Translate this page into:
Ultrasound and Doppler US in Evaluation of Superficial Soft-tissue Lesions
Address for correspondence: Dr. Huseyin Toprak, Department of Radiology, Faculty of Medicine, Bezmialem Vakif University, 34093, Istanbul, Turkey. E-mail: huseyin_toprak@yahoo.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Improved developments in digital ultrasound technology and the use of high-frequency broadband transducers make ultrasound (US) imaging the first screening tool in investigating superficial tissue lesions. US is a safe (no ionizing radiation), portable, easily repeatable, and cheap form of imaging compared to other imaging modalities. US is an excellent imaging modality to determine the nature of a mass lesion (cystic or solid) and its anatomic relation to adjoining structures. Masses can be characterized in terms of their size, number, component, and vascularity with US and Doppler US especially with power Doppler US. US, however, is operator dependent and has a number of artifacts that can result in misinterpretation.
In this review, we emphasize the role of ultrasound, particularly power Doppler, in superficial soft-tissue lesions.
Keywords
Doppler
lesion
soft tissue
ultrasound
INTRODUCTION

Knowledge of basic anatomy and pathology of joints and soft-tissues is essential for evaluation of soft tissue lesions. Power Doppler Ultrasound (PDUS) device with at least a 10 MHz linear transducer is used for joint assessment. Knowledge of the clinical indications, limitations of the technique, and adequate technical experience in US imaging is essential for proper diagnosis. Reliability of power Doppler technology is an issue and requires standardization of the technique. The results can be improved by reducing the three errors of power Doppler scanning: Machine-related error, operator error, and error in interpretation of the images generated.[1] Machine error can be reduced by standardizing machine parameters, such as the power Doppler gain, using a standard pulse repetition frequency (PRF), and by using the same equipment. Operator error can be reduced by having the same operator performing scanning, following the guidelines of musculoskeletal ultrasound scanning, and ensuring that operator uses minimal pressure on the tissue when recording the images for assessment as this minimizes power Doppler blanching.[1234] Excessive pressure during examination can decrease power Doppler signal.[5] Error in image interpretation can be reduced by adequately training radiologists. During examination, low-wall filters and low PRF should be used for detection of low-velocity flows. If the lesion is located in an arm or a leg of a patient, the operator must have the patient extend his/her arm/leg on a table. If the lesion is located in the neck or thoracoabdominal region of the patient the operator must have the patient hold his breath for a while to minimize movement artifacts.
SUPERFICIAL NON-TUMORAL LESIONS
The most common nontumoral lesions are benign cysts, posttraumatic, reactive, and inflammatory lesions. These lesions include synovial cysts, ganglion cysyts, tenosynovitis, epidermoid cysts, and masses (abscess, necrosis, hematoma) that show cystic transformation.
Benign cysts
Benign cystic masses frequently emerge as localized swelling close to or adjacent to a joint. Synovial cysts are frequently associated with joints. The most frequent synovial cysts are popliteal or Baker's cysts [Figure 1]. Power Doppler imaging can confirm the absence of vascular flow within the mass to exclude a popliteal artery aneurysm.[6] They can rupture and give rise to symptoms like pain and swelling in the calf region. This clinical condition named as pseudothrombophlebitis may resemble deep venous thrombosis.[78] Baker's cyst rupture is clinically important because of the difficulty in discriminating the clinical picture from deep vein thrombosis (DVT), which may lead to thromboembolic life-threatening complications. Ruptured Baker's cyst can be differentiated from DVT by color Doppler ultrasound that shows a patent popliteal vein and artery and duplex Doppler scans that reveal a normal flow pattern. The second most frequent location of synovial cysts is the hip joint.
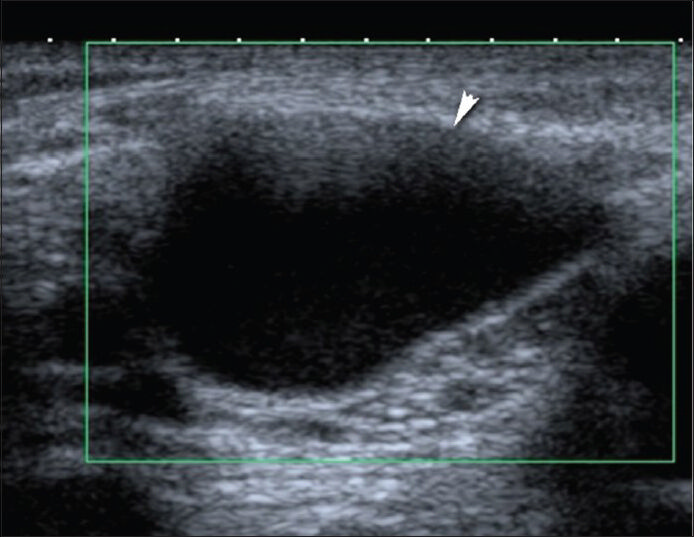
- Avascular Baker's cyst in popliteal fossa. Transverse Doppler US image with Toshiba Applio machine (Toshiba Medical Systems, Tokyo, Japan) using 12-MHz probe demonstrates a 3.5 × 2.5 cm cystic mass.
Ganglion cysts can occur anywhere. They commonly occur in the hand, wrist, foot, and ankle.[9] The most common cause of a palpable mass in the hand and wrist is a ganglion cyst. They are frequently seen in young women and nearly 10% of patients have a history of trauma.[9] These cysts typically contain mucin and approximately 60-70% of ganglion cysts are localized on dorsal part of the wrist. Dorsal ganglia usually originate from dorsal scapholunate ligament.[10] Approximately 20% of the ganglia arise on the volar side of the wrist.[11] Ganglia may extend with a thin neck into the joint space. Ganglia are frequently anechoic on US and demonstrate no vascularization on color Doppler US [Figure 2]. They can be easily aspirated with US guidance under local anesthesia followed by treatment with steroid injection.
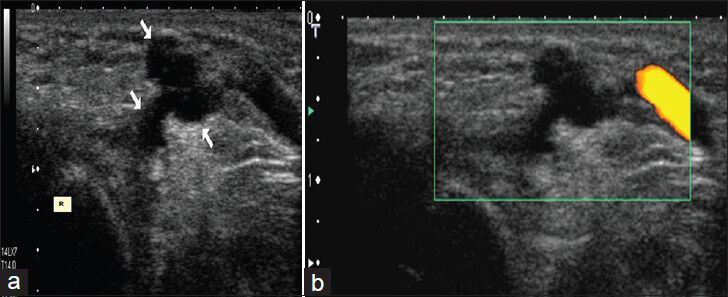
- Ganglion cyst in a girl. (a)Transverse US image shows multilobulated anechoic cystic structure (arrows) on the dorsal part of the left wrist. R. distal radius (b) Power Doppler image shows absence of vascularization in the lesion.
Epidermoid cysts are clinically mostly seen as epithelial-lined cysts arising in the hair bearing areas of the human body. On sonography, epidermoid cysts are most often hypoechoic.[9] Lamellar sonographic pattern is diagnostic in epidermoid cysts.[10] These keratin-filled cysts may sometimes contain calcifications. When epidermal inclusion cysts rupture, they may show color Doppler signal, which can mimic vascularization in a solid mass.[12]
Post-traumatic masses
Muscle ruptures, hematomas, and tendon ruptures are usually identified by clinical history and physical examination. Partial rupture of the muscles can be demonstrated as a separate mass; secondary muscle hypertrophy can be seen in chronic cases.[10] Tendon ruptures in patients with rheumatoid arthritis (RA) and in other rheumatologic diseases with chronic synovitis may develop spontaneously or due to trauma [Figure 3]. Spontaneous rheumatoid-related tendon ruptures are almost always due to synovitis/pannus eroding the tendon. In case of rupture, normal tendon paths cannot be followed using US. Fluid collection can be seen in the neighborhood of the torn segment and large hematomas are very rare.[11] US may be helpful in imaging the proximal segment and in determining the location for surgical incision. The sonographic appearance of hematomas may differ according to the stage of the lesions. Myositis ossificans may occur secondary to trauma or surgery. In myositis ossificans, calcifications are hyperechoic in US images and peripherally located. In these cases, there are no flow signals on PDUS examination.
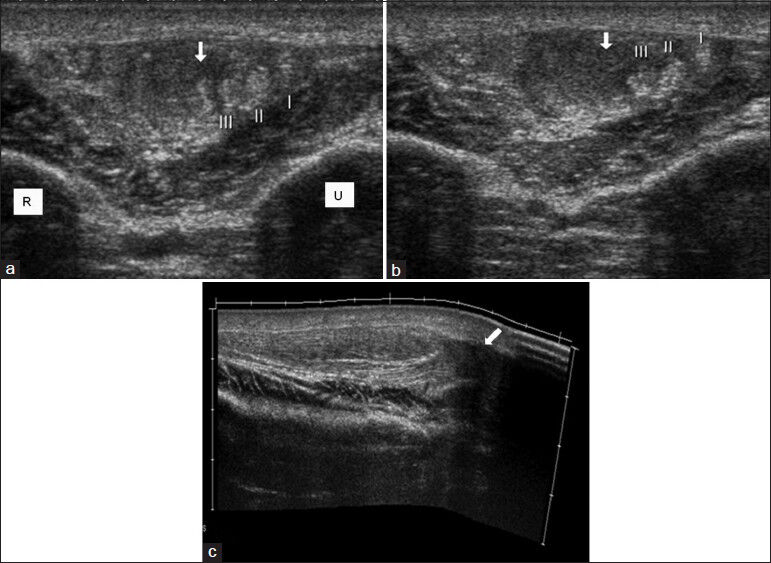
- Right extensor carpi radialis brevis tendon rupture in patient with Behçet's disease. (a and b) Contiguous transverse and (c) longitudinal extended field of view sonogram images show partial rupture and hematoma (arrow). (I, II, III; normal appearing other tendons).
Reactive and inflammatory masses
Inflammatory masses may be infectious or non-infectious in origin. Non-infectious reactive and inflammatory masses are neuromas, giant cell tumors of the tendon sheaths, foreign body granulomas, and rheumatoid nodules. Infectious inflammatory masses are abscesses, phlegmons, pyomyositis, or cellulitis.
Neuromas are a proliferative response to nerve injury. On US, neuromas appear as hypoechoic nodules and on color Doppler US, they typically show minimal vascularity.
Giant cell tumors of the tendon sheaths are the second most common masses after the ganglion cysts in the hand.[13] They tend to be seen on the volar aspect of the fingers. On US, they are hypoechoic masses adjacent to the joints and on Doppler US they are highly vascular structures.[10]
Foreign body granulomas emerge as hard and painful swellings. Penetrating traumas may or may not precede foreign body granulomas. In demonstrating the radiolucent foreign bodies (e.g., tree parts), US is superior to conventional radiography.[14] Foreign bodies are usually hyper-echoic and surrounded by a hyperemic hypoechoic halo. Color Doppler demonstrates an increased vascularization surrounding the foreign body.[11]
Rheumatoid nodules are present in nearly 20% of patients and seen as elongated hypoechoic nodules adjacent to tendons or within the tendon.[13]
Abscesses and cellulitis present with similar clinical findings. Differential diagnosis can be made easily by Doppler US. Abscesses are frequently seen as irregular-walled, septated, complex cystic lesions containing pus with debris or internal echoes inside. Prominent flow around the abscess on power Doppler confirms the diagnosis and is useful in differentiation it from organized hematomas. US-guided aspiration can be tested to confirm the diagnosis [Figures 4 and 5].
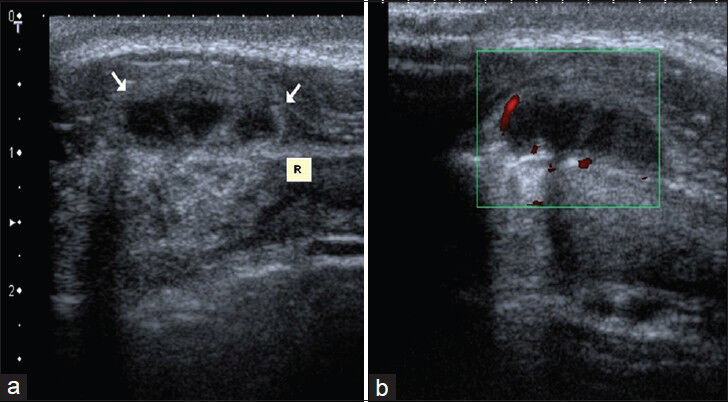
- Patient with Brucellosis. (a) Transverse sonogram shows 2 × 1 cm septated complex cystic mass (arrows) adjacent to 6th anterior rib (R). (b) Power Doppler image shows prominent flow around the lesion.
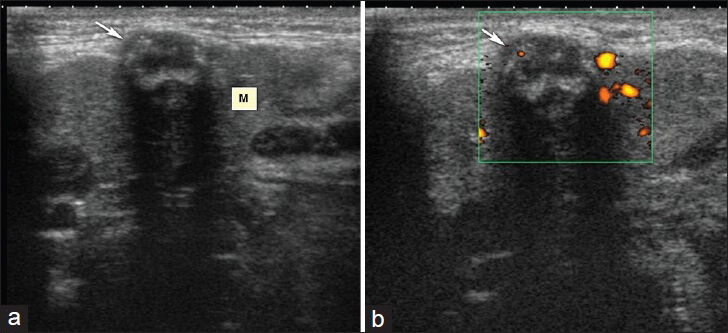
- Right submandibular abscess. (a) Longitudinal sonogram shows 1 × 0.5 cm heterogeneous mass containing thick hyperechoic wall and septae in right submandibular gland (M). (b) Power Doppler image shows prominent flow around the lesion.
In cellulitis, there is subcutaneous edema and subcutaneous fatty tissue appears thickened and echogenic on US [Figure 6]. Tortuous hypoechoic areas may be seen. Skin thickening and increased vascularity may also be seen on Doppler US.[10]
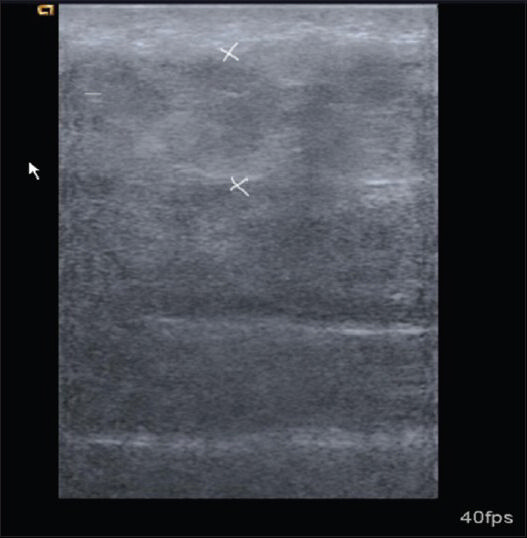
- Cellulitis at right femoral region. Transverse sonogram shows thickening of subcutaneous tissues and increase in echogenicity.
Tenosynovitis is inflammation of the sheath of tendons due to various etiologies. Rheumatoid arthritis is one of the most common inflammatory disease, which causes tenosynovitis.[15] Acute suppurative tenosynovitis is often seen in the case of a penetrating injury and may be hematogenous in origin. Tenosynovitis can be diagnosed sonographically by fluid distending the tendon sheath and/or thickening of the sheath. Thickening of the tendon sheath may be diffuse and smooth or eccentric and nodular. During the active phase of inflammation, increased vascularization can be detected on color and PDUS[10] [Figures 7 and 8].
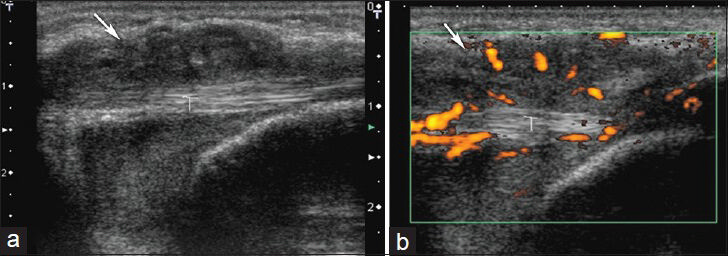
- Tenosynovitis in left wrist. (a) Longitudinal sonogram shows hyopoechoic mass around the flexor carpi ulnaris tendon (T) and (b) power Doppler images show vascularization in and around the lesion.
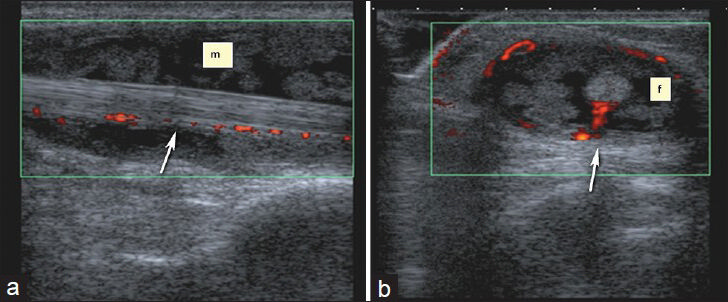
- Nonspecific tenosynovitis of flexor carpi radialis. (a) Longitudinal and (b) transverse Doppler US images show vascularization in and around the heterogeneous soft tissue mass. There is also extensive synovitis and fluid (f) and around the tendon.
Scar (abdominal wall) endometriosis
The scar (abdominal wall) endometriosis can be diagnosed easily based on history in patients who have undergone cesarean surgery or pelvic surgical operations. The mass in the region of the operation becomes more prominent during the menstrual period. On US, endometrial implants usually appear hypoechoic but may also appear hyper-vascular or avascular on Doppler US[16] [Figure 9].
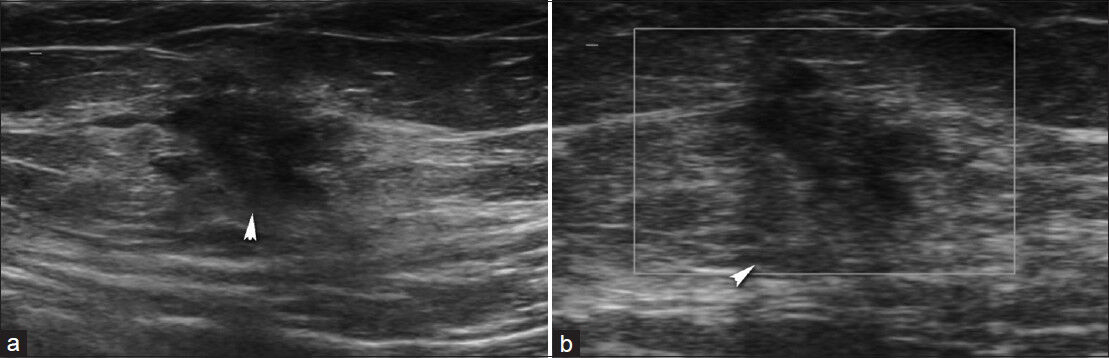
- Abdominal wall scar endometriosis. (a) Transverse sonogram shows irregular shaped, hypoechoic solid mass in the subcutanous fat. (b) Power Doppler sonography shows absence of vascularization.
Superficial neoplastic masses
With US and Doppler US, superficial soft-tissue tumors can be evaluated in terms of echogenicity, size, vascularity, quantity, and relationship with neighboring structures. In some of the cases, specific diagnosis can be made. However, detailed characterization of the tumor requires further imaging with computed tomography (CT) and magnetic resonance imaging (MRI).
Benign neoplastic masses
Lipomas
Lipomas are benign soft-tissue masses frequently localized subcutaneously, intramuscularly, and intermuscularly. They appear hypovascular, homogenous, and predominantly hyper-isoechoic to surrounding fat tissue on US [Figure 10]. They can be seen as encapsulated masses within the subcutaneous fatty tissue. Diagnosis of a lipoma with US should be made carefully because angiolipomas and low-grade liposarcomas containing both benign and malignant fat tissue may mimic lipomas.[10]
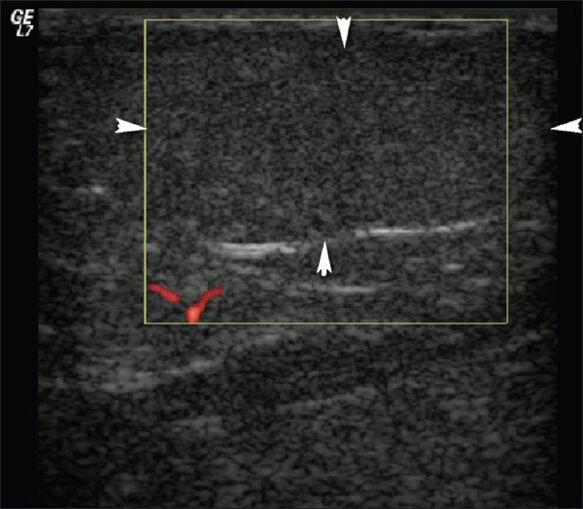
- Lipoma. The patient had a painless soft-tissue mass over the right neck. Doppler US shows a 3 × 1 cm soft-tissue mass (arrowheads) within the subcutaneous fat with similar echogenicity to the adjacent fat, without vascularization.
Fibromatosis
Superficial fibromatosis is a benign condition characterized by fibrous proliferations, which are typically slow-growing subcutaneous lesions. On US, they appear hypoechoic and show variable vascularity.[17]
Extra-abdominal desmoid tumors
Extra-abdominal desmoid tumors are locally aggressive fibroblastic tumor arising from the connective tissue of muscle and its overlying aponeurosis or fascia of the extra-abdominal muscles.[18] They do not have metastatic potential, but they have a high incidence (87%) of recurrence and locally aggressive growth pattern.[19] On US, they appear as infiltrative, hard, and heterogeneous masses. Typically, sonography shows variable degrees of vascularity.[18]
Myxomas
Soft-tissue myxomas can be seen in association with fibrous dyplasia. On US, they appear as soft, fluctuating hypoechoic masses. They are encapsulated and often contain cystic component.
Nerve sheath tumors
Benign nerve sheath tumors are schwannomas and neurofibromas. Schwannomas tend to be hypoechoic, round, and well-defined masses on US that taper distally and have an eccentric cystic component. Neurofibromas often appear elongated and parallel to the long axis of the nerve. Both tumors show vascularization on Doppler US[2021] [Figure 11.]
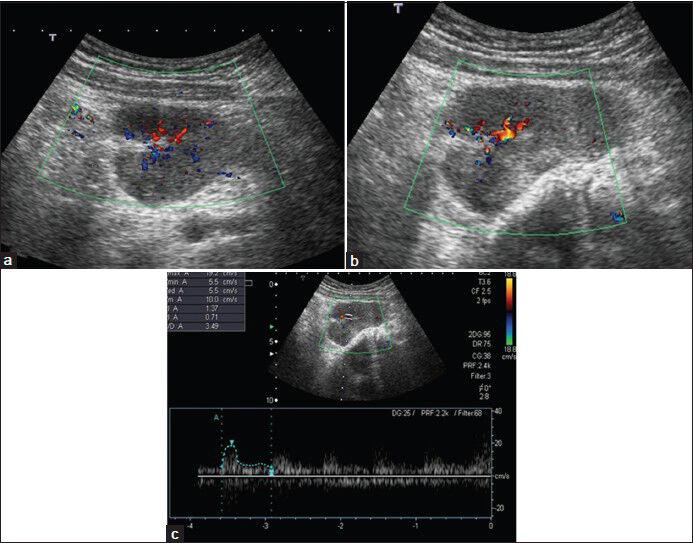
- Neurofibroma in a 13-year-old girl. (a) Transverse and (b) longitudinal color Doppler images show hypoechoic solid mass with central vascularization. (c) Spectral Doppler image shows low-resistance arterial flow.
Pilomatricoma
Pilomatricoma is a benign tumor that is thought to arise from skin appendages.[22] Although pilomatrioma accounts for less than 1% of skin tumors, it is the most common solid cutaneous tumor in patients aged 20 and younger,[23] with a secondary peak occurrence among mature adults (age: 50-65 years). The most common locations are head, neck, face, and arms.[2324] On sonography, pilomatricoma frequently appears as a subcutanous mass containing internal echogenic foci, internal calcifications, and hypoechoic rim. Calcification, which is more typically central, is seen in about 85% of the lesions. Internal vascularity is seen in 50% of these lesions [Figure 12].
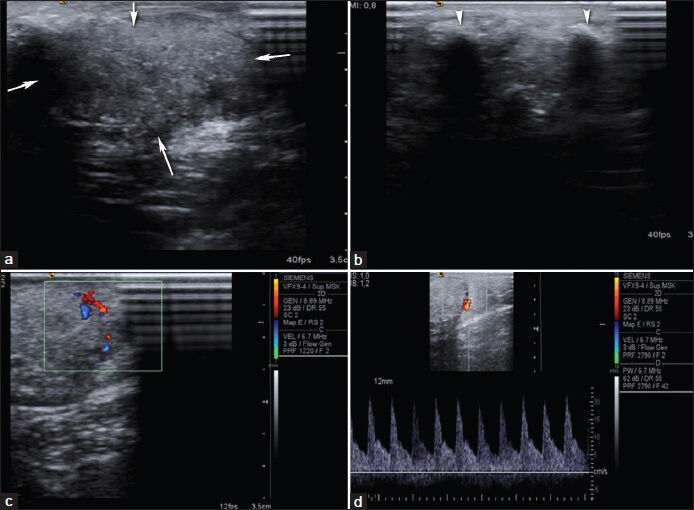
- Pilomatricoma. a and b) Transverse sonograms demonstrate a 6 × 4 × 2 cm subcutaneous hyperechoic mass lesion (arrows) with calcifications (arrowheads) at the right shoulder. (c) Color Doppler US demonstrates internal vascularity and (d) spectral Doppler US demonstrates arterial flow in the lesion.
Vascular malformations
Hemangiomas are frequently seen vascular malformations and constitute 7% of all soft-tissue masses. They are seen more frequently in women. Most commonly diagnosed soft-tissue tumors in pediatric age group are hemangiomas.[25] Capillary hemangiomas are the most common form and often spontaneously regress.[10] Cavernous hemangiomas are larger than capillary hemangiomas and often localized intramuscularly. They occur in the later stages of life and no spontaneous regression is observed. On US, prominent vascular channels, phleboliths and fat can be seen [Figures 13 and 14]. Arteriovenous malformations (AVMs) can be differentiated from hemangiomas as they show high vascularization on Doppler US. Mean peak venous velocity in hemangiomas is significantly lower than in AVMs.[26]
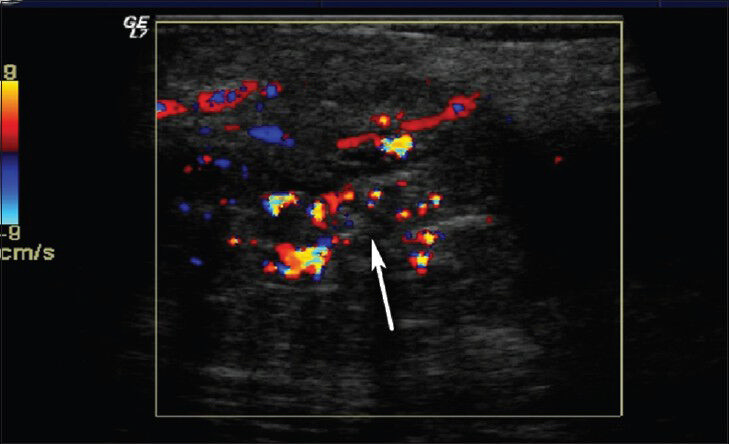
- Hemangioma in left thigh. Color Doppler US shows heterogeneous hypoechoic soft tissue mass with prominent vascularization in the subcutaneous area.
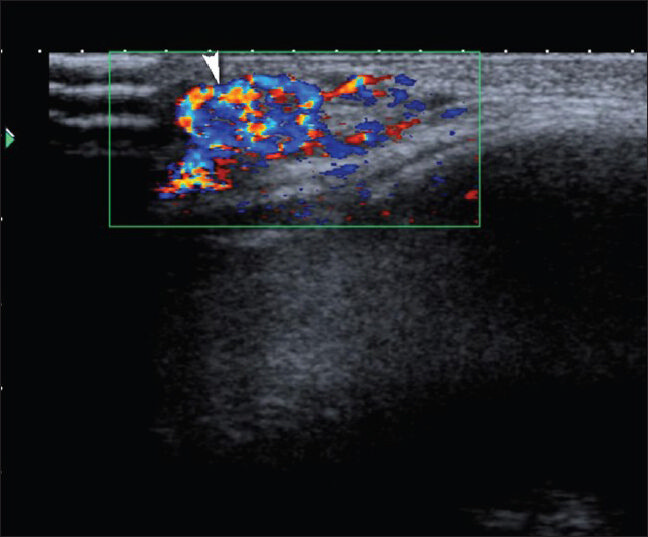
- Hemangioma. Color Doppler image reveals a highly vascular mass consistent with capillary hemangioma at the superolateral margin of the right orbit.
Malignant masses
Malignant superficial soft-tissue masses are primary or metastatic neoplasms and the incidence of sarcomas increases with age. The most common malignant sarcoma in first two decades is rhabdomyosarcoma and in fifth to seventh decades is malignant fibrous histiocytoma.[27] On US, malignant soft-tissue tumors commonly appear hypoechoic and hyper-vascular and can be well-defined. Cystic components, areas of necrosis, and dystrophic calcifications may also be seen. Tumor vascularity correlates with the degree of neoangiogenesis. Many authors have suggested that color and PDUS may be useful in differentiation of benign and malignant tumors and in staging of malignant tumors[282930] [Figure 15]. Absence of flow are observed only in the benign lesions and the negative predictive value is high. The presence of flow is not very discriminative and can be seen in both benign and malignant lesions. Hyperemia is not a constant feature of all malignant tumors. Again, absence of hyperemia is not a decisive feature for all benign tumors. Linear and regular course of the vessels are more suggestive of benignity, whereas scattered and irregular course of the vessels and sudden change in diameter of the vessel might be associated with irregular tumor angiogenesis and suggestive of malignancy.[31]
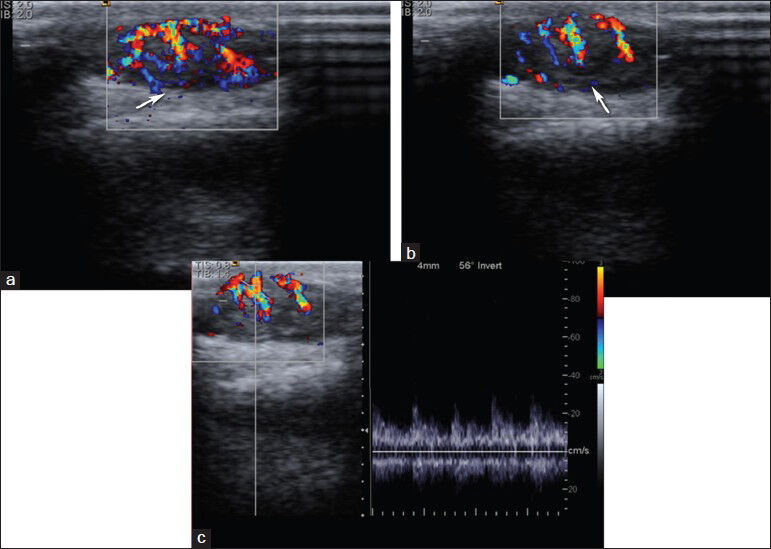
- Basal cell carcinoma. (a and b) Color Doppler and (c) spectral Doppler images show a hypervascular hypoechoic mass lesion with arterial waveform at the right occipital region. Histopathological diagnosis was pigmented basal cell carcinoma.
The use of resistive index (RI) was not found to be successful in differentiation of malignity and benignity.[32] Also, diastolic and venous velocities and pulsatility index (PI) were not found to be useful in distinguishing between malignant and benign lesions. Mean systolic peak flow velocity was found to be higher in malignant tumors.[283334] Mean peak velocity was found to 27 cm/s in benign lesions, whereas mean peak velocity in malignant lesions was 55 cm/s. Flow velocities greater than 50 cm/s have been reported to be useful in distinguishing benign from malignant lesions.[31] Although spectral analysis is not beneficial all the time, it is correlated with overall vascularization of the tumor. Color Doppler US is an important imaging modality in follow-up of response to chemotherapy and radiotherapy. On color Doppler US, the presence or absence of Doppler flow, number of vessels in the mass, orientation, and course of the vessels in the lesion (regular or irregular, linear or tortuous) and the presence or absence of sudden change in diameter of vessel are used as a criterion. Increased vascularization (three or more), irregular distribution of tumoral vessels, tortuosity, the presence of spotted flow, and the presence of sudden change in vessel diameter are indicative of malignancy. Diagnosis of malignant soft-tissue tumor is done when at least two of these findings are present. Using these criteria, color Doppler US provided 85% sensitivity, 88% specificity, 91% negative predictive value, 80% positive predictive value, and 87% accuracy in distinguishing malignant tumors from benign soft-tissue tumors.[31] Flow was observed in all of the 20 malignant lesions, and 3 or more vessels were present in 17 of them (85%) in the study by Belli et al.[31] Another study reported that color Doppler US could not distinguish malignant masses from benign masses, but spectral analysis was found to be useful for distinguishing acute inflammatory diseases (that show reduced impedance) from neoplasms (that show increased impedance).[35] Ultrasonographic appearance of benign and malignant lesions may show similar features. Color Doppler and spectral Doppler data are more useful in differentiating these lesions [Figure 16]. US plays an important role in biopsy of malignant lesions. A study has demonstrated that percutaneous core needle biopsy with sonographic guidance yields highly concordant histopathologic findings of tumors as compared with surgical biopsy.[36] Sonography can also be used for following the therapeutic response and recurrence with a relatively high accuracy.[10]
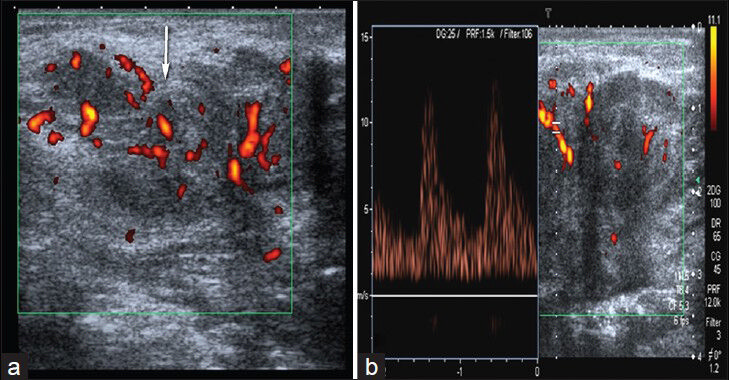
- Skin metastases of gastric carcinoma. (a) Power Doppler image shows irregular shaped, hypoechoic soft tissue mass with internal vascularity. (b) Spectral Doppler US demonstrates arterial flow in the lesion.
CONCLUSION
Ultrasound and power Doppler ultrasound (PDUS) are practical and useful imaging modalities, which also have the advantage of being low in cost, easily available, and highly efficient in the assessment and monitoring of rheumatic disease and other soft tissue lesions.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2014/4/1/12/127965
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Power Doppler ‘blanching’ after the application of transducer procedure. Australas Radiol. 2005;49:18-21.
- [Google Scholar]
- Recommendations for musculoskeletal ultrasonography by rheumatologists: Setting global standards for best practice by ecperct consensus. Arthritis Rheum. 2005;53:83-92.
- [Google Scholar]
- Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis. 2001;60:641-9.
- [Google Scholar]
- Recommendations for the content and conduct of European League against rheumatism (EULAR) musculoskeletal courses. Ann Rheum Dis. 2008;67:1017-22.
- [Google Scholar]
- Doppler US in rheumatic disease with special emphasis on rheumatoid arthritis and spondyloarthritis. Diagn Interv Radiol. 2014;20:72-7.
- [Google Scholar]
- Doppler ultrasonography and exercise testing in diagnosing a popliteal artery adventitial cyst. Cardiovasc Ultrasound. 2009;7:23.
- [Google Scholar]
- Rupture of Baker's cyst producing pseudothrombophlebitis in a patient with Reiter's syndrome. Kaohsiung J Med Sci. 2004;20:600-3.
- [Google Scholar]
- Pseudothrombophlebitis in a patient with Behcet's syndrome: Doppler ultrasound and magnetic resonance imaging findings. Clin Rheumatol. 2002;21:60-2.
- [Google Scholar]
- Sonographic evaluation of the musculoskeletal soft tissue masses. Ultrasound Q. 2005;21:259-70.
- [Google Scholar]
- Relationship between sonographic and pathologic findings in epidermoid inclusion cysts. J Clin Ultrasound. 2001;29:374-83.
- [Google Scholar]
- Giant cell tumor of tendon sheath: Case series and review of literature. J Hand Microsurg. 2010;2:67-71.
- [Google Scholar]
- Soft tissue masses: The underutilization of sonography. Semin Musculoskeletal Radiol. 1999;3:115-34.
- [Google Scholar]
- Advances in the treatment of inflammatory arthritis. Best Pract Res Clin Rheumatol. 2012;26:251-61.
- [Google Scholar]
- Abdominal wall endometriosis: Clinical presentation and imaging features with emphasis on sonography. AJR Am J Roentgenol. 2006;186:616-20.
- [Google Scholar]
- Sonography of the benign palpable masses of the elbow. J Ultrasound Med. 2011;30:113-9.
- [Google Scholar]
- Differentiation between schwannomas and neurofibromas in the extremities and superficial body. J Ultrasound Med. 2008;27:161-6.
- [Google Scholar]
- Sonographic characteristics of peripheral nerve sheath tumors. AJR Am J Roentgenol. 2004;182:741-4.
- [Google Scholar]
- Pilomatrixoma or calcifying epithelioma of Malherbe invading bone. Histopathology. 1992;21:79-81.
- [Google Scholar]
- Pilomatrical neoplasms in children and young adults. Am J Dermatopathol. 1992;14:87-94.
- [Google Scholar]
- Pilomatricomas: The diagnostic value of ultrasound. Skeletal Radiol 2010:39.243-50.
- [Google Scholar]
- Soft tissue vascular anomalies: Utility of US for diagnosis. Radiology. 2000;2014:747-54.
- [Google Scholar]
- Tumor vascularization: Assesment with Duplex sonography. Radiology. 1991;181:241-4.
- [Google Scholar]
- Color Dopplersonography of focallesions of the skin and subcutaneous tissue. J Ultrasound Med. 1999;18:89-93.
- [Google Scholar]
- Potential uses of color Doppler in periskeletal soft tissue neoplasms. Radiol Med. 1997;94:583-90.
- [Google Scholar]
- Role of color Doppler sonography in the assesment of musculoskeletal soft tissue masses. J Ultrasound Med. 2000;19:823-30.
- [Google Scholar]
- Spectral Doppler sonography of musculoskeletal soft tissue masses. J Ultrasound Med. 2003;22:1333-6.
- [Google Scholar]
- Differentiation of benign and malignant tumors of the parotid gland: Value of pulsed Doppler and color Doppler sonography. Eur Radiol. 1998;8:1462-7.
- [Google Scholar]
- Intratumoral peak systolic velocity as a new possible predictor for detection of adnexal malignancy. Am J Obstet Gynecol. 1995;172:1496-500.
- [Google Scholar]
- Image-directed color Doppler ultrasonography in the evaluation of superficial solid tumors. J Clin Ultrasound. 1995;23:233-8.
- [Google Scholar]
- Sonographically guided core needle biopsy of the soft tissue neoplasms. J Clin Ultrasound. 2004;32:294-8.
- [Google Scholar]






