Translate this page into:
Sequential Venoplasty for Treatment of Inferior Vena Cava Stenosis Following Liver Transplant
Address for correspondence: Dr. Ron Charles Gaba, Department of Radiology, Division of Interventional Radiology, University of Illinois Hospital and Health Sciences System, 1740 West Taylor Street, MC 931, Chicago, IL 60612, USA. E-mail: rgaba@uic.edu
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Obstruction of the inferior vena cava (IVC) is a rare complication of liver transplantation with significant consequences including intractable ascites and hepatic dysfunction. Although venoplasty and stenting are effective in many cases, patients who fail first-line treatment may require surgical intervention or re-transplantation. Scheduled sequential balloon dilation, an approach frequently used to treat fibrotic, benign biliary strictures, but less commonly vascular lesions, may avert the need for such high-risk alternatives while achieving favorable clinical and angiographic response. Herein, we report the case of a 36-year-old woman with transplant-related, initially angioplasty-resistant IVC stenosis that was successfully treated with sequential balloon dilation.
Keywords
Inferior vena cava
liver transplant
stenosis
venoplasty
INTRODUCTION

Conventional venoplasty of post-liver transplant inferior vena cava (IVC) stenoses is limited by a high primary treatment failure rate and problematic durability.[1] While IVC stenting may deliver a successful outcome after failed angioplasty,[1] this approach, however, is not always suitable. Hepatic vein ostial coverage may not be desired, device migration may be a concern, and excessive risk for intra-stent stenosis may be perceived in specific cases, rendering definitive balloon dilation therapy as the lone possible minimally invasive treatment option. Recognizing that single-session balloon venoplasty may not successfully and consistently address IVC stenoses, the percutaneous management approach of fibrotic, benign biliary strictures, namely, scheduled sequential balloon dilation sessions, may be adapted for the treatment of analogously scarred vascular lesions.[2] Herein, we describe the case of a young patient with an initially angioplasty-resistant post-transplant IVC stenosis who was successfully treated with programmed successive balloon dilation, and highlight the utility of this approach in managing cases where stent insertion is undesirable.
CASE REPORT
Institutional review board approval is not required for single retrospective case study at our institution. A 36-year-old woman with a history of autoimmune hepatitis and two prior orthotopic liver transplants in 1998 and 2008 (the second transplant was performed due to rejection of the first graft) presented 5 years after her most recent transplant with a chief complaint of progressive bilateral lower extremity edema and weight gain over the preceding 8 weeks. On physical examination, the patient had bilateral 3+ pitting edema to the level of the buttocks. Duplex ultrasonography and computed tomography (CT) imaging revealed a focal thrombus within the hepatic level IVC. The patient's liver function was preserved on lab assessment. Compression stockings and diuretic therapy provided minimal symptomatic relief, and the patient's condition continued to worsen in terms of leg swelling despite initiation of therapeutic anticoagulation. The patient was subsequently referred to interventional radiology (IR) for catheter-directed venous thrombolysis. She received intra-thrombus, low-dose (0.5 mg/h) tissue plasminogen activator (TPA) for 48 h, which resulted in complete clearance of the IVC clot, but revealed an underlying high-grade stenosis of the hepatic IVC at the level of the end-to-side transplant anastomosis just superior to the hepatic vein ostium [Figure 1]. The lesion was dilated using a 10-mm balloon after thrombolysis termination, but recoil afforded no luminal caliber improvement. The patient's lower extremity edema thus persisted [Figure 2] despite systemic anticoagulation given for 2 months post-procedure. The patient also developed a mild increase in her bilirubin (1.7 mg/dl) and creatinine (1.5 mg/dl) levels; further intervention was thus clinically indicated. Her case was discussed among a multidisciplinary team comprising transplant surgeons, hepatologists, and interventional radiologists. Stenting of the lesion was considered, but was felt to be undesirable given the relative young age of the patient, which placed her at high lifetime risk of intra-stent stenosis and future recurrent complications, while the preference of the transplant surgeon to avoid impeding the hepatic vein ostium limited the choice of stent to shorter models with a concomitantly higher risk of migration. Therefore, further balloon angioplasty was selected as the management choice.
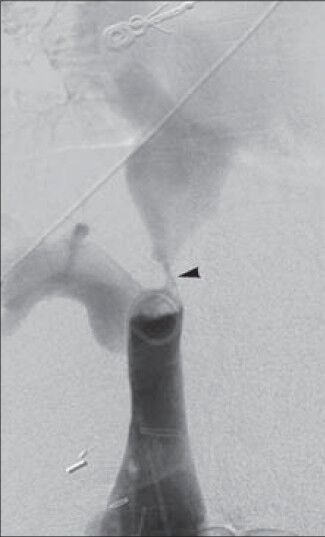
- 36-year-old woman with a history of autoimmune hepatitis and two prior orthotopic liver transplants who presented 5 years after her most recent transplant with a chief complaint of progressive bilateral lower extremity edema and weight gain was diagnosed with transplant-related, initially angioplasty-resistant IVC stenosis. Inferior vena cavagram reveals high-grade stenosis of hepatic level IVC (arrowhead), with 2-3 mm luminal caliber at this site.
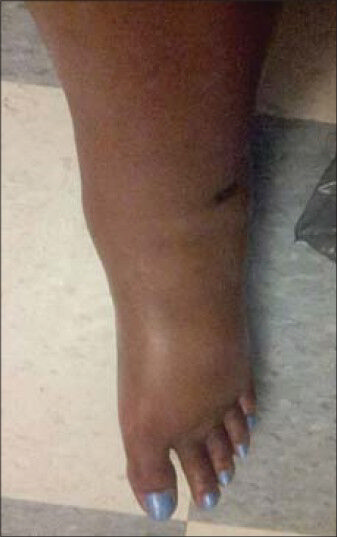
- 36-year-old woman with a history of autoimmune hepatitis and two prior orthotopic liver transplants who presented 5 years after her most recent transplant with a chief complaint of progressive bilateral lower extremity edema and weight gain was diagnosed with transplant-related, initially angioplasty-resistant IVC stenosis. Pre-treatment photograph shows marked lower extremity edema.
Venoplasty procedures were performed in the IR suite under intravenous moderate sedation. Sequential treatment sessions were scheduled at 2-3 week intervals. In general, the patient was positioned supine and her right neck prepared in standard sterile fashion. The right internal jugular vein was localized with ultrasound, after which the overlying soft tissue was anesthetized with 2% lidocaine and the vessel accessed with a 21-gauge needle under real-time sonographic guidance. The percutaneous access was then dilated to accept a 12-French vascular sheath, which was then advanced into the upper IVC. At the first treatment session, angioplasty of the lesion was performed using a 10-mm balloon (Mustang; Boston Scientific, Natick, MA, USA) with prolonged (2-3 min) inflation. The IVC caliber improved to 6 mm. At the second treatment session, an 8-mm cutting balloon (Peripheral Cutting Balloon; Boston Scientific) and a 12-mm high-pressure balloon (Mustang) were sequentially dilated at the site of the stenosis three times each, with the idea that the atherotomes would assist with disruption of fibrotic and scarred tissue, as in the case of benign anastomotic biliary strictures. Further angioplasty was then performed using a 16-mm balloon (XXL Vascular; Boston Scientific) with three inflations until the balloon waist was fully expanded, followed by prolonged inflation. The IVC diameter enlarged to 8 mm. At the third treatment session, sequential dilation was performed using 14-mm (XXL Vascular), 16-mm (XXL Esophageal; Boston Scientific), and 18-mm (MAXI LD; Cordis, Bridgewater Township, NJ, USA) balloons that were advanced to the stenosis and sequentially inflated twice each for 2-3 min per inflation. Each of the balloons reached its nominal diameter except for the 18-mm balloon, which had a small residual waist. The IVC size increased to 9 mm. A fourth venoplasty session was similarly performed using 16-mm (XXL Esophageal) and 18-mm (Maxi LD) balloons sequentially inflated twice each for 2-3 min per inflation. Upon completion of the sequential dilations, completion cavogram demonstrated an excellent response to treatment [Figure 3].
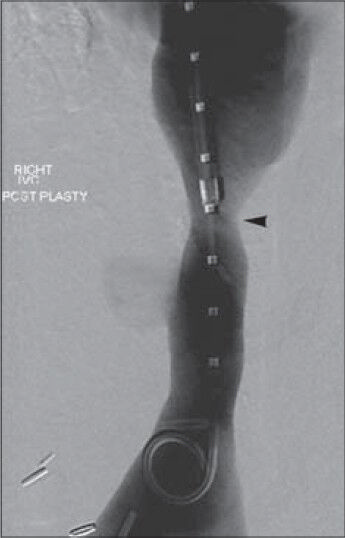
- 36-year-old woman with a history of autoimmune hepatitis and two prior orthotopic liver transplants who presented 5 years after her most recent transplant with a chief complaint of progressive bilateral lower extremity edema and weight gain was diagnosed with transplant-related, initially angioplasty-resistant IVC stenosis. Inferior vena cavagram performed following multiple venoplasty sessions reveals excellent response to therapy, with luminal diameter enlargement to 10-11 mm (arrowhead).
Over the course of the next 3-4 weeks, the patient reported a fluid weight loss of approximately 90 pounds with concurrent reduction in her lower extremity edema [Figure 4]. Moreover, her serum creatinine value normalized from 1.5 mg/dl pre-procedure to 1.0 mg/dl post-procedure. A low-level pre-procedure total bilirubin elevation also normalized (from 1.7 to 0.9 mg/dl). The patient did not have ascites. At 3-month follow-up, the patient had returned to her baseline level of functioning without recurrence of edema, and CT venography confirmed stable caliber of the previously dilated hepatic IVC segment. Follow-up at 6-months showed no relapse of clinical signs or symptoms. Future follow-up with clinical assessment and repeat CT venography at 6-month intervals was planned.
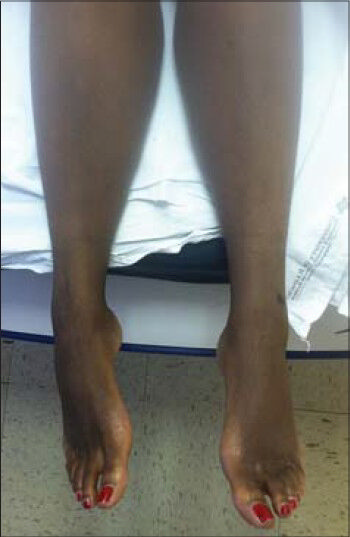
- 36-year-old woman with a history of autoimmune hepatitis and two prior orthotopic liver transplants who presented 5 years after her most recent transplant with a chief complaint of progressive bilateral lower extremity edema and weight gain was diagnosed with transplant-related, initially angioplasty-resistant IVC stenosis. Post-treatment photograph demonstrates complete resolution of lower extremity edema.
DISCUSSION
In this report, we described the case of a 36-year-old woman with a recalcitrant IVC stenosis related to liver transplantation. After failing to respond to initial balloon angioplasty, the patient underwent four additional sessions over the course of 2 months, ultimately achieving dramatic clinical improvement in conjunction with expansion of the caval lumen from 2-3 mm to 10-11 mm. This case exemplifies the potential role of a programmed approach to balloon venoplasty employing multiple sessions and including use of a cutting balloon, similar to that used for benign biliary stricture dilation.[2] This method affords a more vigorous approach to treatment of the fibrotic neointimal hyperplasia of vascular anastomotic stenoses refractory to single-session therapy, while averting the need for stent deployment. The small incremental gains in IVC luminal caliber achieved at each treatment session may have been deemed ineffective when taken on its own, but were markedly successful in enhancing IVC patency when taken together. Ideally, the sequential dilation approach will result in sustained clinical and angiographic response; at minimum, it may be employed as a temporizing measure that does not preclude the possibility of subsequent stenting or increase the technical difficulty of future surgical intervention. Notably, however, IVC stenosis and/or hepatic veno-occlusive disease may increase the technical complexity of liver transplant surgery, and such circumstances may require arduous dissection requiring meticulous care, and may necessitate auxiliary or advanced vascular reconstructive maneuvers.
Venous outflow obstruction is a rare complication of liver transplantation, with an incidence below 2%.[3] Signs and symptoms of caval stenosis resemble those of Budd–Chiari syndrome: Abdominal pain, lower extremity edema, ascites, and hepatic dysfunction. Obstruction occurring in the early postoperative period is typically the result of technical factors, whereas delayed presentation may be related to intimal hyperplasia, perivascular fibrosis, or extrinsic caval compression.[4] Recently, immune-mediated processes have been shown to contribute to graft failure and development of hepatic venous outflow obstruction,[5] suggesting a role for preventative immunosuppressive strategies. Data regarding post-transplant IVC obstruction, although limited to retrospective studies and case series, suggest that such lesions typically respond favorably to conventional percutaneous treatment.[6] In a recent retrospective study, Lorenz et al., reported technical and clinical success rates of 96% and 88%, respectively, in 25 patients with IVC obstruction who underwent primary venoplasty with additional stent placement for refractory or recurrent stenosis. However, cumulative primary patency in this cohort was only 57.1% at 6 months and 51.4% at 7 years, highlighting the poor durability of primary venoplasty. In contrast, among the 17 patients stented for refractory or recurrent stenosis, the cumulative primary and primary assisted patency rates were 86% and 100% at 6 months and 7 years, respectively.[1]
Given the proven and long-lasting efficacy of stent placement, several authors have advocated this approach as first-line treatment.[78] However, the limitations of stenting may preclude its use in some patients. Stenting may increase the technical difficulty of future surgical interventions, while the risk of migration or fracture, though minor, must be considered.[89] In the current report, stenting was deferred due to concern regarding the high likelihood of in-stent stenosis over the patient's lifetime coupled with the need for a short stent with a high risk of migration. Serial balloon dilation was pursued as an alternative to re-transplantation, which is a resource-intensive procedure associated with mortality rates as high as 41.6%.[10] Although long-term stricture recurrence may ensue in the reported patient, whose stenosis was felt to be most likely related to anastomotic perivascular fibrosis or intimal hyperplasia, long-term primary assisted patency may be nonetheless achieved;[1] the patient was thus enrolled in a clinical and imaging surveillance program to help identify any need for repeat balloon angioplasty intervention.
CONCLUSION
In summary, sequential balloon angioplasty may produce a favorable outcome in cases of initially angioplasty-resistant IVC obstruction. Although the report of a single case does not constitute evidence of efficacy, operators should not consider non-response to one session of balloon dilation a treatment failure, and may consider use of successive interventional procedures to achieve therapeutic success. As a final note, such a treatment plan warrants substantial patient cooperation, with an expectation for responsible follow-up for scheduled sessions.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2014/4/1/50/141557
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Long-term outcomes of percutaneous venoplasty and Gianturco stent placement to treat obstruction of the inferior vena cava complicating liver transplantation. Cardiovasc Intervent Radiol. 2014;37:114-24.
- [Google Scholar]
- Long-term follow-up of percutaneous transhepatic balloon cholangioplasty in the management of biliary strictures after liver transplantation. Transplantation. 2004;77:110-5.
- [Google Scholar]
- Incidence, management, and results of vascular complications after liver transplantation. Transplant Proc. 2011;43:749-50.
- [Google Scholar]
- Management of venous outflow complications after liver transplantation. Tech Vasc Interv Radiol. 2007;10:240-5.
- [Google Scholar]
- Impact of post-transplant flow cytometric panel-reactive antibodies on late-onset hepatic venous outflow obstruction following pediatric living donor liver transplantation. Transpl Int. 2014;27:322-9.
- [Google Scholar]
- Outcome of percutaneous transhepatic venoplasty for hepatic venous outflow obstruction after living donor liver transplantation. Radiology. 2006;239:285-90.
- [Google Scholar]
- Long-term efficacy of stent placement for treating inferior vena cava stenosis following liver transplantation. Liver Transpl. 2010;16:513-9.
- [Google Scholar]
- Primary Gianturco stent placement for inferior vena cava abnormalities following liver transplantation. J Vasc Interv Radiol. 2000;11:177-87.
- [Google Scholar]
- Stenosis of the inferior vena cava after liver transplantation: Treatment with Gianturco expandable metallic stents. Cardiovasc Intervent Radiol. 1995;18:212-6.
- [Google Scholar]
- Treatment of vascular complications following liver transplantation: Multidisciplinary approach. Hepatogastroenterology. 2001;48:179-83.
- [Google Scholar]






