Translate this page into:
Role of 123I-Iobenguane Myocardial Scintigraphy in Predicting Short-term Left Ventricular Functional Recovery: An Interesting Image
Address for correspondence: Dr. Mauro Feola, Department of Cardiovascular Rehabilitation-Heart Failure Unit, Ospedale SS Trinità, via Ospedale, 4, 12045 Fossano, Cuneo, Italy. E-mail: m_feola@virgilio.it
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
123I-iobenguane myocardial scintigraphy (MIBG) has been shown to be a predictor of sudden cardiac mortality in patients with heart failure. One patient with recent anterior myocardial infarction (MI) treated with coronary angioplasty and having left ventricular ejection fraction (LVEF) of 30% underwent early MIBG myocardial scintigraphy/tetrofosmin single-photon emission computed tomography (SPECT) in order to help evaluate his eligibility for implantable cardioverter defibrillator (ICD). The late heart/mediastinum (H/M) ratio was calculated to be 1.32% and the washout rate was 1%. At 40-day follow-up after angioplasty, LVEF proved to be 32%, New York Heart Association (NYHA) class was still II–III, and an ICD was placed in order to reduce mortality from ventricular arrhythmias. MIBG myocardial scintigraphy might be a promising method for evaluating left ventricular recovery in post-MI patients.
Keywords
Congestive heart failure
implantable cardioverter defibrillator
MIBG myocardial scintigraphy

INTRODUCTION
The current American guidelines recommend a “waiting period” before placement of implantable cardioverter defibrillator (ICD) to reduce mortality from ventricular arrhythmias, in patients with chronic heart failure (CHF), who are classified as New York Heart Association (NYHA) functional class of II or III, with a left ventricular ejection fraction (LVEF) ≤35%, to at least 3 months after a cardiac revascularization or 40 days after an acute myocardial infarction (MI).[12] However, a large percentage of ICD devices have been found to never deliver therapy during their lifetime, and nearly 33% ofpatients ineligible for an ICD (LVEF >35%) die of sudden cardiac death (SCD). Moreover, a “waiting period” of 40 days or 3 months can put patients in a situation of high risk of major arrhythmic events.
Cardiac imaging MIBG scintigraphy can detect impaired global left ventricle (LV) sympathetic innervation and regional myocardial sympathetic heterogeneity, which are highly arrhythmogenic and could be useful in the decision process for an early ICD implantation.
We performed an early MIBG scintigraphy in order to evaluate the prognostic value of this exam in the selection for ICD implantation.
CASE REPORT
A 79-year-old male had a large anterior ST segment elevation myocardial infarction (STEMI) due to a single vessel disease (of the left anterior descending coronary artery) and was treated after 4 h with coronary angioplasty and bare metal stent. A 99mTc-tetrofosmin myocardial scintigraphy at rest and an I23I-iobenguane (MIBG) myocardial scintigraphy was performed within 15 days after angioplasty [Figure 1]. The late heart/mediastinum (H/M) ratio was calculated to be 1.32% and the washout rate was 1%. The images demonstrated a large perfusion defect in the anterior wall, apex, infero-apical and latero-apical segments; the Summed Rest Score was calculated as 26; the scintigraphic ejection fraction was 27% with a clear ventricular enlargement (end-diastolic volume 153 ml). The MIBG scintigraphic image showed a severe reduction of the tracer uptake in the anterior wall, apex, infero-apical and latero-apical segments demonstrating, as usual, a larger defect in the sympathetic innervations in comparison with the perfusion defect. The echocardiographic examination confirmed the severe systolic dysfunction (LVEF = 30%) underlying enlargement of LV as well (diastolic volume 143 ml); in the segmental kinesis analysis, there was evidence of an anterior and apical akinesia. At 40-day follow-up after the angioplasty, a transthoracic echocardiogram was repeated which gave a similar value of LVEF (32%) and an unchanged pattern of segmental kinesis, and the patient was diagnosed with NYHA class of II–III CHF, though he was already put on optimal pharmacologic therapy. An ICD was implanted after few days as prescribed by the current guidelines that underline the implantation of an ICD as effective in reducing mortality from ventricular arrhythmias in patients with CHF, classified as NYHA class II or III, and with an LVEF ≤35%.[12] He was then re-evaluated some months later by a transthoracic echocardiogram, which showed a stable LVEF of 30%, NYHA class of II–III CHF; the device had not yet delivered any therapy because no repetitive ventricular arrhythmias were registered in those months. ICD in primary prevention usually delivered electric therapy in no more than 5–6% of implanted CHF patients and our case report confirms that a longer follow-up and larger experiences are needed to observe ICD functioning in primary prevention. The interpretation of MIBG scan in our patient helped us to predict the absence of LVEF recovery and, therefore, the need for ICD implantation after the “waiting period” of 40 days following an acute MI as specified in the current guidelines.
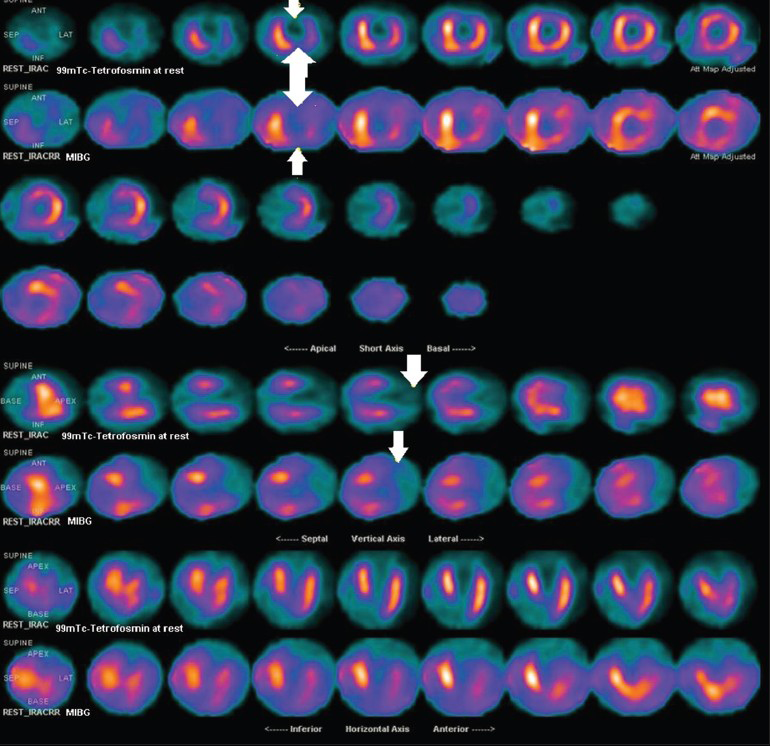
- 79-year-old male with a large anterior STEMI due to a single vessel disease (left anterior descending coronary artery) was treated after 4 h with coronary angioplasty and bare metal stent. MIBG scintigraphic tetrofosmin myocardial scintigraphy at rest and the I23I-iobenguane (MIBG) myocardial scintigraphy performed after 15 days show a severe reduction of the tracer uptake in the anterior wall, apex, infero-apical and latero-apical segments demonstrating, as usual, a larger defect in the sympathetic innervations in comparison with the perfusion defect (upper series). The white arrows point to the larger defect of the uptake of MIBG versus 99mTc-tetrofosmin myocardial scintigraphy in the different left ventricle segments. An enlargement of the left ventricle is also seen.
DISCUSSION
Many devices never deliver electric therapy that is effective in stopping ventricular arrhythmias during the lifetime of a patient, whereas many patients, ineligible for ICD placement under current LVEF-based criteria in the guidelines, die of SCD.[3] Moreover, according to the current guidelines, a period of at least 3 months after a cardiac revascularization or optimization of medical therapy in CHF, or 40 days after MI should elapse before implanting an ICD and during this “waiting period,” patients could be at high risk of major arrhythmic events.[4] 123I-iobenguane myocardial scintigraphy (MIBG), which identifies sympathetic nervous system dysfunction, has been shown to be an effective predictor of SCD mortality in patients with CHF and could be useful in identifying early adverse clinical events.[567]
The “waiting period” can place a patient at high risk of major arrhythmic events and an early evaluation and stratification could be important in finding a reliable instrument that may help in selection of patients for ICD implantation. In fact, interesting data derived from the Wearable Cardioverter-Defibrillator (WCD) registry[8] show that the 3-months mortality rate after revascularization in patients with LVEF < 35% was 7% after coronary artery bypass graft (CABG) and 10% after percutaneous transluminal coronary angioplasty (PTCA) in non-WCD users. All these arrhythmic deaths occurred in the “waiting period” when cardiologists are restricted in deciding about an ICD implantation in primary prevention.
CONCLUSION
In this case report, we followed a patient listed for ICD, who underwent an early MIBG scintigraphy (within 15 days after a revascularization for an MI) before the implantation, in order to assess the prognostic value of a cardiac MIBG in predicting appropriate indications for ICD based on LV functional recovery. Cardiac imaging MIBG scintigraphy can detect impaired global LV sympathetic innervation and could be useful for stratifying prognosis in heart failure patients in NYHA class II–III and with severe systolic LV dysfunction (LVEF <35%). The impaired global LV sympathetic innervations and regional myocardial sympathetic heterogeneity are highly arrhythmogenic and MIBG scintigraphy could be useful in the decision-making process of selecting ICD implantation for primary prevention. This case report might be considered as a hypothetical work which suggests that an early MIBG scintigraphy in ischemic patients after a coronary revascularization can predict LV function, in order to assess the appropriate indications for ICD (after the waiting period) based on the LV functional recovery.
Financial support and sponsorship
This study was not supported by any grant.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We sincerely acknowledge the contribution and help rendered by Terulla Alessandra, MD (Nuclear Medicine Service, Ospedale S Croce-Carle, Cuneo, Italy).
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2015/5/1/56/168707
REFERENCES
- 2012 ACCF/AHA/HRS focused update of the 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60:1297-313.
- [Google Scholar]
- AmericanCollege of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1-62.
- [Google Scholar]
- Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction. Two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161-6.
- [Google Scholar]
- Defibrillator implantation early aftermyocardial infarction. N Engl J Med. 2009;361:1427-36. Available on: “http://www.ncbi.nlm.nih.gov/pubmed/20730742” \o“Kardiologia polska.” Kardiol Pol 2010;68:977-8
- [Google Scholar]
- I-123-mIBG myocardial imaging for assessment of risk for a major cardiac event in heart failure patients: Insights from a retrospective European multicenter study. Eur J Nucl Med Mol Imaging. 2008;35:535-46.
- [Google Scholar]
- Predicting the need for an implantable cardioverter defibrillator using cardiac metaiodobenzylguanidine activity together with plasma natriuretic peptide concentration or left ventricular function. J Nucl Med. 2008;49:225-33.
- [Google Scholar]
- ADMIRE-HF Investigators. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView myocardial imaging for risk evaluation in heart failure) study. J Am Coll Cardiol. 2010;55:2212-21.
- [Google Scholar]
- Early risk of mortality after coronary revascularization in patients with left ventricular dysfunction and potential role of the wearable cardioverter defibrillator. Circ Arrhythm Electrophysiol. 2013;6:117-28.
- [Google Scholar]






