Translate this page into:
Struma Ovarii with Papillary Thyroid Carcinoma
Address for correspondence: Dr. Daniel M. Alvarez, Interventional Radiology Fellow, Hospital San Jose Tec de Monterrey, Ave. Morones Prieto No. 3000 Pte. Col. Doctores, Monterrey, N.L., Mexico C.P. 64710 E-mail: alvarezmauricio@gmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Struma ovarii is an uncommon condition, in which thyroid tissue is the predominant or exclusive element in an ovarian teratoma. Thyroid tissue may demonstrate the same spectrum of pathological features as in the normal thyroid including benign and malignant changes. We present a case of papillary thyroid carcinoma arising in a struma ovarii of the left ovary in a 21-year-old female.
Keywords
Dermoid cyst
mature cystic teratoma
papillary carcinoma
struma ovarii
ultrasound
INTRODUCTION

Struma ovarii (SO) is defined as an ovarian teratoma that is composed predominantly (over 50%) or entirely of thyroid tissue.[1] It may demonstrate pathologic features that can be seen in the normal thyroid including benign and malignant changes.[2] Although 5–15% of all ovarian teratomas may contain some thyroid tissue; only 2% of these are made exclusively of SO.[2] The vast majority of SO are benign tumors (95%).
CASE REPORT
Our patient is a 21-year-old African American female, gravida 1 para 1; with no significant medical history, who had been on surveillance, for the past 3 years, for a left pelvic mass found incidentally during routine perinatal ultrasound (US). At the time of initial discovery, the left adnexal mass measured 7.1 × 7.4 × 5.1 cm and contained both cystic and solid components [Figure 1]. No significant internal blood flow was appreciated by US at that time. The patient was given the diagnosis of a mature cystic teratoma of the left ovary and managed with follow-up of the lesion with US imaging. The patient was lost to follow up until 2 years later, when she presented to the emergency room with complaints of 3 days of right lower quadrant pain and vomiting. Transvaginal US [Figure 2] as well as abdominal computed tomography (CT) was obtained. A large multilobular mixed cystic and solid lesion arising from the left ovary was observed once again with no significant change in its size from the prior ultrasound. CT demonstrated enhancement of the solid portions of the mass and, in addition, calcifications were noted [Figure 3]. Tumor markers (CA-125, AFP, and beta hCG) were negative. The patient was discharged from the emergency department with the diagnosis of mature cystic teratoma.
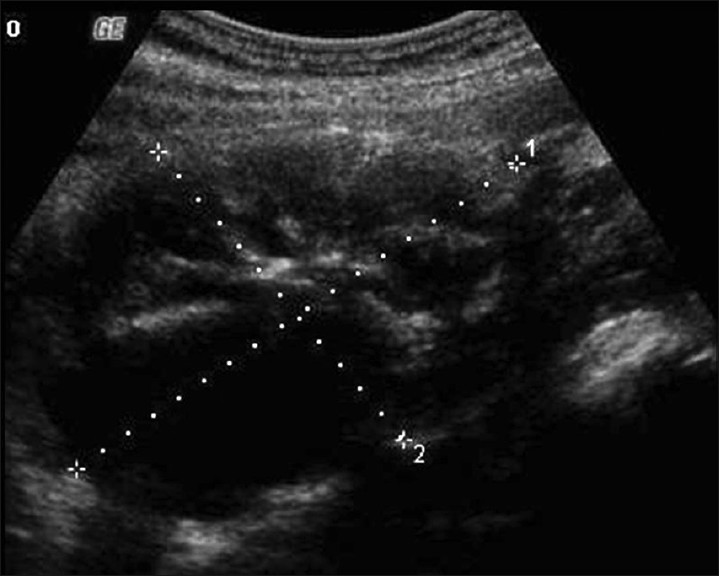
- Transabdomen grayscale US image shows a heterogeneous lesion demonstrating solid and cystic portions arising from the left ovary.
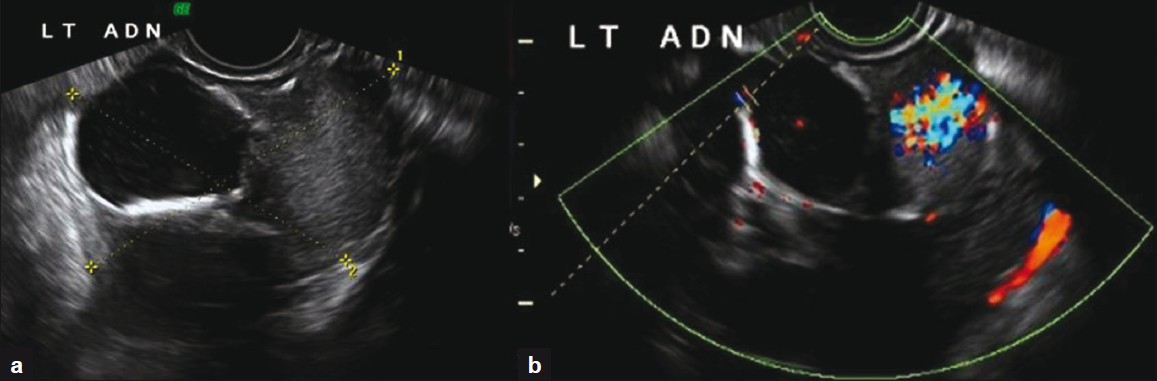
- (a) Transvaginal US image shows a complex mass arising from the left ovary with solid and cystic components (b) Color Doppler examination of the same mass demonstrates high vascularity in the solid portion of the left ovarian mass.
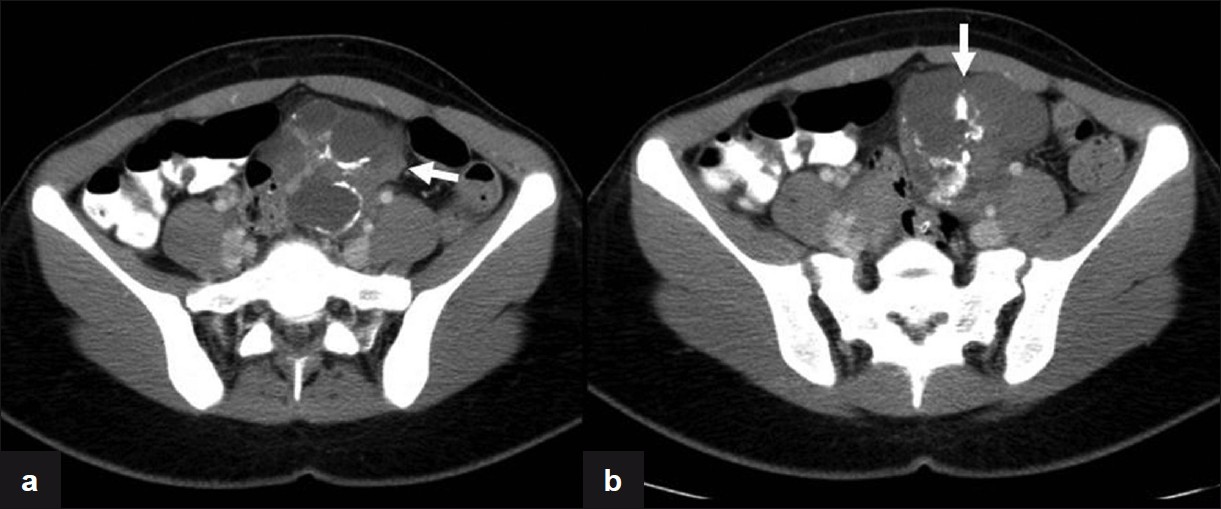
- Axial CT images with IV contrast demonstrate the presence of a heterogeneous mass in the left adnexa (arrows) with macro calcifications and enhancement of the solid components.
After 1 year of US imaging follow up [Figure 4] and intermittent episodes of left lower quadrant pain, the patient elected to have a laparoscopic operative procedure. A left salpingo-oophorectomy was performed with removal of a 9.5 × 5.5 × 3.5 cm tan-gray, multilobulated cystic mass along with the adherent segment of the left fallopian tube. The histopathology report was papillary thyroid carcinoma arising in SO [Figure 5], with no capsular invasion.
As part of her treatment, the patient underwent a total thyroidectomy, which demonstrated no evidence of thyroid malignancy. No further treatment was deemed necessary at this time.
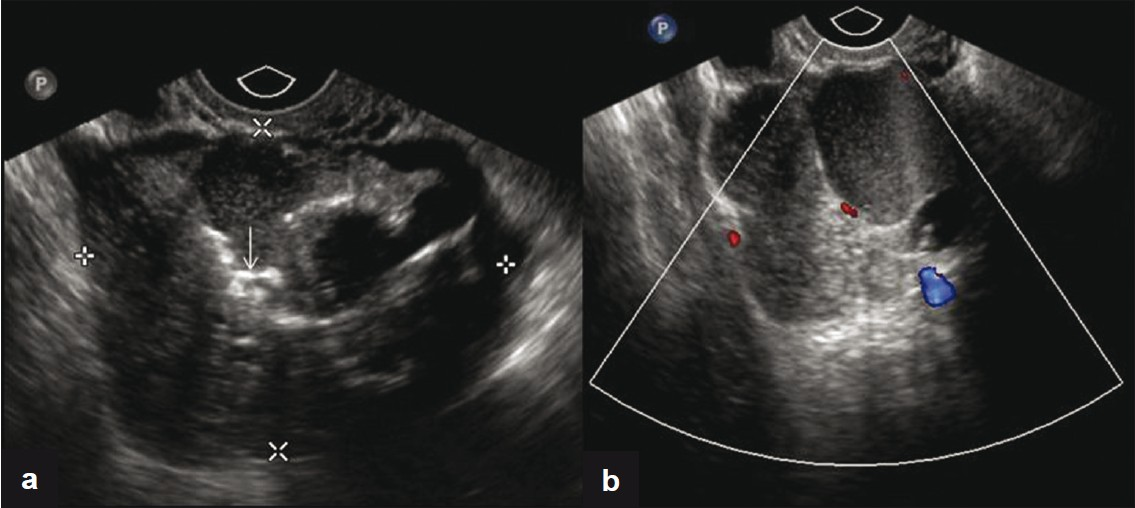
- (a) Transvaginal US image shows a complex mass with solid and cystic components and calcifications (arrow). (b) Corresponding color flow Doppler image of the lesion.
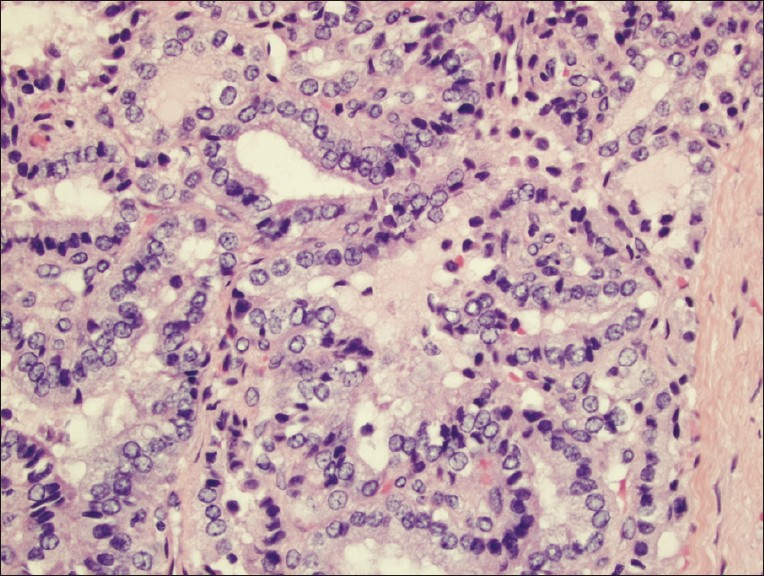
- Hematoxylin and eosin-stained tissue (400×) displaying branching papillae with atypical cytologic features including nuclear groves, clearing, overlapping, and enlargement, consistent with papillary thyroid carcinoma arising in a struma ovarii.
The patient has subsequently been followed up for many years with no evidence of metastasis.
DISCUSSION
SO had been described earlier, but Ludwig Pick first recognized in 1901 that this tumor was composed of thyroid tissue and suggested that ovarian goiters are actually teratomas in which thyroidal elements have overgrown the other tissues.[3]
The age incidence of SO is 20–40 years, similar to that of mature cystic teratoma.[1] The peak age incidence of SO is in the fifth decade, but cases have been reported in postmenopausal women and uncommonly occur in prepubertal girls.[1]
The patient usually presents signs and symptoms of a pelvic mass, ascites in one-third of cases, and occasional patients have Meigs′ syndrome.[1] Less than 5% of SO are associated with hyperthyroidism. Because 95% of SO are benign and usually occur in premenopausal women, preoperative diagnosis is important to avoid unnecessary surgery such as hysterectomy and dissection of pelvic lymph nodes.[4]
Sonographically, SO appears similar to mature cystic teratomas, with a mean tumor diameter of 5.7 cm (3–9.5 cm). Zalel Y et al described a typical finding in a series of 251 cystic teratomas where they demonstrate the presence of abundant blood flow in the center of the SO tumors and the reason for this finding can be related to the existence of highly vascularized thyroid tissue in comparison to the avascular fat and hair found in common teratomas.[5]
Although imaging findings of SO are nonspecific, US and CT demonstrate its complex appearance with multiple cystic and solid areas and a multilobulated surface, reflecting the gross pathologic appearance of the tumor.[6] At magnetic resonance imaging (MRI), the signal intensity of these components varies. The classic MRI appearance includes multiple intracystic areas with low signal intensity on T2-weighted images and intermediate signal intensity on T1-weigthed images, findings that represent thyroid tissue.[7]
In most instances, the thyroid tissue within the tumor does not produce a significant amount of thyroid hormone. In a minority of cases, however, the tumor behaves autonomously and produces excess thyroid hormone. This in turn suppresses the normal (cervical) thyroid and causes thyrotoxicosis. Scintigraphy performed with Iodine-131 is useful for diagnosing a hyperfunctioning SO on the basis of higher uptake of the radionuclide by the ovarian mass than by the thyroid gland in the neck.
When SO is not associated with hyperthyroidism, the differential diagnosis should include mature cystic teratoma without fatty tissue, cystadenoma or cystadenocarcinoma, endometriosis, tuboovarian abscess, and metastatic tumor because the imaging features of these tumors may resemble those of SO.[8]
Histopathology of SO typically consists of normal-appearing thyroidal tissue composed of thyroid follicles of various sizes and often is associated with mature cystic teratoma.[1] Occasionally, the thyroid tissue may show changes associated with hyperactivity, hypoactivity, or have the appearance of an adenomatous nodule or nodular goiter. Uniform accepted criteria for malignancy in SO have yet to be established. Because of varying usage and the lack of a precise definition, it is recommended that the term “malignant struma ovarii” no longer be utilized for cases of malignancy that develop in ovarian struma. The term “thyroid-type carcinoma originating in struma ovarii” (specifying the type) is more appropriate to describe this entity.[1]
Papillary carcinoma is the most common thyroid-type carcinoma to occur in SO; follicular carcinoma is the second most common type of carcinoma and varies in its degree of differentiation.[1] It is important to note that histological changes of malignancy in SO, however, often do not equate with clinical malignant behavior, and most cases of thyroid-type carcinoma arising in SO do not have a clinically aggressive course.[1]
Due to its rarity, there is no agreement in the diagnosis and management of these tumors.[9] Although these tumors are relatively indolent, most authors recommend surgery and debulking of metastasis followed by radioactive iodine. Total thyroidectomy is advocated to allow effective ablative therapy with Iodine-131, to facilitate follow-up and to exclude by careful histologic examination, a primary thyroid cancer.[10]
Follow up examinations of patients with thyroid-type carcinoma include assessment of thyroglobulin levels and Iodine-131 scanning, the same procedures used in the case of thyroid carcinoma.[9] The metastatic rate is around 5–6% and it may occur even up to 26 years after the primary disease.
In summary, this case demonstrates the imaging findings and the correlation with the histopathology of papillary thyroid carcinoma originating in SO. SO is a rare entity that may be suggested when there are findings of a complex ovarian mass with highly vascular or enhancing solid portions seen by cross-sectional imaging. As evidenced in this case report, it is important to remember that a minority of SO are associated with thyroid-type carcinoma and this may significantly alter patient management.
Source of Support: Nil
Conflict of Interest: None declared.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2011/1/1/44/84322
REFERENCES
- A selective historical survey of ovarian pathology emphasizing neoplasms. In: Roth LM, Czernobilsky B, eds. Tumors and tumor like conditions of the ovary. Vol 6. New York: Churchill Livingstone; 1985. p. :269-85.
- [Google Scholar]
- New and unusual aspects of ovarian germ cell tumors. Am J Surg Pathol. 1993;17:1210-24.
- [Google Scholar]
- Sonographic and clinical characteristics of struma ovarii. J Ultrasound Med. 2000;19:857-61.
- [Google Scholar]
- Imaging findings of complications and unusual manifestations of ovarian teratomas. Radiographics. 2008;28:969-83.
- [Google Scholar]
- Thyroid carcinoma on an ovarian teratoma; A case report and review of literature. Gynecol Endocrinol. 2002;16:207-11.
- [Google Scholar]
- Malignant struma ovarii treated by ovariectomy, thyroidectomy and 131 I administration. Cancer. 1987;60:178-82.
- [Google Scholar]






