Translate this page into:
Subpleural Lung Changes Adjacent to Enlarged Internal Mammary Artery in Patients with Coarctation of Aorta and Pulmonary Hypoplasia: Some Thoughts on an Interesting Observation

*Corresponding author: Venkatraman Bhat, Imaging Services, Narayana Health, Bengaluru - 560 099, Karnataka, India. bvenkatraman@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhat V, Gadabanahalli K. Subpleural lung changes adjacent to enlarged internal mammary artery in patients with coarctation of aorta and pulmonary hypoplasia: Some thoughts on an interesting observation. J Clin Imaging Sci 2020;10:14.
Abstract
Irregular pleural interface, minimally reduced lung density and/or focal subpleural cystic lung changes were noted in two patients with coarctation of aorta and in a patient with the right pulmonary hypoplasia. Lesions were distributed in the anterior segments of upper lobes, adjacent to internal mammary arteries (IMA). In view of unusual location of lung changes with unique distribution pattern prompted us to look for ‘cause and effect’ relation of events specific to long standing vascular pulsations, thus explaining the lung changes. While there are multiple factors for cystic lung disease, special distribution the lung changes seen in our patients leads us to explore mechanical process such as water hammer effect by dilated tortuous pulsatile IMA on the lungs as an additional etiology. A brief note on clinical and imaging appearance of our patients and discussion regarding water hammer theory are presented.
Keywords
Coarctation of aorta
Water hammer effect
Subpleural cyst
Internal mammary artery
Computed tomography pulmonary
Pulmonary hypoplasia
INTRODUCTION
There is no well-established relation between coarctation of aorta and cystic lung disease. Single case of aortic coarctation is reported in association with congenital lung cysts in the right upper lobe.[1] Subpleural cysts, commonly seen in adolescents, are typically located at the lung apices and do not show distribution along vascular structures. Review of the literature does not provide evidence of clear association between aortic coarctation and focal lung aeration abnormality in upper lobes or cystic lung changes in the vicinity of collateral arteries.[2,3] Mechanism leading to selective location of cystic lung changes in our patients is of interest considering the fact that there are tortuous pulsating internal mammary arteries (IMAs) in the vicinity. In general, the mechanisms which can cause lung damage are repetitive direct mechanical pressure, indirect damage secondary to repeated expansion-contraction of tissues due to exaggerated pulsations or due to genetic predisposition. The effect of mechanical pressure is reported in airways when sudden pressure rise produced by glottal closure in the sub-gliotic tract during vocal fold oscillation. Flow turbulence, which can cause tissue damage due to oscillation can be computed as a water hammer effect in engineering. In this report, we propose water hammer effect caused by hypertrophic, pulsatile IMA as a possible mechanism for cystic lung changes.
CASE REPORT
Case 1
An 18-year-old male patient was incidentally detected to have hypertension with blood pressure reading of 173/78 mmHg. Peripheral pulses were present in all limbs, 55/min with slight delay of pulse on the right upper limb. Evaluation of cardiovascular system revealed ejection systolic murmur and evidence of the left ventricular hypertrophy on clinical examination and electrocardiogram. Chest radiograph revealed subtle changes like inconspicuous aortic arch with relative prominence of descending thoracic aorta. Lung parenchyma was unremarkable. There was no rib notching. Echocardiography showed normal right atrium, mildly enlarged left atrium and left ventricle. Ascending aorta was normal in size, whereas aorta beyond the left subclavian artery could not be visualized. Based on echo observations, a provisional diagnosis of severe coarctation of aorta was suspected. Multidetector computed tomography (MDCT) evaluation was performed and confirmed severe narrowing of aortic arch distal to the left subclavian artery [Figure 1a]. Markedly enlarged, tortuous intercostal and IMAs were demonstrated constituting collateral circulation. Lungs revealed gross surface irregularities [Figure 1b], subpleural cystic changes, distributed mainly adjacent to the path of IMA [Figure 2a-c]. Few small lung cysts were noted at the deeper aspect of upper lobes. Lung changes were best demonstrated with volumetric minimal intensity projection (MinIP). Subpleural upper lobe anterior lungs also showed reduced caliber of branches of pulmonary arteries and focal reduction in attenuation values. There was no evidence of generalized emphysema. The patient was recommended to have surgical correction of aortic coarctation. The patient did not undergo surgical correction due to financial limitations
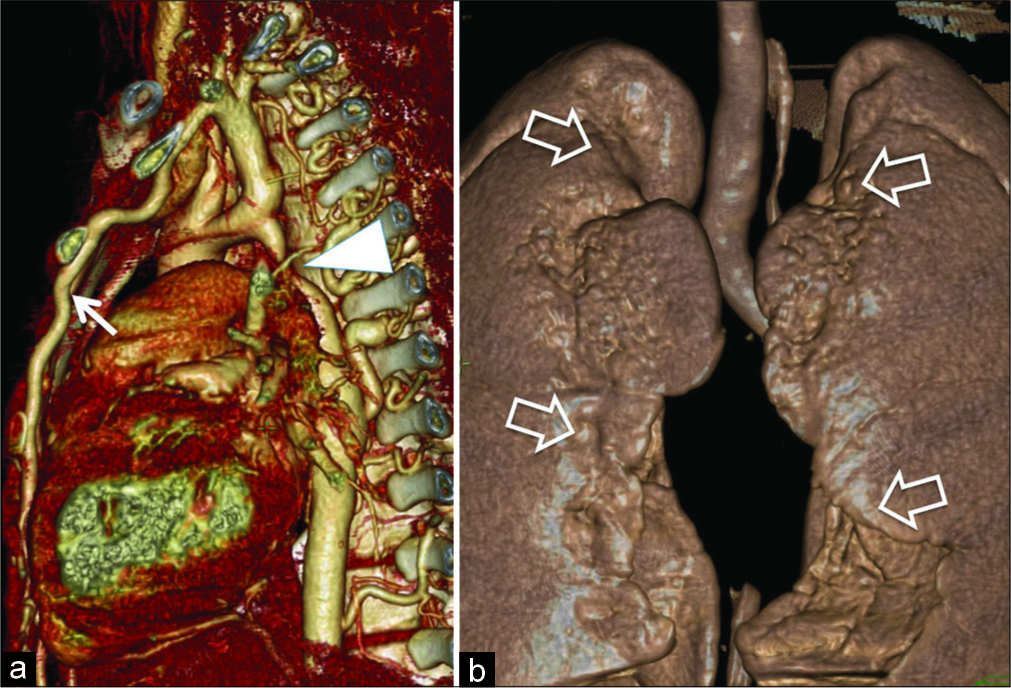
- An 18-year-old male with coarctation of aorta presented with incidentally detected hypertension. Computed tomography 3D angiogram (a) demonstrates post-ductal coarctation (arrowhead) and enlarged, hypertrophic internal mammary artery (white arrow). (b) On a 3D surface rendered anterior view, there is an irregular pleural interface along internal mammary arteries (open arrows).
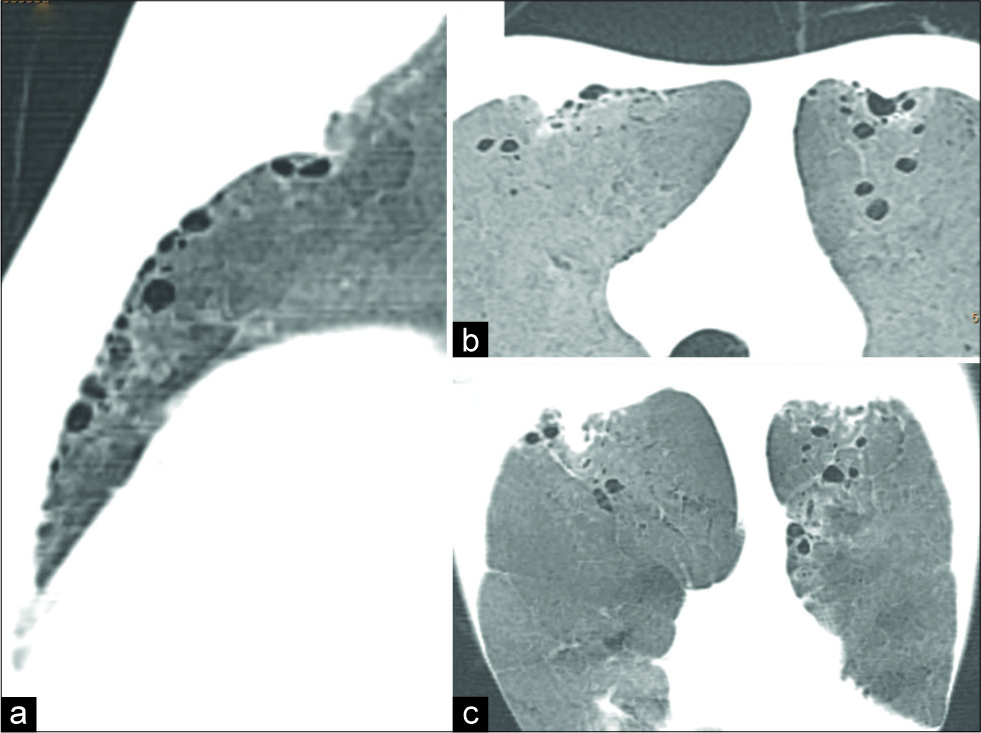
- An 18-year-old male diagnosed with coarctation presented with incidentally detected hypertension. Computed tomography minimal intensity projection images (a) sagittal, (b) axial, and coronal (c) orientation demonstrate subpleural cysts in anterior part of upper lobes along the path of internal mammary arteries.
Case 2
A 22-year-old male patient presented with an episode of throbbing headaches and exertional dyspnea. On examination, he was found to have hypertension with blood pressure reading of 184/88 mmHg. Peripheral pulses were present in all limbs, regular, 75/min. Cardiovascular evaluation revealed left ventricular cardiomegaly and evidence of post- ductal coarctation on echocardiography. There was no rib notching. MDCT confirmed severe narrowing/interruption of aorta distal to the left subclavian artery [Figure 3a]. Markedly enlarged, tortuous IMAs were demonstrated causing prominent impression on anterior lung segments [Figure 3b]. Lungs revealed subpleural irregularly defined hyperlucent areas located adjacent to the path of IMA [Figure 3c and d]. Lesions were more extensive on the left side. There were no well-defined lung cysts. MinIP images clearly showed typical distribution of the lesion. Subpleural upper lobe anterior lungs also showed a paucity of pulmonary arteries and focal reduction in attenuation values highlighted in color map. There was no evidence of generalized emphysema. Pulmonary function test, done as a part of pre-operative evaluation, was normal. The patient underwent successful surgical correction of aortic coarctation without any post-operative complications.
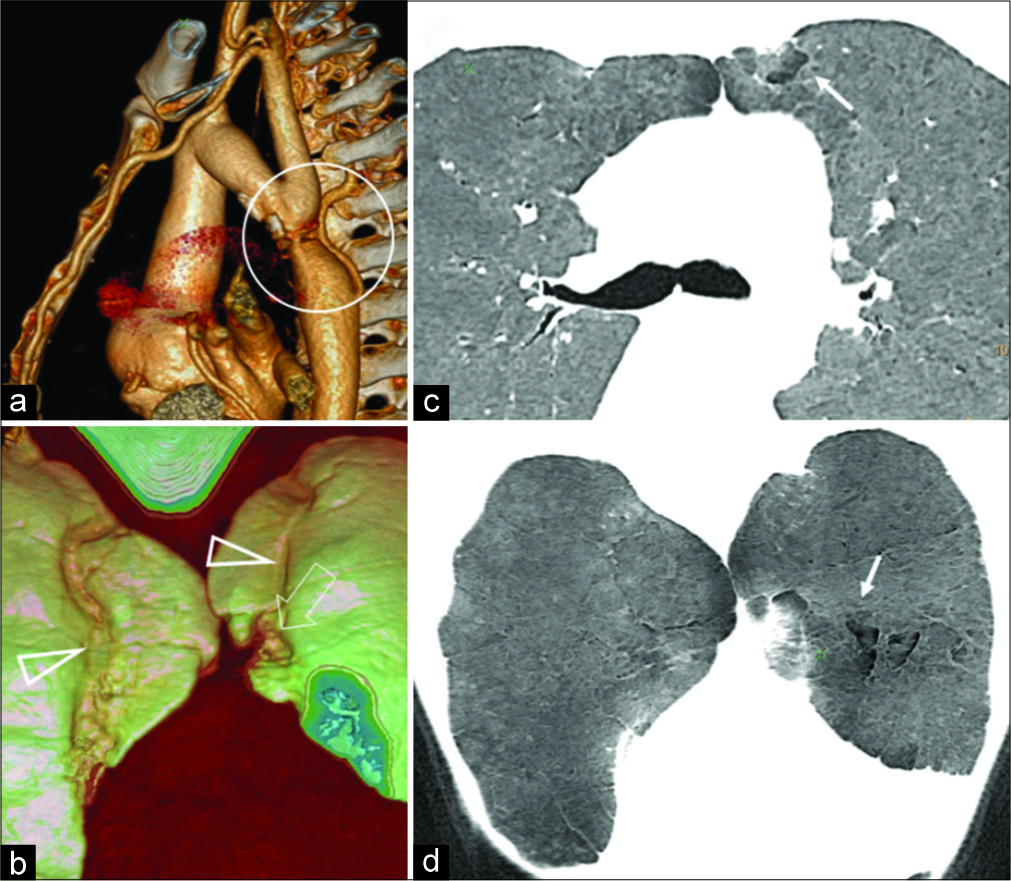
- A 22-year-old male with coarctation of aorta presented with throbbing headache, exertional dyspnea shows (a) post-ductal coarctation (circle) with dilated internal mammary artery on surface rendered 3D image. (b) 3D surface rendered image of the anterior lungs shows prominent groove for internal mammary arteries with irregular pleural surface on the left side. Axial and coronal (c and d) minimal intensity projection image demonstrates vertically oriented hyperlucency bilaterally, with more extensive change on the left side (arrow).
Case 3
A 7-month-old male with ventricular septal defect (VSD) was investigated with MDCT for the evaluation of associated anomalies. Examination confirmed a large perimembranous VSD and additionally showed right pulmonary lower lobe branch stenosis, right lung hypoplasia, and enlarged right IMA, supplying the hypoplastic lung. Furthermore, subpleural cystic changes are noted around the enlarged IMA branches [Figure 4]. The left lung was normal except for subtle radiolucent area in the lower lobe.

- A 7-month-old male presented with failure to thrive, on examination diagnosed with ventricular septal defect and enlarged internal mammary arteries (IMA) secondary to pulmonary branch stenosis. Subpleural cystic changes are noted (arrows) around the enlarged branches of IMA (triangle) (a-c and e). Irregular pleural surface (open arrow) is noted on 3D surface rendered images of the lungs (d).
DISCUSSION
Review of the literature for various forms of cystic lung disease does not suggest direct association with “regional causative factor” for pathogenesis or evolution of disease process.[2,3] Pulsations from vascular structures are not the known to be etiological factor in the causation of cystic changes in adjacent lungs. Location, distribution of lung cysts, and anatomical environment in our cases make it a compelling case for cause and effect relation. While exploring possible mechanisms, established physical hydrodynamic principles appear to provide a possible explanation for the association in our case. Water hammer effect (pressure surges within fluid transients in pipelines) is an oscillatory form of unsteady flow generated by sudden changes due to the rapid closing or opening of valves often caused by a pumping action. The term “water hammer” was initially coined to indicate pressure variations causing damage to liquid-filled pipe systems. Now, the term is broadly applicable to all types of transient flow in pipelines. Water hammer analyses provide information on the operational conditions, namely, pressure and velocity within a pipe system. Additional confounding effects such as unsteady friction, acoustic radiation to the surroundings, or fluid-structure interaction (FSI) are also considered operational in the general context.[4] FSI can be presented to include mechanisms acting both all along the entire pipe and at specific points in the system. In the biomedical context, water hammer theory has been proposed to account for effects in biofluids under mechanical stress, such as explaining Korotkoff sounds during blood pressure measurement,[5,6] or to the development syrinx within the spinal cord;[7] same principle has also been suggested in relation to trachea and the supralaryngeal tract in the context of voice production, allowing prediction of the intraoral and intratracheal pressure fluctuations, induced by vocal fold motion in the phonatory system.[8]
The effect of repeated pressure waves caused by pulsating arteries on air containing lungs has not been explained on the basis of water hammer theory. It is very tempting to consider such a phenomenon in our cases, to explain the distribution of subpleural cysts adjacent to IMA. We have seen definitive evidence of the distribution of lesions along IMA with irregular pleural interface and focal areas of low attenuation in our patients. Cystic changes, however, were evidenced in only one patient. Lung changes appear secondary to the enlargement of IMA (and secondary effects) due to any etiology, rather than limited to patients with coarctation, as indicated by the third case. Close scrutiny of disease pattern in the images of our patients certainly appears to justify this possibility. It is very likely that there may be more than one factor in the susceptibility of lung parenchyma apart from repetitive mechanical insult, explaining interindividual variability. Validity of such effect, perhaps, needs to be substantiated by recording and reviewing clinical contexts, wherein pulsating vascular structures are located in the vicinity of lungs, in a larger cohort of patients. Such clinical contexts include patients with enlarged collateral arteries, patients with aortic aneurysm or large arteriovenous malformations, etc. Clinical significance of these observations at the moment is not clear. In view of limited morphological disease burden on the lungs, it is unlikely that these changes will be associated with clinical symptoms. Only one patient underwent pulmonary function test, which was normal. Larger cross-sectional study with detailed clinical, laboratory, and histological evaluation may throw additional light on the complex topic. Thus, concept presented in this report may open doors for exploration in that direction.
CONCLUSION
Pulmonary and pleural changes in the form of regional radiolucency, subpleural cystic changes and irregular pleuro-pulmonary interface on reconstructed CT images are demonstrated in patients with coarctation of aorta and right pulmonary hypoplasia with enlarged internal mammary arteries. Exact aetiology of this unique observation is unclear. Brief discussion on the possible mechanisms, including the role of Water hammer effect is presented. Need for further exploration and research in such similar clinical setting is recommended.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Atypical aortic coarctation (author's transl) Thoraxchir Vask Chir. 1976;24:107-18.
- [CrossRef] [PubMed] [Google Scholar]
- Cystic and cavitary lung diseases: Focal and diffuse. Mayo Clin Proc. 2003;78:744-52.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges in pulmonary fibrosis. 3: Cystic lung disease. Thorax. 2007;62:820-29.
- [CrossRef] [PubMed] [Google Scholar]
- Fluid-structure interaction in liquid-filled pipe systems: A review. J Fluids Struct. 1996;10:109-46.
- [CrossRef] [Google Scholar]
- Characterization of the korotkoff sounds using joint time-frequency analysis. Physiol Meas. 2004;25:107-17.
- [CrossRef] [PubMed] [Google Scholar]
- Hypothesis on the pathophysiology of syringomyelia based on simulation of cerebrospinal fluid dynamics. J Neurol Neurosurg Psychiatry. 2003;74:344-7.
- [CrossRef] [PubMed] [Google Scholar]
- A water hammer analysis of pressure and flow in the voice production system. Speech Commun. 2009;51:344-51.
- [CrossRef] [Google Scholar]






