellowship-trained breast radiologists and categorized by consensus into 1 through 4 using the ACR BI-RADS categories with category 1: almost entirely fatty, category 2: scattered fibroglandular tissue, category 3: heterogeneous fibroglandular tissue, and category 4: extreme fibroglandular tissue.[34] The BPE was recorded using the axial first postcontrast subtracted T1-weighted images obtained at 90 s on both the pre- and post-NAC MRI. Using the ACR BI-RADS MRI lexicon, BPE was also visually assessed by 2 fellowship-trained breast radiologists and categorized by consensus into 1 through 4 with category 1: minimal, category 2: mild, category 3: moderate, and category 4: marked enhancement.[34] The size of breast cancer and the biopsy-proven benign lesion was measured in centimeters (cm) on the first postcontrast axial and sagittal fat-suppressed subtracted T1-weighted images for the maximum dimension on the pre- and post-NAC MRI. In only one patient, cancer and the benign lesion were not well seen on the first postcontrast sequence, and the second postcontrast sequence was used for the measurement. The enhancement of breast cancer and the benign lesion was assessed by 2 fellowship-trained breast radiologists and categorized by consensus as 1 through 4 based on the enhancement characteristics above background glandular enhancement using the first postcontrast axial fat-suppressed subtracted T1-weighted image for both the pre- and post-NAC MRI. The enhancement was categorized as follows (1) minimal enhancement, (2) mild enhancement, (3) moderate enhancement, and (4) marked enhancement with the enhancing aorta as a visual reference for marked enhancement.
Pathology
A pathologic review of the tumor was performed for tumor type, grade, and tumor markers. Based on the classification proposed by Dupont and Page,[3738] the benign pathology was divided into nonproliferative lesions and proliferative lesions.
Statistical analysis
A 2 (intervention: Pre, post) ×2 (tumor type: cancer, benign) repeated measures ANOVA was conducted on the size and enhancement characteristics of benign lesions and cancers. Post hoc tests were conducted using the Sidak method for multiple comparisons. A repeated measures t-test was conducted to examine changes in background breast glandularity and background enhancement over time, and two-tailed P values are reported. The analysis by independent t-tests was preformed to evaluate for any potential differences between the proliferative (p) and nonproliferative (np) groups with respect to changes in benign lesion size, benign lesion enhancement, cancer size, cancer enhancement, and background glandularity and enhancement with two-tailed P values are reported. All analyses were considered statistically significant at the P < 0.05 threshold.
RESULTS
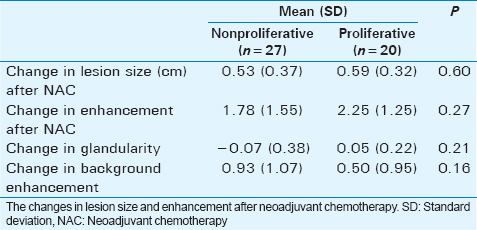
The mean patient age was 53 years; all were females, 55.4% were African-American, 40.4% were Caucasian, and 4.2% were hispanic. Of the 38 breast cancer patients who underwent NAC, pre- and post-NAC MRI and had an ipsilateral or contralateral benign breast biopsy, 37 (97%) had invasive ductal carcinoma and one patient (3%) had invasive lobular carcinoma. Among the 47 benign lesions, 27 (57.4%) lesions were classified as nonproliferative and 20 (42.6%) as proliferative. The effects of NAC in 38 cancers were compared to the effects of NAC in 47 benign lesions in these 38 patients.
Neoadjuvant chemotherapy effects on lesion size
Both cancers and the benign lesions showed a significant decrease in size (P < 0.001) after NAC (Figure 1 and Table 1). The mean cancer size decreased from 4.09 cm (±standard deviation [SD] =2.51 cm) on pre-NAC MRI to 1.54 cm (±SD = 2.32 cm) on the post-NAC MRI. The benign masses also showed a significant decrease in size from the mean size of 0.83 cm (±SD = 0.51 cm) on pre-NAC MRI to 0.28 cm (±SD = 0.51 cm) on post-NAC MRI (Figure 1 and Table 1). Although both benign and malignant lesions decreased in size after NAC, the size reduction in malignant lesions was significantly greater than in benign lesions [F (1, 46) =42.36, P < 0.001].
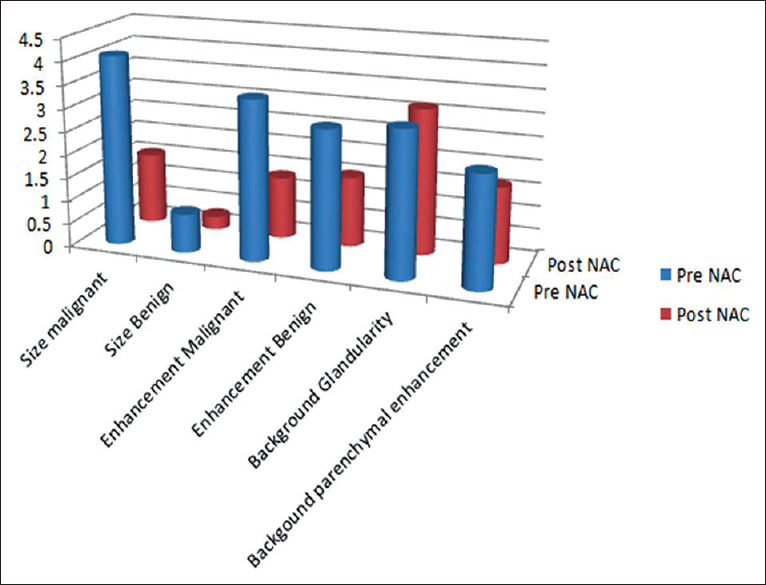
- Effects of neoadjuvant chemotherapy on the size and enhancement of malignant and benign lesions, background breast glandularity, and background parenchymal enhancement.
Neoadjuvant chemotherapy effects on lesion enhancement (scale 1–4)
Cancers showed a decrease from a moderate enhancement value (M = 3.43; ±SD = 0.80) on pre-NAC MRI to a mild enhancement value (M = 1.02; ±SD = 1.34) on post-NAC MRI (P < 0.001) (Figure 1 and Table 1). The benign lesions also showed a decrease from moderate enhancement value (M = 2.96; ±SD = 1.04) to a mild enhancement value (M = 0.98; ±SD = 1.51). There was a significant overall decrease in the enhancement for both benign and malignant lesions after NAC and both types of lesions showed a similar decrease in enhancement without a significant difference in the enhancement patterns (P = 0.23).
Breast glandularity (scale 1–4)
There was no change in the breast glandularity from pre-NAC (M = 3.11; SD = 0.84) to post-NAC treatment (M = 3.13, SD = 0.82); t (46) =0.44, (P = 0.66) (Figure 1).
Breast background parenchymal enhancement (scale 1–4)
There was a significant decrease in BPE from pre-NAC MRI (M = 2.38, SD = 0.97) to post-NAC MRI (M = 1.64, SD = 0.70); t (46) =4.95, (P < 0.001).
Proliferative versus nonproliferative benign lesions
The size reduction from pre-NAC to post-NAC in nonproliferative lesions versus proliferative lesions was not significantly different (M = 0.53 cm, SD = 0.37 cm vs. M =0.59 cm, SD = 0.32 cm; P = 0.60) (Table 2). There was also no significant difference between the enhancement patterns (P = 0.27).
DISCUSSION
Breast MRI is considered helpful to determine the extent of disease in patients with known breast cancer, evaluate for occult malignancy, and assess response in breast cancer patients treated with NAC. MRI can detect occult disease in the ipsilateral breast in about 12–27% of patients and in the contralateral breast in about 4% of patients.[20] In evaluating the response to chemotherapy, breast MRI is better than the clinical examination or mammography.[12131516]
In patients with newly diagnosed breast cancer who receive an MRI, further management not only depends on the extent of known cancer but also on discovery of additional ipsilateral and/or contralateral lesions. Many times, additional work-up with targeted ultrasound and/or additional biopsies is recommended before either any definitive surgical treatment is provided or treatment with NAC is started. In general, an additional lesion having suspicious morphology/dynamic characteristics will be biopsied before the treatment to rule out additional malignancy if an additional cancer diagnosis will change the further management decision. However, the additional lesions having benign or probably benign morphology are frequently left unbiopsied, and many of these patients will proceed to NAC treatment for their cancers. A management dilemma may arise when such a lesion responds to the NAC in a similar fashion as the patient's known cancer responds. This may raise the radiologist's or clinician's level of suspicion about these unbiopsied lesions for possible additional cancer foci.
The results of our study demonstrate that NAC affects benign lesions in a similar fashion as it affects cancers [Figure 2]. Although the size reduction happens to a lesser degree compared to cancers, there is no significant difference in the degree of decrease in the lesion enhancement. The response of benign lesion to NAC can be explained based on the previous work of Yeh et al., and Moll and Chumas.[58] They showed histologic changes such as lobular atrophy, decreased cellularity, and increased fibrosis occurring in normal glandular tissue after NAC treatment. It can be postulated that the same processes can probably happen in benign lesions as well leading to a decrease in size. Since cancers are generally excessively cellular, their size reduction can be expected to be more than the benign lesions. In addition, NAC affects the endothelial lining of the vessels leading to loss of vascular support. Similar changes may be expected to be seen in benign lesions leading to decreased enhancement. Protopapa et al., reported a positive correlation between the degree of tumor angiogenesis and the efficacy of chemotherapy.[39] They proposed increased angiogenesis in a cancer leads to a better delivery of the chemotherapeutic agent within the tumor increasing its chemosensitivity. One may intuitively think that the proliferative benign lesions would be expected to respond more than the nonproliferative benign lesions because they tend have more cellularity and/or microvasculature. However, our study did not show any difference in response between proliferative and nonproliferative lesions. This could be due to a small sample size or a number of other factors in the tumor microenvironment that may affect the response to NAC.
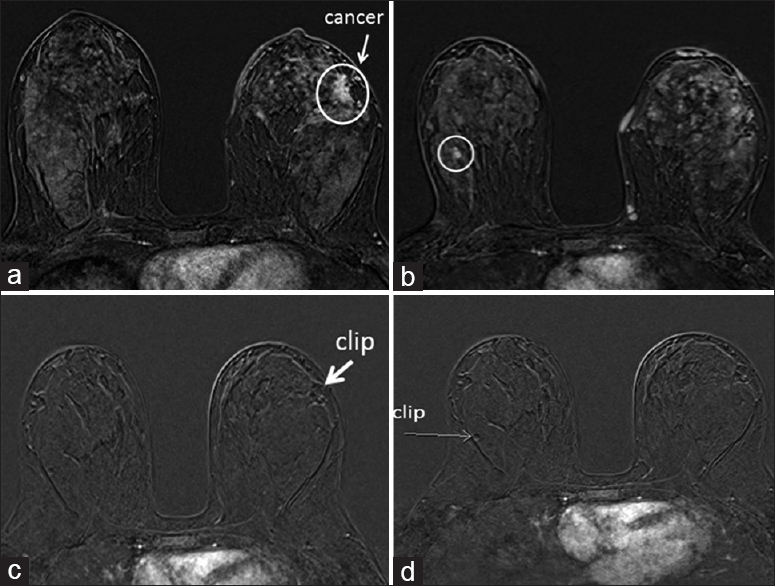
- A 44-year-old female with a history of newly diagnosed left breast cancer at 3 o’clock position (a). Subtraction T1-axial images from the first dynamic sequence of bilateral contrast-enhanced breast magnetic resonance imaging before neoadjuvant chemotherapy (a and b) and after neoadjuvant chemotherapy (c and d) are shown. Another enhancing mass in the right breast (circled image b) was found to be a fibroadenoma on subsequent biopsy. Images c and d from the respective slices after neoadjuvant chemotherapy show disappearance of enhancement in cancer as well as benign lesion (fibroadenoma).
Our study showed a significant decrease in BPE after NAC treatment [Figure 2a and c] which is consistent with the results reported in several previous studies.[40414243] Li et al., reported a decrease in BPE in both the treated and the untreated breast.[40] Due to the changes of lobular atrophy and fibrosis that occurs after the NAC, one may also expect an overall decrease in breast glandularity after NAC treatment. A decrease in breast glandularity after NAC was demonstrated by Chen et al.,[44] when they measured it by quantitative MRI technique. They showed a 10–16% decrease in amount of the glandular tissue after the NAC treatment. Our study did not show any significant decrease in glandularity which could be due to the fact that it was only visually assessed.
The limitations of our study include a small sample size and retrospective nature of the study. The other limitation was that the mean size of the benign lesions was smaller than the mean size of the malignant lesions which could have impacted on some of our results. A future larger study including larger benign lesions may give better comparative results.
CONCLUSION
Benign breast lesions also decrease in size and enhancement after NAC; however, the size reduction is to a lesser degree than breast cancers. Therefore, additional ipsilateral or contralateral lesions seen on preoperative MRI that respond to NAC similar to the patient's known cancer may not be assumed to be cancers. The management decisions should still rely on the initial level of suspicion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2016/6/1/39/190899
REFERENCES
- Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672-85.
- [Google Scholar]
- Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796-804.
- [Google Scholar]
- Preoperative chemotherapy in patients with operable breast cancer: Nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96-102.
- [Google Scholar]
- Neoadjuvant chemotherapy in breast cancer: Significantly enhanced response with docetaxel. J Clin Oncol. 2002;20:1456-66.
- [Google Scholar]
- Morphologic effects of neoadjuvant chemotherapy in locally advanced breast cancer. Pathol Res Pract. 1997;193:187-96.
- [Google Scholar]
- Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48:3342-54.
- [Google Scholar]
- Neoadjuvant chemotherapy in breast-conserving surgery – Consequences on margin status and excision volumes: A nationwide pathology study. Eur J Surg Oncol. 2016;42:986-93.
- [Google Scholar]
- Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. 2005;184:868-77.
- [Google Scholar]
- Effect of neoadjuvant chemotherapy on breast cancer phenotype, ER/PR and HER2 expression – Implications for the practising oncologist. Eur J Cancer. 2016;60:40-8.
- [Google Scholar]
- Typing breast cancer following primary chemotherapy. Histopathology. 1999;35:584-5.
- [Google Scholar]
- The effect of chemotherapy on the morphology of human breast carcinoma. S Afr Med J. 1987;72:103-6.
- [Google Scholar]
- Evaluation of neoadjuvant chemotherapeutic response of locally advanced breast cancer by magnetic resonance imaging. Cancer. 1996;78:91-100.
- [Google Scholar]
- Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy – results from ACRIN 6657/I-SPY TRIAL. Radiology. 2012;263:663-72.
- [Google Scholar]
- Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 2003;181:1275-82.
- [Google Scholar]
- MRI evaluation of pathologically complete response and residual tumors in breast cancer after neoadjuvant chemotherapy. Cancer. 2008;112:17-26.
- [Google Scholar]
- Impact of MRI-evaluated neoadjuvant chemotherapy response on change of surgical recommendation in breast cancer. Ann Surg. 2009;249:448-54.
- [Google Scholar]
- Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst. 2013;105:321-33.
- [Google Scholar]
- Early prediction of pathologic response to neoadjuvant therapy in breast cancer: Systematic review of the accuracy of MRI. Breast. 2012;21:669-77.
- [Google Scholar]
- Dynamic enhanced MRI predicts chemosensitivity in breast cancer patients. Eur J Radiol. 2006;60:270-4.
- [Google Scholar]
- ACR Practice Parameter for the Performance of Contrast-Enhanced Magnetic Resonance Imaging (MRI) of the Breast. Available from: http://www.acr.org/guidelines/Breast.MRI
- [Google Scholar]
- Background parenchymal enhancement of the contralateral normal breast: Association with tumor response in breast cancer patients receiving neoadjuvant chemotherapy. Transl Oncol. 2015;8:204-9.
- [Google Scholar]
- Early changes in functional dynamic magnetic resonance imaging predict forpathologic response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2008;14:6580-9.
- [Google Scholar]
- Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: Normal contrast medium enhancement and cyclical-phase dependency. Radiology. 1997;203:137-44.
- [Google Scholar]
- Background parenchymal enhancement on breast MRI: Impact on diagnostic performance. AJR Am J Roentgenol. 2012;198:W373-80.
- [Google Scholar]
- Impact of menopausal status on background parenchymal enhancement and fibroglandular tissue on breast MRI. Eur Radiol. 2012;22:2641-7.
- [Google Scholar]
- Background parenchymal enhancement at breast MR imaging: Normal patterns, diagnostic challenges, and potential for false-positive and false-negative interpretation. Radiographics. 2014;34:234-47.
- [Google Scholar]
- The lactating breast: Contrast-enhanced MR imaging of normal tissue and cancer. Radiology. 2005;237:429-36.
- [Google Scholar]
- Menstrual cycle and age: Influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology. 1997;203:145-9.
- [Google Scholar]
- Physiological changes in breast magnetic resonsnce imaging during the menstrual cycle: Perfusion imaging, signal enhancement, and influence of the T1 relaxation time of breast tissue. Breast J. 2005;11:236-41.
- [Google Scholar]
- Hormonal replacement therapy in postmenopausal women: Breast tissue perfusion determined with MR imaging-initial observations. Radiology. 2005;235:36-41.
- [Google Scholar]
- Assessment of breast tissue changes on hormonal replacement therapy using MRI: A pilot study. J Comput Assist Tomogr. 1999;23:407-13.
- [Google Scholar]
- The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology. 2007;244:356-78.
- [Google Scholar]
- ACR BI-RADS® magnetic resonance imaging. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
- [Google Scholar]
- American College of Radiology Mammography Accreditation Requirements. Available from: http://www.acr.org/~/media/ACR/Documents/Accreditation/Mammography/Requirements.pdf
- [Google Scholar]
- American College of Radiology Breast MR Accreditation Guidelines. Available from: http://www.acr.org/~/media/ACR/Documents/Accreditation/MRI/Requirements.pdf
- [Google Scholar]
- Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146-51.
- [Google Scholar]
- Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. Cancer. 1993;71:1258-65.
- [Google Scholar]
- Vascular density and the response of breast carcinomas to mastectomy and adjuvant chemotherapy. Eur J Cancer. 1993;29A:1391-3.
- [Google Scholar]
- Breast MRI after conservation therapy: Usual findings in routine follow-up examinations. AJR Am J Roentgenol. 2010;195:799-807.
- [Google Scholar]
- Background parenchymal enhancement in the contralateral normal breast of patients undergoing neoadjuvant chemotherapy measured by DCE-MRI. Magn Reson Imaging. 2013;31:1465-71.
- [Google Scholar]
- Breast cancer: Influence of taxanes on response assessment with dynamic contrast-enhanced MR imaging. Radiology. 2015;277:687-96.
- [Google Scholar]
- Background parenchymal enhancement in breast MRI before and after neoadjuvant chemotherapy: Correlation with tumour response. Eur Radiol. 2016;26:1590-6.
- [Google Scholar]
- Decrease in breast density in the contralateral normal breast of patients receiving neoadjuvant chemotherapy: MR imaging evaluation. Radiology. 2010;255:44-52.
- [Google Scholar]






