Translate this page into:
The efficacy of cone-beam computed tomography-guided transcatheter arterial chemoembolization in hepatocellular carcinoma survival: A systematic review

*Corresponding author: Levent Akman Solim, Department of Radiology, Frankfurt University Hospital, Frankfurt, Hessen, Germany. leventsolim@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Solim LA, Atasoy D, Vogl TJ. The efficacy of cone-beam computed tomography-guided transcatheter arterial chemoembolization in hepatocellular carcinoma survival: A systematic review. J Clin Imaging Sci. 2024;14:25. doi: 10.25259/JCIS_32_2024
Abstract
Cone-beam computed tomography (CBCT)-guided transcatheter arterial chemoembolization (TACE) represents an alternative treatment option for advanced hepatocellular carcinoma (HCC) patients, yet a comprehensive evaluation of CBCT guidance on this procedure and CBCT’s impact on patient survival remains lacking. We aimed to assess the efficacy and benefits of CBCT-guided TACE in improving survival outcomes for patients with HCC and show the importance of CBCT in interventional radiology. Meta-analysis was conducted to evaluate CBCT-guided TACE compared to conventional TACE in the treatment of HCC. PubMed and Cochrane library databases were searched for studies published. Outcomes of interest included 1- or 3-year local progression-free survival (LPFS) rates, overall survival (OS) rates, and tumor response results. A total of eight studies were included in the meta-analysis, comprising 1176 patients. The analysis showed that CBCT-guided TACE improved 1-year LPFS (odds ratio [OR] = 2.81, P < 0.001), 3-year (OR = 4.42, P = 0.002), and the 3-year OS rates (OR = 3.03, Confidence Interval = 1.65–11.80, P = 0.14) compared to conventional TACE. CBCT-guided TACE enhances survival outcomes for patients with HCC; by addressing this research gap, our study endeavors to encourage clinicians and researchers to pursue this medical technology by providing a robust synthesis of current evidence.
Keywords
Cone-beam computed tomography
Transcatheter arterial chemoembolization
Hepatocellular carcinoma
Oncology
INTRODUCTION
HCC is the most common form of primary liver cancer with increasing incidence, globally.[1] It is an aggressive cancer with a poor prognosis.[2-5] The management of HCC requires a multidisciplinary approach; surgical resection, liver transplantation, and ablation are the standard curative treatments for the early-stage HCC. However, these treatments may not be suitable for patients with advanced HCC or those with underlying severe liver cirrhosis.[4,6-10]
Cone-beam computed tomography (CBCT) is a type of 3D angiography method that uses a cone-shaped X-ray beam and a flat-panel detector to capture images of the body in a single C-arm rotation. CBCT-guided transcatheter arterial chemoembolization (TACE) has been introduced to enhance the precision and safety of TACE by providing images of the liver, and arteries relative to liver parenchyma, respective location of the catheter, contrast, and surrounding anatomy during the procedure; therefore, CBCT-guided TACE is widely preferred in the treatment of advanced hepatocellular carcinoma (HCC) if it is available. Yet still, the majority of the interventional radiology units do not have enough resources to use CBCT guidance during their procedures.[11,12]
While there is existing literature on the use of various imaging modalities and treatment techniques in the management of HCC, there is a notable scarcity of comprehensive systematic reviews and meta-analyses specifically focusing on the efficacy and survival outcomes associated with CBCT-guided TACE to our best knowledge. Although studies may individually highlight the benefits and disadvantages of CBCT in guiding TACE procedures, there is a need to synthesize available evidence to determine the overall impact on patient survival and the comparative advantages over alternative imaging methods.
Despite the growing use of CBCT-guided TACE in the treatment of HCC, there is still no published consensus on its efficacy and survival benefits over conventional TACE alone. While several studies have reported benefits, others have reported limited advantages compared to conventional TACE alone.[13] Addressing this gap is essential for a more thorough understanding of the role of CBCT in guiding TACE for HCC treatment, and it can contribute valuable insights for clinicians, researchers, and policymakers involved in cancer care decision-making. In addition, a comprehensive meta-analysis can help identify potential variations in outcomes across different studies, populations, and methodologies, providing a nuanced perspective on the overall effectiveness of this specific approach in improving survival rates for patients with HCC and other pathologies of multiple disciplines. The primary aim of this meta-analysis is to study the available evidence on the effectiveness of CBCT-guided TACE to the survival of HCC patients and provide the current state. Beyond this, we strive to augment the prevalence of CBCT in TACE units, aiming to inspire manufacturers and researchers to invest in enhancing the engineering and refine the device.
MATERIAL AND METHODS
Literature search
We used PubMed and Cochrane Library to search for published data until March 2023. Our search strategy contained combinations of the following:
(“cone beam” [tiab] OR cbct [tiab] OR “c-arm” [tiab] OR “volume computed tomography” [tiab] OR “volume ct” [tiab] OR “CBCT” [mesh]) AND (Liver [tw] OR hepatic* [tw] OR “liver” [mesh]) AND (“tace” [tiab] OR chemoembolization [tiab] OR “chemoembolization” [tiab] OR “TACE” [tiab] or “transarterial chemoembolization” [tiab]) AND English [lang])
The articles screened for eligibility based on their titles and abstracts, and the full text of the articles that meet the inclusion criteria were evaluated for further eligibility. The selection of articles was studied independently by two reviewers, and any discrepancies were resolved through consensus.
Criteria
Inclusion and exclusion criteria were established for the systematic review of studies related to the efficacy of CBCT-guided TACE in the treatment of HCC. Inclusion criteria contain studies which are as follows; randomized controlled trials, observational studies, and case series, providing outcomes relative to the topic, and the efficacy of CBCT-guided TACE in the treatment of HCC. In addition, studies were required to be published in English and to furnish sufficient data. The key outcome indicators of interest were 1 or 3-year overall survival (OS) rates.
Exclusion criteria were set to filter out studies that did not align with the research objectives. These encompassed studies that failed to report the outcomes of interest, such as tumor response and OS. Studies were excluded if they did not disclose the number of patients who received CBCT-guided TACE or conventional TACE. Furthermore, studies that only focused on patients receiving drug-eluting bead TACE (DEB-TACE) or excluded adult patients with HCC, were also excluded from the analysis.
The superiority of DEB over conventional TACE has not been conclusively demonstrated and some studies yield controversial results about this comparison.[14-16] Due to these controversies and the fact that DEB and conventional TACE are different methods, we excluded DEB in this study to obtain more refined results and prevent any biases. After the last assessment for eligibility, nine studies out of seventeen were excluded due to; DEB-TACE execution (n = 3), not enough or missing data (n = 5), and containing other multiple reasons that decrease the quality (n = 1) [Figure 1].

- PRISMA flow diagram on literature research strategy. (DEB-TACE: Drug-eluting bead transcatheter arterial chemoembolization.)
Collection of data
Two independent authors collected the data for this meta-analysis. Each article was screened individually, and data were extracted strictly according to predetermined criteria. The collected information includes author information, year of publication, institute location, baseline patient characteristics, population demographics, intervention details, and outcome indicators. The primary outcomes observed in this meta-analysis were the effects on 1-year and 3-year survival rates, while the secondary outcome indicator was tumor response.
Quality and bias evaluation
The publications included in our meta-analysis were analyzed according to the Cochrane Handbook for Systematic Reviews of Interventions (ROBIN 1 tool). The literature was evaluated and cross-checked by two independent authors blindly. Any disagreements during the evaluation of quality were resolved through discussion, and a final decision was made by consensus of the two authors.
Statistic methods
All statistical analyses were conducted using MetaXL software (version 5.3, Epigear). We utilized Cochran`s Q test and I2 test to analyze and evaluate the heterogeneity of the results. For I2 value and P value, %50 and 0.10 were used respectively as cutoff for heterogeneity. To overcome heterogeneity due to a small number of studies, a random effect model was used for meta-analysis, and odds ratios (ORs) were calculated. P < 0.05 was considered statistically significant. Sensitivity analysis was also conducted using MetaXL software by excluding each study one by one, which can be seen in supplementary tables 1-3.
RESULTS
Literature search results
During the initial search, a total of 252 publications were obtained. After eliminating duplicates, 247 abstracts were screened, and 78 studies were identified as eligible for full-text review based on our criteria. Following a thorough page-by-page review of the full text, we selected 17 publications for data extraction. Ultimately, after data extraction, only eight studies met our inclusion/exclusion criteria and provided sufficient data for inclusion in our meta-analysis [Figure 1].[17-24] Unfortunately, only four of the included studies had a control group, and five of the eight publications reported on elements of tumor response.[17-20]
The characteristics of included publications
All of the publications included in our analysis were conducted in various countries and were retrospective studies, except Miyayama 2014.[17-24] Specifically, we identified three articles from the same author, Miyayama, which were published in 2013, 2014, and 2021.[19,21,24] We included only those three articles of the writer while they were using different sets of patients. In total, our analysis involved eight studies that included 1176 patients with HCC, with 838 patients receiving CBCT-guided TACE and 338 patients receiving digital subtraction angiography (DSA) TACE. We have presented the baseline characteristics and features of the eight included publications in Tables 1 and 2. Because not all publications had control groups for their study, we analyzed the studies in two separate ways; with articles which had control group and all articles. In all articles’ groups, we analyzed data for the CBCT-guided TACE arm only, while analyzing both DSA-TACE and CBCT-guided TACE arm for articles with control groups.
| Study | Country | AFP Software | Application | Number of patients | Mean age, years (SD) | Sex (F/M) | Mean tumor size, mm (SD) | Child-Pugh Score (A/B/C) |
|---|---|---|---|---|---|---|---|---|
| Bannangkoon (2021)[17] | Thailand | Yes | CBCT guided | 196 | 62.3 (21-91)† | 56/140 | 3.1 (01-7.0)*† | 144/37/15 |
| DSA (control group) | 141 | 63.3 (37-90)† | 47/94 | 3.2 (1.1-7.0)*† | 96/27/18 | |||
| Lee (2019)[18] | South Korea | No | CBCT guided | 55 | 60.53 (9.7) | 7//48 | 21.2 (7.4)* | 49/6/0 |
| DSA (control group) | 58 | 60.02 (11.1) | 19//39 | 21.9 (0.96)* | 51/7/0 | |||
| Miyayama (2013)[19] | Japan | No | CBCT guided | 79 | 70.7 (7.9) | 32/47 | 19.9 (9.1) | 56/18/5 |
| DSA (control group) | 70 | 68.9 (8.8) | 31/39 | 22.2 (10.1) | 57/12/1 | |||
| Iwazawa (2012)[20] | Japan | No | CBCT guided | 61 | 70 (41-85)† | 21/40 | 22 (7-90)*† | 43/17/1 |
| DSA (control group) | 69 | 71 (50-84)† | 22/47 | 21 (10-100)*† | 54/14/1 | |||
| Miyayama (2021)[21] | Japan | Yes | CBCT guided | 259 | 73.4 (8.2) | 105/154 | 17.20 (5.9) | 202/49/8 |
| Orlacchio (2021)[22] | Italy | No | CBCT guided | 50 | 66.7 (8.2) | 10/40 | 21.4 (11) | 44/6/0 |
| Choi (2017)[23] | South Korea | No | CBCT guided | 57 | 61.1 (11.5) | 14/43 | NS | 51/6/0 |
| Miyayama (2014)[24] | Japan | Yes | CBCT guided | 81 | 73.3 (8.1) | 40/41 | 17.4 (7.4) | 60/19/2 |
SD: Standard Deviation, CBCT: Cone-Beam Computed Tomography, DSA: Digital Subtraction Angiography
| Study | Method | Total Bilirubin, mg/dL (SD) | Serum Albumin, g/dL (SD) | Alpha Fetoprotein, ng/mL | Platelet x10^9/L (SD) | ALT IU/dL (SD) | AST IU/dL (SD) | Hepatitis B (positive/negative) | Hepatitis C (positive/negative) |
|---|---|---|---|---|---|---|---|---|---|
| Bannangkoon (2021)[17] | CBCT guided | 0.87 (0.18-2.87)† | 3.6 (2.2-4.7)† | 37.26 | 99 (30-435)† | NS | NS | 97/99 | 54/142 |
| DSA (control group) | 0.98 (0.20-2.95)† | 3.4 (1.8-4.8)† | 34.28 | 106 (35-318)† | NS | NS | 65/76 | 36/105 | |
| Lee (2019)[18] | CBCT guided | 1.13 (0.66) | 4.00 (0.59) | 83.55 | 120.67 (74.01) | NS | NS | 38/17 | 9/46 |
| DSA (control group) | 1.22 (0.63) | 3.96 (0.44) | 71.49 | 105.72 (51.72) | NS | NS | 44/14 | 6/52 | |
| Miyayama (2013)[19] | CBCT guided | 1.1 (0.7) | NS | NS | NS | 45.3 (32.7) | 51.9 (29.2) | NS | NS |
| DSA (control group) | 0.9 (0.4) | NS | NS | NS | 46.7 (27.9) | 53.8 (30.5) | NS | NS | |
| Iwazawa (2012)[20] | CBCT guided | 0.9 (0.4-2.3)† | 3.9 (2.2-4.7)† | 19 | 112 (44-302)† | 12 (13-188) | 46 (23-370) | 10/51 | 44/17 |
| DSA (control group) | 0.8 (0.3-2.8)† | 3.7 (2.2-3.6)† | 25 | 105 (30-365)† | 46 (11-470) | 53 (20-277) | 4/65 | 54/15 | |
| Miyayama (2021)[21] | CBCT guided | NS | NS | 85.7 | NS | NS | NS | 51/208 | 147/112 |
| Orlacchio (2021)[22] | CBCT guided | 1.28 (0.6) | NS | NS | 116.20 (59.51) | NS | NS | 4//46 | 24//26 |
| Choi (2017)[23] | CBCT guided | NS | NS | 32.1 | NS | NS | NS | NS | NS |
| Miyayama (2014)[24] | CBCT guided | NS | NS | NS | NS | NS | NS | 9/72 | 58/23 |
CBCT: Cone-Beam Computed Tomography, DSA: Digital Subtraction Angiography, NS: Not Significant, ALT: Alanine Transaminase, AST: Aspartate Transaminase, SD: Standard Deviation, IU: International Unit
Quality and bias assessment
The risk of bias assessments is presented in Figures 2 and 3. Out of the included studies, four studies indicated the use of randomization, which was classified as low risk of selection bias. However, the other four studies were not clear on their use of randomization, leading to a moderate risk of selection bias. Regarding blinding methods, all studies applied proper blinding, resulting in a low risk of reporting bias. One of the studies lacked a satisfactory statement on missing data, resulting in a moderate risk of missing data bias classification. There were two disagreements during classification between low risk and moderate risk, both of them were resolved by the consensus of two authors and classified as moderate risk. Overall, the analysis revealed that there was no high-risk publication, while four studies were identified as having a moderate risk of bias, and the others were evaluated as having a low risk of bias.
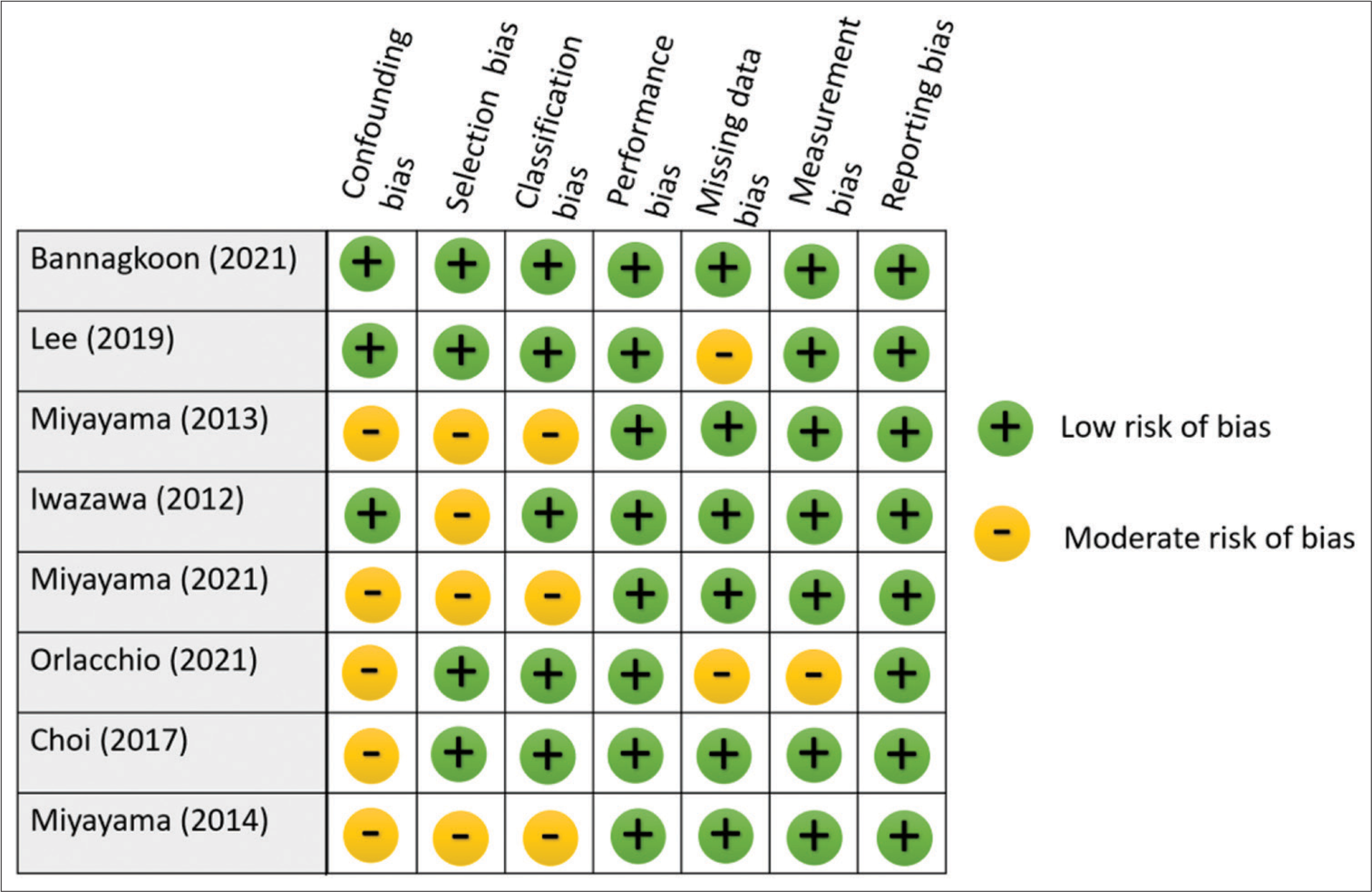
- Risk of bias assessment for each study.
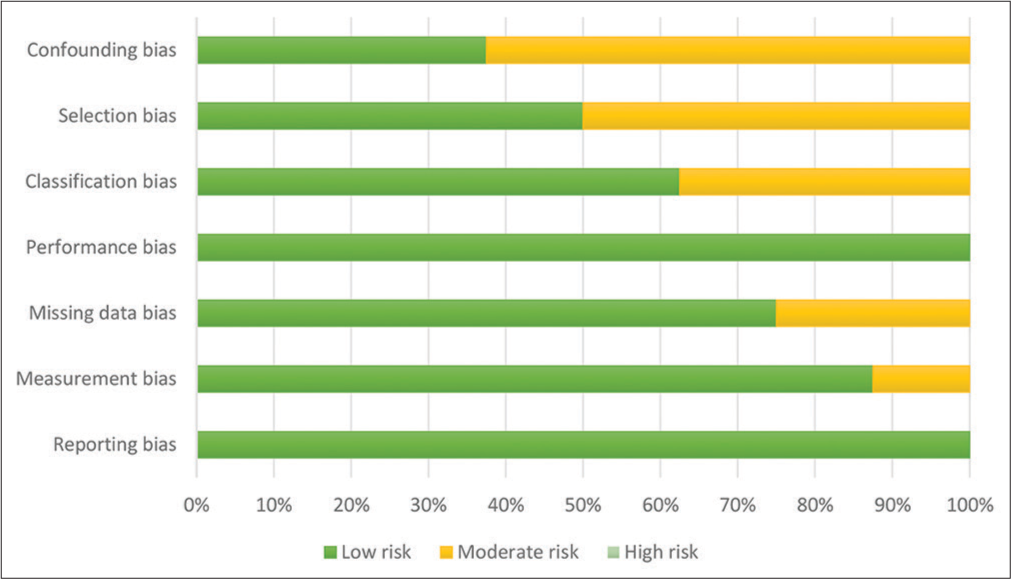
- A risk of bias graph classified by each bias category.
Meta-analysis
Analysis of studies without control group
Survival
Out of the eight articles included in this meta-analysis, five had data on OS and local progression-free survival (LPFS) rates. The 1-and 3-year OS rates for HCC were analyzed using a random effect model. The analysis showed that the pooled 1-year OS rate [Table 3] was 94% (87–99%) and the 3-year OS rate [Table 4] was 74% (54–90%). Sensitivity analysis of 1-year OS and 3-year OS results are given in [Supplementary Tables 1 and 2], respectively. The analysis revealed that the 1-year LPFS rate was 61% (50–72%) and the 3-year LPFS rate was 39% (27–52%).
| Study | Prevalence | LCI 95% | HCI 95% | Weight (%) |
|---|---|---|---|---|
| Iwazawa 2012 | 93.9 | 86.3 | 98.8 | 18.7 |
| Choi 2017 | 100 | 97 | 100 | 18.5 |
| Lee 2019 | 85.5 | 74.7 | 93.7 | 18.3 |
| Miyayama 2021 | 97.1 | 94.7 | 98.9 | 22.5 |
| Bannangkoon 2021 | 87 | 81.9 | 91.4 | 22.0 |
| Pooled | 94.2 | 87.3 | 98.7 | 100 |
| Study | Prevalence | LCI 95% | HCI 95% | Weight (%) |
|---|---|---|---|---|
| Iwazawa 2012 | 71 | 58.9 | 81.8 | 19.6 |
| Choi 2017 | 88.4 | 78.6 | 95.6 | 19.5 |
| Lee 2019 | 74.5 | 62.1 | 85.3 | 19.4 |
| Miyayama 2021 | 82.8 | 78 | 87.2 | 20.8 |
| Bannangkoon 2021 | 44 | 37.1 | 51 | 20.7 |
| Pooled | 73.6 | 53.8 | 89.7 | 100 |
Tumor response
Out of the eight articles included, five had data on tumor complete response, four had data on partial response, and four had data on stable disease. In addition, five studies had data on progressive disease. The analysis showed that the complete response rate was 71% (48–90%), while the partial response rate was 17% (0–40%). The stable disease rate was 4% (1–9%), and the progressive disease rate was 4% (0–12%).
Overall recurrence rate
Out of the eight articles included, four had data on overall recurrence rates. The analysis showed that the overall recurrence rate was 51% (23–78%) [Table 5]. Sensitivity analysis of the overall recurrence rate was given in [Supplementary Table 3].
| Study | Prevalence | LCI 95% | HCI 95% | Weight (%) |
|---|---|---|---|---|
| Iwazawa 2012 | 62.3 | 49.7 | 74.1 | 24.5 |
| Bannangkoon 2021 | 77.9 | 71.8 | 83.5 | 25.4 |
| Miyayama 2013 | 31.1 | 21.4 | 41.8 | 24.8 |
| Miyayama 2014 | 30.4 | 22.9 | 38.4 | 25.2 |
| Pooled | 50.7 | 23 | 78.3 | 100 |
Analysis of studies with control group
Survival
Out of the eight articles included, four had a control group. All four have LPFS data and three of them have data on OS. The analysis revealed that the 1-year OS rate was 2.1 with an unsatisfying confidence interval (CI), of 0.44–10.48 [Table 6]. The 3-year OS rate was 3.03 times (CI = 1.65–11.80) better in the CBCT-guided group (P = 0.14) [Figure 4]. In 1-year LPFS, the CBCT-guided group had 2.81 times (CI = 1.15–6.91) better results than the DSA alone group [Figure 5]. Furthermore, in 3-years LPFS, the CBCT-guided group had 4.42 times (CI = 1.65–11.80) better results than the DSA alone group [Figure 6]. Both 1-year (P < 0.001) and 3-year (P = 0.002) LPFS rates showed significant results in favor of the CBCT-guided group.
| Study | Odds Ratio | LCI 95% | HCI 95% | Weight (%) |
|---|---|---|---|---|
| Iwazawa 2012 | 4.13 | 1.24 | 13.71 | 32.08 |
| Lee 2019 | 0.32 | 0.08 | 1.28 | 30.25 |
| Bannangkoon 2021 | 5.7 | 3.35 | 9.71 | 37.66 |
| Pooled | 2.15 | 0.44 | 10.48 | 100 |
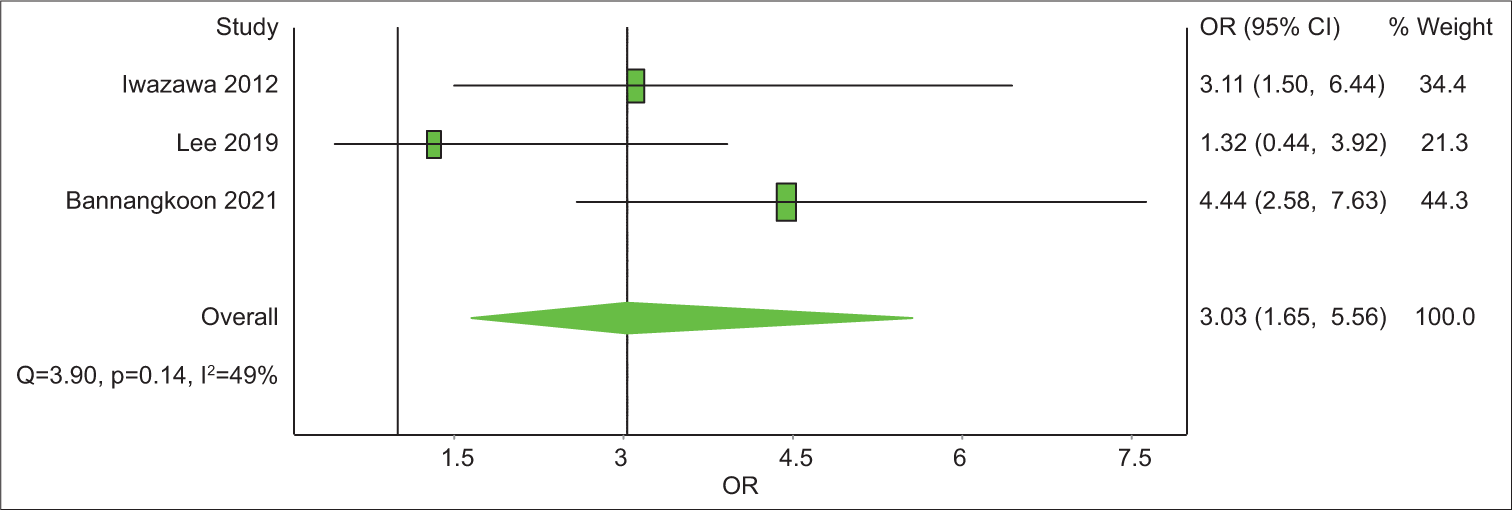
- A forest plot of 3-year overall survival rate odds ratios comparing cone-beam computed tomography (CBCT)-guided transcatheter arterial chemoembolization (TACE) and digital subtraction angiography (DSA)-guided TACE. Odds ratios (OR) above 1 favor CBCT-TACE over DSA-TACE.
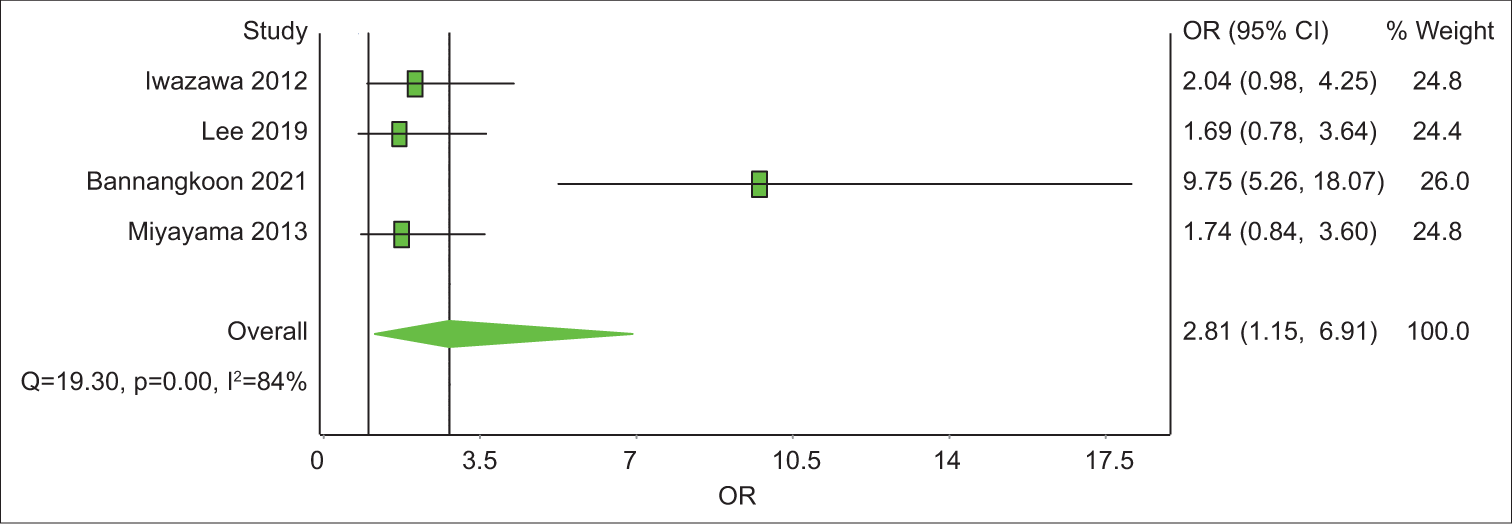
- A forest plot of 1-year local progression-free survival odds ratios comparing cone-beam computed tomography (CBCT)-guided transcatheter arterial chemoembolization (TACE) and digital subtraction angiography (DSA)-guided TACE. Odds ratios (OR) above 1 favor CBCT-guided TACE over DSA-guided TACE.
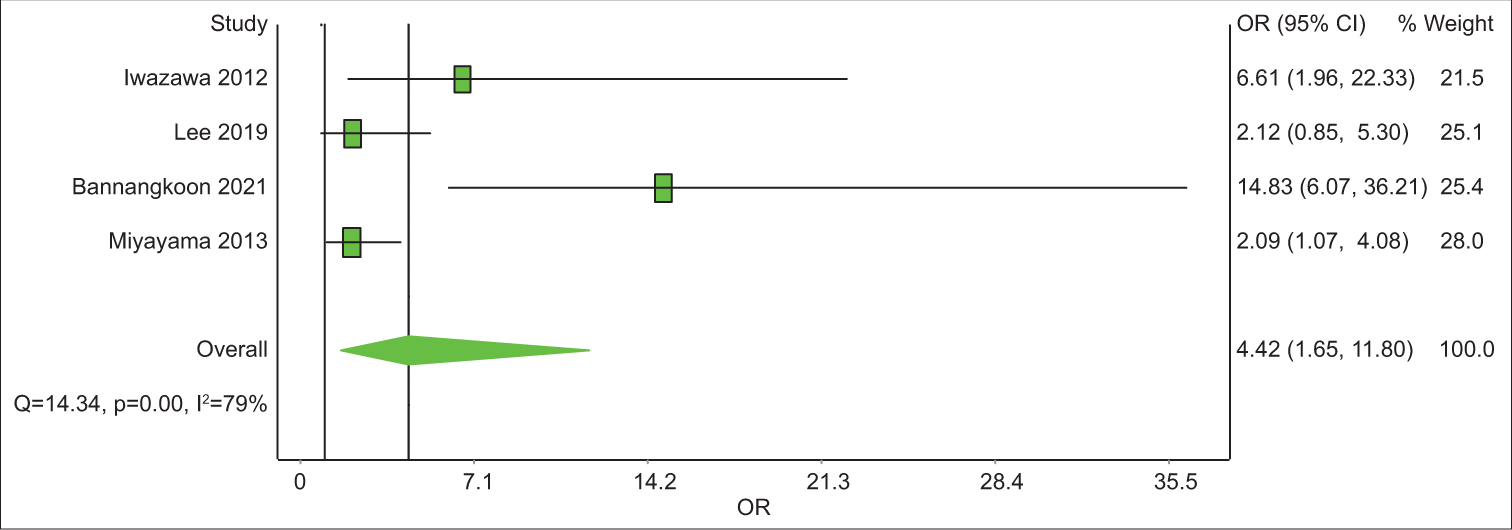
- A forest plot of 3-year local progression-free survival odds ratios comparing cone-beam computed tomography (CBCT)-guided transcatheter arterial chemoembolization (TACE) and digital subtraction angiography (DSA)-guided TACE. Odds ratios (OR) above 1 favor CBCT-guided TACE over DSA-guided TACE.
Tumor response
Out of the four articles with the control group, only two studies had data on complete response and progressive disease. Complete response results were in favor of the CBCT-guided group with 3.63 times better results than the DSA alone group with a CI from 0.89 to 14.79. Lee et al.[18] showed a higher progression rate in the CBCT-guided group, while Bannangkoon et al.[17] demonstrated a higher progression rate in the DSA-guided group. Overall analysis favored a lower progression rate in the CBCT-guided group with a pooled OR of 0.22 and CI between 0.01 and 3.78.
Overall recurrence rate
Three articles out of four articles had data on overall recurrence rates. The analysis showed that the overall recurrence rate was lower in the CBCT-guided group without statistical significance (P = 0.12).
DISCUSSION
The aim of our study was to evaluate the effectiveness of CBCT-guided TACE in the treatment of HCC and provide a comprehensive summary of the current state of the evidence on the survival rates. CBCT-guided TACE has been introduced as a way to enhance the precision and safety of TACE by providing real-time imaging during the procedure and high-resolution images of the liver with surrounding anatomy by a meta-analysis done by Pung et al.[11] However, in the meta-analysis done by Pung et al.,[11] there was no evaluation of the survival or tumor response data of the patients, which we believe is an essential gap that must be filled. Our findings indicate that CBCT-guided TACE is an effective treatment option for patients with HCC.
Our analysis of eight studies involving a total of 1176 patients demonstrated that CBCT-guided TACE was associated with significantly higher 1-year and 3-year LPFS rates compared to conventional TACE. Higher LPFS rates were consistent with previous research showing that the use of CBCT significantly increases the detection of tumors and feeding arteries during the procedure.[13,25,26] However, even though 3-year and 1-year OS rates are higher in the CBCT-guided group; the evidence of its superiority to conventional TACE was statistically inconclusive. As another component of this study, tumor responses and overall recurrence rates were in favor of CBCT-guided TACE as well; complete response results in the CBCT-guided group were 3.63 times better than the DSA alone group. All these results came out during our analysis and observation align with previous studies.[27-31]
We believe that the results of our meta-analysis show that the features of CBCT guidance, such as vessel mapping and three-dimensional tumor detection, would improve the quality of TACE treatment by maximizing its potential by not missing any anatomical detail or applying the treatment from a better location. CBCT angiography is considered vital to improving tumor detection, targeting, and distinguishing healthy liver tissue.[32,33] The current publications and our results favor CBCT to become a routine TACE component against HCC. With this study, one of our objectives was to show the importance of CBCT in interventional radiology and increase the number of CBCT-guided TACE units; also, to motivate manufacturers and researchers to invest in and improve the CBCT devices with more efficient software and engineering. Of course, there is a need for more data and studies on this specific topic to discuss whether it should be mandatory to use CBCT during TACE procedures against unresectable HCC.
The overall outcome is consistent with the previous studies that have reported benefits such as increased accuracy in catheter placement, improved tumor response, and reduced toxicity.[34-36] Usually, all treatment-related adverse events in the studies we included were mild and within acceptable limits. The most frequent adverse events observed were fever, pain, and hepatic toxicity. CBCT-guided TACE can be used to identify small tumors that may be difficult to visualize with conventional TACE, allowing for more precise targeting of the tumor and reducing the risk of recurrence.[13] The higher survival rates observed in this study may be due to the improved accuracy and precision of CBCT-guided TACE, leading to better tumor control and fewer complications.[37,38] In addition, CBCT-guided TACE provides high-resolution images of the liver and surrounding anatomy, which can help to identify the blood supply to the tumor and minimize the risk of complications.[13]
In this study, we focused on conventional TACE studies while excluding those involving DEB-TACE. This decision was guided by a comprehensive review of the existing literature, which revealed considerations. Available evidence suggested that the pooled analysis showed no significant advantage of DEB-TACE over conventional TACE in complete or partial response, disease stability, and disease progression control outcomes. Furthermore, DEB-TACE and conventional TACE involve distinct DEBs and embolization techniques, leading to differences in patient cohorts and methodological designs.[14-16] The decision-making process on this specific matter aimed to maintain a more homogeneous dataset, given the ongoing debate and conflicting results in the literature regarding its superiority over conventional TACE.
It is important to note that even after all the efforts, the studies included in our meta-analysis had some limitations as expected. First, the sample sizes of some individual studies were relatively small, and there was significant heterogeneity among the studies in terms of patient characteristics and outcome measures. Second, the majority of the studies were retrospective studies, which are inherently subject to bias. It is also possible that the higher survival rates observed with CBCT-guided TACE may be due to other factors, such as patient selection, rather than the use of CBCT-guided TACE itself, which are evaluated and mentioned under bias and quality sections.
Furthermore, the outcomes of this meta-analysis highlight the need for further research to evaluate the efficacy and safety of CBCT-guided TACE in the treatment of HCC since there is not enough data or satisfying significant results on this particular subject as we mentioned before. The usual aspect of safety is the advantage of reducing the overall patient exposure to iodinated contrast material and ionizing radiation.[34-36]
CONCLUSION
Our meta-analysis provides evidence that CBCT-guided TACE is an effective treatment option for patients with HCC. CBCT-guided TACE was associated with higher 1-year and 3-year LPFS and 3-year OS rates. Despite the limitations of the individual studies included in our meta-analysis, our findings suggest that CBCT-guided TACE has the potential to improve the outcomes of patients with HCC and should be considered as a viable treatment option. We encourage clinicians and researchers to pursue this medical technology by providing a robust synthesis of current evidence. However, further studies with larger sample sizes are needed to create consensus on determining the optimal protocol for CBCT-guided TACE and to identify the patients who will benefit most from this treatment.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Supplementary material available on:
Financial support and sponsorship
Nil.
References
- Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-606.
- [CrossRef] [PubMed] [Google Scholar]
- Trends in the mortality of hepatocellular carcinoma in the United States. J Gastrointest Surg. 2017;21:2033-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prognosis of hepatocellular carcinoma according to new staging classifications. Dtsch Med Wochenschr. 2004;129:1725-30.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatocellular carcinoma: Present status and prospects. J Hepatol. 2003;38(Suppl 1):S136-49.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of 5-year survival rate in hepatocellular carcinoma patients. J Res Med Sci. 2019;24:86.
- [CrossRef] [PubMed] [Google Scholar]
- Goals and targets for personalized therapy for HCC. Hepatol Int. 2019;13:125-37.
- [CrossRef] [PubMed] [Google Scholar]
- 2022 update of BCLC treatment algorithm of HCC: What's new for interventional radiologists? Cardiovasc Intervent Radiol. 2022;45:275-6.
- [CrossRef] [PubMed] [Google Scholar]
- Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28-36.
- [CrossRef] [PubMed] [Google Scholar]
- BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-93.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical management of hepatocellular carcinoma worldwide: A concise review and comparison of current guidelines: 2022 update. Biosci Trends. 2022;16:20-30.
- [CrossRef] [PubMed] [Google Scholar]
- The role of cone-beam CT in transcatheter arterial chemoembolization for hepatocellular carcinoma: A systematic review and meta-analysis. J Vasc Interv Radiol. 2017;28:334-41.
- [CrossRef] [PubMed] [Google Scholar]
- Cone-beam CT angiography for hepatocellular carcinoma: Current status. Dig Dis. 2015;33:759-64.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology. 2011;53:1580-9.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the safety and efficacy of conventional transarterial chemoembolization (cTACE) and drug-eluting bead (DEB)-TACE in the management of unresectable hepatocellular carcinoma: A systematic review. Cureus. 2023;15:e41943.
- [CrossRef] [Google Scholar]
- Conventional transarterial chemoembolization versus drug-eluting beads in patients with hepatocellular carcinoma: A systematic review and meta-analysis. Cancers (Basel). 2021;13:6172.
- [CrossRef] [PubMed] [Google Scholar]
- Conventional vs. drug-eluting beads transarterial chemoembolization for unresectable hepatocellular carcinoma-a propensity score weighted comparison of efficacy and safety. Cancers (Basel). 2022;14:5847.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of cone-beam computed tomography with automated feeder detection software on the survival outcome of patients with hepatocellular carcinoma during treatment with conventional transarterial chemoembolization. BMC Gastroenterol. 2021;21:419.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of transarterial chemoembolisation with cone-beam CT in patients with hepatocellular carcinoma within the Milan criteria: A retrospective cohort study. Clin Radiol. 2019;74:407, e19-28
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of local control in transcatheter arterial chemoembolization of hepatocellular carcinoma =6 cm with or without intraprocedural monitoring of the embolized area using cone-beam computed tomography. Cardiovasc Intervent Radiol. 2014;37:388-95.
- [CrossRef] [PubMed] [Google Scholar]
- Survival after C-arm CT-assisted chemoembolization of unresectable hepatocellular carcinoma. Eur J Radiol. 2012;81:3985-92.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of superselective conventional transarterial chemoembolization using guidance software for hepatocellular carcinoma within three lesions smaller than 3 cm. Cancers (Basel). 2021;13:6370.
- [CrossRef] [PubMed] [Google Scholar]
- Role of cone-beam CT in the intraprocedural evaluation of chemoembolization of hepatocellular carcinoma. J Oncol. 2021;2021:8856998.
- [CrossRef] [PubMed] [Google Scholar]
- Cone beam CT-guided chemoembolization of probable hepatocellular carcinomas smaller than 1 cm in patients at high risk of hepatocellular carcinoma. J Vasc Interv Radiol. 2017;28:795-803.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Ultraselective transcatheter arterial chemoembolization for small hepatocellular carcinoma guided by automated tumor-feeders detection software: Technical success and short-term tumor response. Abdom Imaging. 2014;39:645-56.
- [CrossRef] [PubMed] [Google Scholar]
- 90Y radioembolization versus drug-eluting bead chemoembolization for unresectable hepatocellular carcinoma: Results from the TRACE phase II randomized controlled trial. Radiology. 2022;303:699-710.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of automated tumor-feeder detection software using cone-beam computed tomography technology in transarterial embolization through extrahepatic collateral vessels for malignant hepatic tumors. Hepatol Res. 2016;46:166-73.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between progression-free survival and overall survival in randomized clinical trials of targeted and biologic agents in oncology. J Cancer. 2019;10:3717.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6:e204564.
- [CrossRef] [PubMed] [Google Scholar]
- Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151:1155-63.e2.
- [CrossRef] [PubMed] [Google Scholar]
- Initiative on superselective conventional transarterial chemoembolization results (INSPIRE) Cardiovasc Intervent Radiol. 2022;45:1430-40.
- [CrossRef] [PubMed] [Google Scholar]
- Yttrium-90 radioembolization for the treatment of solitary, unresectable HCC The LEGACY study. Hepatology. 2021;74:2342-52.
- [CrossRef] [PubMed] [Google Scholar]
- The role of immediate post-procedural cone-beam computed tomography (CBCT) in predicting the early radiologic response of hepatocellular carcinoma (HCC) nodules to drug-eluting bead transarterial chemoembolization (DEB-TACE) J Clin Med. 2022;11:7089.
- [CrossRef] [PubMed] [Google Scholar]
- Three-dimensional evaluation of lipiodol retention in HCC after chemoembolization: A quantitative comparison between CBCT and MDCT. Acad Radiol. 2014;21:393-9.
- [CrossRef] [PubMed] [Google Scholar]
- Recognizing extrahepatic collateral vessels that supply hepatocellular carcinoma to avoid complications of transcatheter arterial chemoembolization. Radiographics. 2005;25(suppl_1):S25-39.
- [CrossRef] [PubMed] [Google Scholar]
- Cone-beam computed tomography imaging: therapeutic staff dose during chemoembolisation procedure. J Radiol Prot. 2014;34:843-51.
- [CrossRef] [PubMed] [Google Scholar]
- Radiation exposure to operating staff during rotational flat-panel angiography and C-arm cone beam computed tomography (CT) applications. Eur J Radiol. 2012;81:4138-42.
- [CrossRef] [PubMed] [Google Scholar]
- Selective and sequential transarterial chemoembolization: Survival in patients with hepatocellular carcinoma. Eur J Radiol. 2012;81:2290-7.
- [CrossRef] [PubMed] [Google Scholar]
- Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: Relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol. 2007;18:365-76.
- [CrossRef] [PubMed] [Google Scholar]






