Translate this page into:
Role of Cross-sectional Imaging (CT/MRI) in Characterization and Distinguishing Benign from Malignant/Potentially Malignant Cystic Lesions of Pancreas

*Corresponding author: Betty Simon, Department of Radiodiagnosis, Christian Medical College and Hospital, Vellore - 632 004, Tamil Nadu, India. drbettysimon@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Abraham AS, Simon B, Eapen A, Sathyakumar K, Chandramohan A, Raju RS, et al. Role of Cross-sectional Imaging (CT/ MRI) in Characterization and Distinguishing Benign from Malignant/ Potentially Malignant Cystic Lesions of Pancreas. J Clin Imaging Sci 2020;10:28.
Abstract
Objectives:
The aim of the study was to evaluate the accuracy of computed tomography/magnetic resonance imaging (CT/MRI) in characterizing cystic lesions of the pancreas and in differentiating between benign and malignant/potentially malignant lesions.
Material and Methods:
A retrospective study was performed on patients with pancreatic cystic lesions who underwent pre-operative imaging and surgery between October 2004 and April 2017 at a tertiary care teaching hospital. The images were reviewed for specific characteristics and diagnoses recorded independently by two radiologists who were blinded to the histopathological examination (HPE) report. Radiological diagnostic accuracy was assessed with HPE as reference standard.
Results:
A total of 80 patients fulfilled the inclusion criteria (M: F = 27:53). The final HPE diagnoses were solid pseudopapillary neoplasm (32.5%), walled off necrosis/pseudocyst (27.5%), mucinous cystadenoma (15%), serous cystadenoma (11.25%), intraductal papillary mucinous neoplasm (8.75%), mucinous cystadenocarcinoma (2.5%), simple epithelial cyst (1.25%), and unspecified benign cystic lesion (1.25%). Observer1 correctly identified the diagnosis in 73.75% of cases while observer 2 did so in 72.5%. Sensitivity for distinguishing benign versus malignant/potentially malignant lesions was 85.1% for observer 1 and 80.9% for observer 2. On multivariate logistic regression analysis: Solid cystic morphology, presence of mural nodule, and female gender were associated with premalignant/malignant lesions.
Conclusion:
Cross-sectional imaging is a valuable tool for characterization of pancreatic cystic lesions within its limitations.
Keywords
Pancreas
Cysts
Neoplasms
Computed tomography
Magnetic resonance imaging
INTRODUCTION
Cystic lesions of pancreas are increasingly encountered in clinical practice. They encompass a diverse group of pathologies ranging from benign to pre-malignant and malignant lesions. The commonly encountered cystic lesions of pancreas are listed in Table 1. Cross-sectional imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) continue to be the primary modalities used in the evaluation of these lesions.[1] Distinguishing benign from potentially malignant/malignant lesions is crucial to triage patients into those requiring surveillance and those who need to undergo surgical resection. Although certain morphological features are helpful in characterizing cystic lesions of pancreas, there is considerable overlap and some lesions have non-specific morphology. Unilocular cysts and macrocystic variants of serous cystadenomas often pose diagnostic challenges to the radiologist.[2] The objective of this study was to determine the accuracy of CT/MRI in characterizing cystic lesions of the pancreas and in differentiating between benign and potentially malignant/malignant cystic lesions of the pancreas.
| Benign | Premalignant | Malignant |
|---|---|---|
| Neoplastic: | IPMN | IPMN |
| Serous cystadenoma | Mucinous cystic neoplasm | Mucinous cystadenocarcinoma |
| IPMN | Solid tumors undergoing cystic degeneration: | |
| Dermoid cyst/Teratoma | SPN | |
| Non-neoplastic: | Neuroendocrine tumor | |
| Pseudocyst | Adenocarcinoma pancreas | |
| Simple epithelial cyst | Metastasis | |
| Lymphatic cyst | ||
| Parasitic cyst | ||
MATERIAL AND METHODS
A retrospective study was done in patients who underwent an operation for cystic lesions of the pancreas between October 2004 and April 2017 at our hospital, a major tertiary care referral center in South India. These patients had pre-operative imaging (CT/MRI) and post-operative histopathological examination (HPE). Those patients with imaging done in other centers and those with solid lesions were excluded from the study.
Demographic profile and clinical presentation of the patients were recorded. The primary investigator in conjunction with radiologist 1 reviewed the CT/MRI images. Specific imaging features and most likely diagnosis were recorded. Radiologist 2 independently reviewed the images and recorded the diagnosis. Both radiologists had more than 5 years’ experience in abdominal imaging. The investigators were blinded to the HPE reports.
The imaging features that were evaluated and recorded are shown in Table 2. Morphological features incorporated the classification proposed by Sahani et al.[1] The lesion was classified as microcystic if there were >6 cysts with individual cysts being <2 cm in size and macrocystic if the size of one or more individual cysts was more than 2 cm in size in a multicystic lesion. If there were fewer number of cysts (≤6) with size of individual cysts being <2 cm, it was classified as micro-oligocystic. A lesion was classified as septated cyst when it was predominantly unilocular with an occasional incomplete or single complete septation. Solid cystic morphology had a large solid component whereas a lesion with predominant cystic component and small nodular soft-tissue component was classified as a cyst with mural nodule. The contour of the lesions was assessed and classified as smooth or lobulated. If lobulated, it was recorded as microlobulated if the lobulations were closely spaced giving rise to almost a mamillated appearance and macrolobulated if lobulations were larger or a combination of these two. The extracapsular cystic sign was taken to be positive if there was any small and independent daughter cyst seen around and protruding from the dominant mother cyst as described by Chen et al.[3]
| Location of lesion (head/uncinate process/body/tail/> one region ) Number of lesions If multiple lesions – location (head/uncinate process/body/tail/pancreatic + extrapancreatic/>one region) Maximum dimension – transverse/anteroposterior/craniocaudal diameter Morphology (unilocular/microcystic/macrocystic/cyst with mural nodule/mixed solid-cystic /micro-oligocystic/septated) Size of unilocular cyst ( ≤ /> 3 cm ) Size of cyst when morphology was cyst with mural nodule (≤/>2 cm) Number of cysts if multilocular (≤/>6) Contour (smooth/lobulated/ macrolobulated/microlobulated/mixed micro and macrolobulated) Wall (thin/thick irregular) Maximum septal thickness Cyst wall thickness (≤3mm/>3mm) Cyst wall/lesion enhancement(>/=/< pancreas) Septal enhancement (present/absent) Central scar (Present/absent) Debris/hemorrhage within cysts (present/absent) Differential density of locules (present/absent) Calcification and its morphology(central stellate/peripheral egg shell like/septal /coarse/ combination of above) Mural nodule and its maximum anteroposterior and transverse diameters Signal intensity of mural nodule on T2W MRI Additional features on MRI (Hemorrhage/Debris/ick irregular septa/None) MPD (size/communication with lesion) Vascular involvement (SMV,SMA,PV,SV)- Abutting/Encasement/Acute thrombosis/Chronic thrombosis) Pancreas morphology (Acute/chronic/acute on chronic/thinning/upstream atrophy) Extracapsular cystic sign |
Contrast enhanced multi-detector CT scans of the abdomen and pelvis were performed using the following machines – (1) 64 slice GE Discovery 750 HD, Milwaukee, WI, USA scanner, (2) 16 slice Siemens Somatom Emotion, Essen, Germany scanner, (3) 6 slice Philips 6 Brilliance, OH 44143, USA, and (4) 2 slice Siemens Somatom Emotion, Forchheim, Germany. The tube voltage was 100–130Kv, automatically controlled tube current, pitches 0.98–1.2 and reconstructed slice thickness 2.5–5 mm.
MRI was done on the following machines: Siemens Magnetom Avanto 1.5Tesla, Siemens Avanto Fit 1.5 Tesla scanner, Germany, Philips Ingenia 3Tesla scanner, and Philips Achieva 1.5Tesla scanner, the Netherlands.
The MRCP protocol used for imaging is provided in Appendix 1.
Diagnostic accuracy was assessed with HPE as reference standard.
A sample size of 75 was calculated with the precision of 10% and z-value for 95% confidence interval of 1.96 taking an average absolute agreement rate of 75% between CT and histopathology based on a prior study by Visser et al.[4]
Statistical analysis was performed using STATA/IC 13.1 Lakeway Drive, Texas 77845 USA software. Inter-observer agreement was determined using Kappa statistics. Multivariate logistic regression analysis was performed to assess the morphological features that best predicts premalignant/ malignant etiology.
The study was approved by our institutional review board.
RESULTS
A total of 80 patients were included in the final analysis. The study group comprised 27 males and 53 females. The median age of the study population was 29.5 years (range of 18–73 years). Fifty-six patients underwent CT imaging while 24 had both CT and MRI.
The most common presenting complaint was abdominal pain (76.25%), followed by abdominal lump/distension (7%). In six out of 80 patients (7.5%), the pancreatic lesion was incidentally detected.
Review of the clinical record of the patients showed history of pancreatitis recorded in 21/80 patients (26.25%). Fifty- six patients had no record of pancreatitis mentioned in their clinical records.
The different morphologies of the lesions recorded were solid-cystic in 25 (31.25%), unilocular in 15 (18.75%), macrocystic in 12 (15%), microcystic in 5 (6.25%), septated cyst in 12 (15%), and cyst with mural nodule in 11 (13.75 %).
The final HPE diagnoses of the lesions are shown in Table 3. The most frequent diagnoses in each of the different morphological groups were solid pseudopapillary neoplasm (SPN) in the solid cystic morphology (22/25, 88%), walled off necrosis (WON)/pseudocyst in the unilocular category (12/15, 80%), serous cystadenoma in the microcystic group (3/5, 60%), mucinous cystadenoma in the macrocystic group (5/12, 41.66%), intraductal papillary mucinous neoplasm (IPMN) in cystic lesions with mural nodule (5/11, 45.45%), and WON in the septated cyst morphology group (7/12, 58.3%).
| Diagnosis | n=80 | Percentage |
|---|---|---|
| Solid pseudopapillary neoplasm | 26 | 32.5 |
| Walled off necrosis/pseudocyst | 22 | 27.5 |
| Mucinous cystadenoma | 12 | 15 |
| Serous cystadenoma | 9 | 11.25 |
| Intraductal papillary mucinous neoplasm | 7 | 8.75 |
| Mucinous cystadenocarcinoma | 2 | 2.5 |
| Simple epithelial cyst | 1 | 1.25 |
| Unspecified benign cystic lesion | 1 | 1.25 |
Among the 80 lesions studied, the correct diagnosis was obtained in 73.75% cases and 72.5% cases by observers 1 and 2, respectively.
The sensitivity and specificity for distinguishing benign versus malignant/potentially malignant lesions was 85.1% (95% CI: 71.7–93.8 %) and 63.6% (95% CI: 45.1–79.6%) for observer 1 and 80.9% (95% CI: 66.7–90.9%) and 66.7% (95% CI: 48.2–82%) for observer 2, respectively. Inter-observer agreement was moderate (Kappa – 0.4795, SE – 0.1117).
The final HPE diagnoses, distribution of few salient clinical/ radiological features in each of these lesions and the percentages of specific correct diagnoses made by the two observers are summarized in Table 4.
| Diagnosis (number) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Clinical, Radiological features and % specific diagnosis made | SPN (26) | WON/pseudocyst (22) | IPMN (7) | MCA (12) | SCN (9) | MCAC (2) | Simple epithelial cyst (1) | Unspecified benign cyst (1) |
| Median age (Range) years | 26 (18–42) | 35(23–63) | 53 (41–71) | 35.5 (21–73) | 43 (29–54) | 52 (46–58) | 33 | 35 |
| Gender M:F | 5:21 | 15:7 | 6:1 | 0:12 | 1:8 | 0:2 | 0:1 | 0:1 |
| Location (Most frequent) | Body and tail 7 (26.9%) | Pancreatic and extrapancreatic 10 (45.4%) |
Head and Uncinate 4 (57.1%) | Body and Tail 4 (33.3%) | Body and Tail 6 (66.6%) | Body and Tail 2 (100%) | Body and tail 1 (100%) | Head and uncinate 1 (100%) |
| Morphology (Frequently encountered) | Solid cystic 22 (84.6%) Microcystic 2 (7.69%) Cyst with mural nodule 2 (7.69%) |
Unilocular 12 (54.5%) Septated 7(31.82%) |
Cyst with mural nodule 5 (71.4%) |
Macrocystic 5 (41.6%) Unilocular 3 (25%) |
Microcystic 3 (33.3%) Macrocystic 2 (22.2%) Mixed solid cystic 2 (22.2%) Septated 2 (22.2%) |
Macrocystic 1 (50%) Cyst with mural nodule 1 (50%) |
Macrocystic 1 (100%) |
Macrocystic 1 (100%) |
| Contour (Most frequent) | Smooth/macro-lobulated (22/26) 84.6% |
Smooth (15/22) 68.1% |
Macrolobulated (4/7) 57.1% |
Smooth (10/12) 83.3% |
Macrolobulated (5/9) 55.5% |
Smooth (2/2) 100% |
Smooth | Macrolobuated |
| Wall thickness | Not assessed in solid cystic lesions | ick (12/22) 54.55% | in (6/7) 85.7% |
in (10/12) 83.3% |
in (7/9) 77.7% |
ick (2/2) 100% |
ick | in |
| Cyst wall enhancement Equal/Less than/More than pancreas (Most frequent) |
Less 14 (56%) |
Equal 17 (77.2%) |
Equal 5 (71.4%) |
Less 7 (58.33%) |
Equal 5 (55.56%) |
Equal 1 (50%) More 1(50%) |
Equal 1 (100%) |
Equal 1 (100%) |
| Central scar | None | None | None | None | 1/9 11.1% |
Nil | Nil | Nil |
| Debris/ Hemorrhage | 5 (19.2%) | 15 (68.2%) | 1 (14.2%) | 1 (8.3%) | Nil | Nil | Nil | Nil |
| Morphology of calcification (Most frequent) |
Coarse 7 (26.9%) |
Coarse 1 (4.5%) |
Nil | Combination of peripheral and septal 2 (16.6%) |
Coarse 5 (55.5%) |
Combination of peripheral and septal 1 (50%) | Coarse 1 (100%) |
Nil |
| Extracapsular cystic sign | 1 (3.8%) | Nil | 1 (14.2%) | 2 (16.6%) | 6 (66.6%) | Nil | Nil | 1 (100%) |
| MPD dilatation | 2 (7.6%) | 6 (27.2%) | 7 (100%) | 4 (33.3%) | 2 (22.2%) | Nil | Nil | Nil |
| Caliber of MPD if dilated in mm Min- Max | 3.4–6 mm | 3.3–10 mm | 4.1–14mm | 3.5–5 mm | 3–10.4 mm | Nil | Nil | Nil |
| MPD communication | None | None | 6 (85.7 %) | None | None | Nil | Nil | Nil |
| Morphology of background pancreas (Most frequent) | Normal 25 (96.1%) |
inning 10 (45.4%) |
Normal 4 (57.1%) |
Normal 11 (91.6%) |
Normal 8 (88.8%) |
Normal 2 (100%) |
Normal 1 (100%) |
Normal 1 (100%) |
| Correct specific diagnosis (%) Observer 1 |
23 (88.4%) | 18 (81.8%) | 6 (85.7%) | 8 (66.6%) | 3 (33.3%) | 1 (50%) | 0% | 0% |
| Correct specific diagnosis (%) Observer 2 | 22 (84.6%) | 20 (90.9%) | 5 (71.4%) | 9 (75%) | 2 (22.2%) | 0% | 0% | 0% |
Univariate analysis showed significant difference in the following features among the patients with premalignant/ malignant pancreatic cystic lesions and the benign pancreatic cystic lesions: Gender predilection, number of pancreatic lesions, presence of hemorrhage/debris within the lesion, solid cystic morphology, cyst with mural nodule, and associated vascular abnormalities (P < 0.005).
Among above, solid cystic morphology (P< 0.001), cyst with mural nodule (P = 0.003), and female gender (P = 0.022) were identified as the best clinico-radiological predictors of premalignant/malignant pancreatic cystic lesions using multivariate logistic regression analysis.
DISCUSSION
Cystic neoplasms account for about 10–15% of pancreatic cystic lesions.[5] Characterization of these lesions is crucial. While some of the lesions are premalignant/malignant and timely resection is mandatory, unwarranted surgical interventions can be avoided in benign asymptomatic lesions.
While CT remains the primary imaging modality, additional MRI sections, particularly T2-weighted images and magnetic resonance cholangiopancreatography (MRCP) provide valuable information for further characterization of cystic lesions of pancreas.[6] MRI has the advantage of better soft- tissue resolution. Pancreatic duct communication, debris or hemorrhage within the cyst, septae, and small mural nodules are better evaluated on MR, long TR (repetition time) sequences. In our series, 24 patients had additional MRI performed.
The majority of our patients presented with abdominal pain and only 7.5% were incidentally detected lesions. The frequency of incidentally detected pancreatic cystic lesions is on the rise in recent years.[5] Laffan et al. found a prevalence of 2.6% for unsuspected pancreatic cysts discovered on multi-detector CT.[7] A prevalence of 13.5% was reported for incidentally detected pancreatic cysts on MR imaging by Lee et al.[8] Being a surgical series, a greater proportion of symptomatic patients is expected in our study. The final histopathology of the incidentally detected lesions in our series was solid pseudopapillary neoplasia (SPN) in four out of six and serous cystadenoma (SCA) in two out of six incidental lesions.
Studies on characterization of cystic pancreatic lesions on cross-sectional imaging have had variable outcomes. In the study by Procacci et al., correct diagnosis was made on CT in 60% of cases,[9] while it was 39% in the study by Fisher et al.[10] In a study that assessed the relative accuracy of CT and MRI, correct specific diagnosis was made in 46% and 43% of cases on CT by two-independent observers. There is limited literature comparing the relative performance of CT and MRI in cystic pancreatic lesions, Visser et al. in their study showed similar overall accuracy for CT and MRI in the characterization of cystic pancreatic lesions.[4] When Fisher et al. determined the accuracy of CT in predicting malignant potential of cystic pancreatic lesions, accuracy rates were 60.4% and 62.5% for the two radiologists and there was only fair agreement between them with kappa value of 0.28.[10] The slightly higher figures obtained in our series could be due to the fact that being a surgical series, we did not encounter incidental unilocular cysts <3 cm in diameter. This group is very difficult to characterize on cross-sectional imaging alone as also observed by other authors.[10,11]
Moreover, we had a good number of walled off necrosis/ pseudocysts (22/80) and some of these cases had changes related to pancreatitis on imaging. Procacci et al. in their study had excluded cases with the previous history of pancreatitis. We did not do so because pseudocyst is a close differential for mucinous cystic neoplasm and history of pancreatitis is often not forthcoming in many instances. Differentiation becomes especially difficult when the septae in mucinous cystic neoplasm are few and almost imperceptible. A total of four out of 22 WON were diagnosed as mucinous cystadenoma by either one of the observers. All these lesions were unilocular cystic lesions measuring >3 cm in size mimicking mucinous neoplasm. Subtle signs of pancreatitis can sometimes be overlooked and hence a thorough search for them is recommended. Similarly, three mucinous cystadenomas were initially thought of as benign pseudocysts on imaging illustrating the non-specific morphology of unilocular macrocysts especially in the absence of supporting clinical features [Figure 1]. Two cases of benign cysts on histopathology were also diagnosed as mucinous cystadenomas on imaging. The histopathologically diagnosed benign epithelial cyst had thin septations and peripheral focus of calcification radiologically simulating a mucinous cystadenoma once again demonstrating the overlapping morphology exhibited by some lesions [Figure 2]. Procacci et al. in their series observed that 15–20% of pancreatic cystic lesions could not be correctly diagnosed on CT due to non-specific morphology exhibited by these lesions.[9] In practice, a differential diagnosis is mandatory in such situations and further evaluation with endoscopic ultrasound (EUS) and cyst aspiration provide valuable information for clinical decision-making.[10-12]
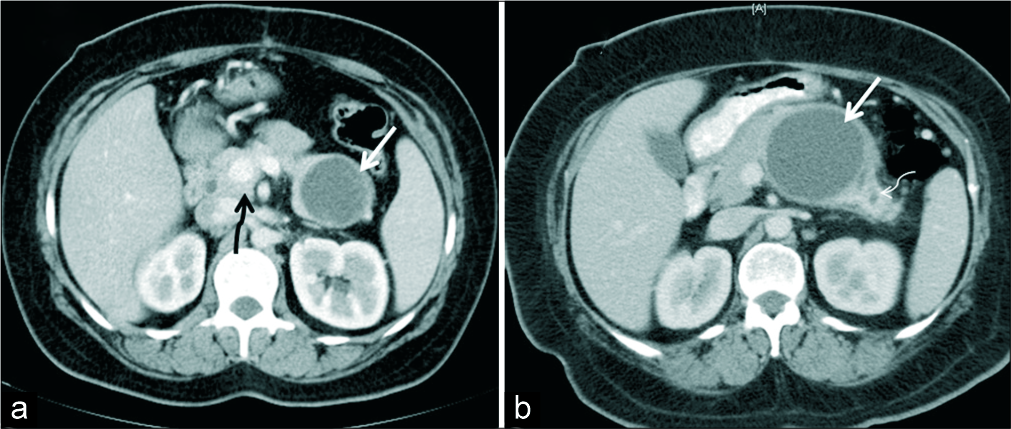
- (a) A 38-year-old woman who presented with abdominal pain, axial IV contrast enhanced CT demonstrates a unilocular thin walled cystic lesion in the tail of pancreas (white arrow). Histopathology confirmed diagnosis of pseudocyst. Incidental finding of portal annular pancreas noted (black squiggly arrow). (b) A 50-year-old woman with abdominal pain, axial IV contrast- enhanced CT demonstrates unilocular cystic lesion with no septations or mural nodule (white thick arrow). Histopathology revealed mucinous cystadenoma. Upstream dilatation of MPD is seen in the tail of pancreas (thin white arrow).
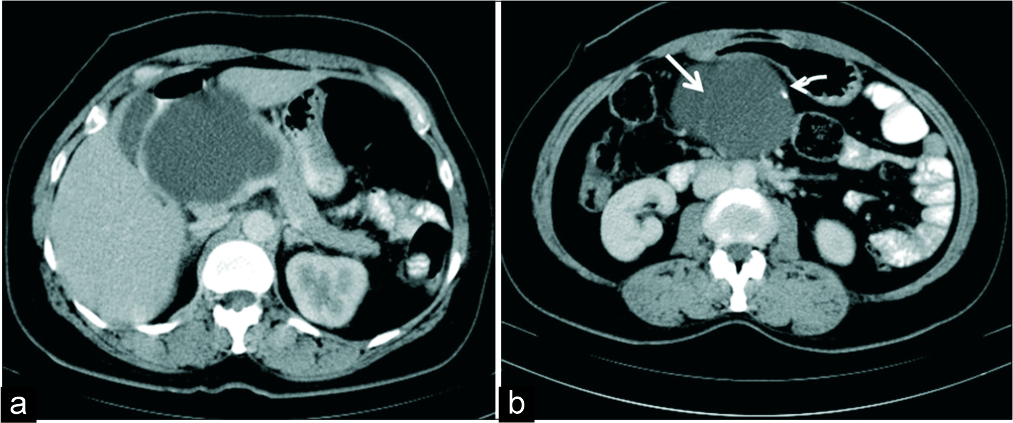
- A 33-year-old woman with a palpable abdominal lump. (a) Axial IV contrast-enhanced CT demonstrates a macrocystic lesion in the head of pancreas. (b) CT section at a caudal level shows thin septation (white thick arrow) and a peripheral focus of calcification (white thin arrow) which mimicked a mucinous cystadenoma morphologically. HPE revealed a simple epithelial cyst.
Transabdominal ultrasound is useful to differentiate between cystic and solid lesions when a hypo-attenuating lesion is encountered on CT. It also helps in evaluation of septae and nodules in the cyst. Although ultrasound has the advantage of being widely available and cost effective, small lesions can be difficult to assess especially if there is a poor sonological window.[13] EUS provides higher resolution imaging of pancreas and allows guided procedures to be performed.[14]
Another challenge we encountered in our study was atypical morphology of serous cystadenoma. Oligocystic and macrocystic variants of serous cystadenomas are well known entities and often difficult to diagnose preoperatively.[1,15] Less than 10% of serous cystadenomas are estimated to be of atypical morphology.[1] The correct diagnosis of serous cystadenoma was made in 33.3% and 22.2% cases by the two observers in our series. In the study by Curry et al., the ability to correctly diagnose serous cystadenoma on CT by the different observers varied between 23 and 41%.[15] Atypical morphology of serous cystadenoma was encountered in six out of nine (66.6%) cases in our series and would partly explain the low sensitivity. [Figure 3]. Johnson et al. assessed pancreatic cystic lesions with CT and sonography and found that 47% of serous lesions had atypical morphology.[16] Cohen - Scali et al. in their series observed three CT findings that assisted in differentiating macrocystic serous cystadenomas from mucinous neoplasms and pseudocyst which included location in head of pancreas, lobulated contour, and absence of wall enhancement.[17] We did not find a site predilection for serous cystadenoma in our series. The contour was lobulated in all our cases and predominantly macrolobulated. Kim et al. in their study assessing CT features in differentiating the various macrocystic neoplasms of pancreas, found the shape of the lesion as the most useful feature.[2] They observed that a lobulated shape was more often seen in serous oligocystic adenomas, smooth shape in mucinous cystadenomas, and pleomorphic cysts with clubbed finger like cysts in IPMN.[2] Low incidence of central stellate scar (11.1%) is similar to the observation by Johnson et al. who identified this only in 2/16 (13%) of their cases.[16] Manfredi et al. observed this finding on MR images in 29.6% (8/27) of serous cystadenomas in their study.[18] Enhancement of peripheral wall on MR images was found to be a useful feature in their study to differentiate mucinous lesions from serous cystic lesions – an absence of peripheral wall enhancement favoring serous neoplasm.[18] Central calcification is described as a CT feature in serous cystadenoma and was found in approximately 18% in one study.[15] Most of our cases had scattered coarse calcification. We also observed that islands of cluster of small cysts in the lesion may not be resolved on routine CT and can sometimes be mistaken as solid areas. One needs to be wary of this. Pseudosolid appearance of the whole lesion also has been described due to the cluster of microscopic cysts.[19] “Extracapsular cystic sign” is a new sign described by Chen et al. as helpful in differentiating serous cystic neoplasm from other pancreatic cystic lesions.[3] “Extracapsular cystic sign” was positive in 66.67% cases of serous cystadenomas in our series. SCN is traditionally described as a “grandmother” lesion with median age of diagnosis being >60 years.[20,21] The median age of SCN in our study was 43 years (range 29–54 years).
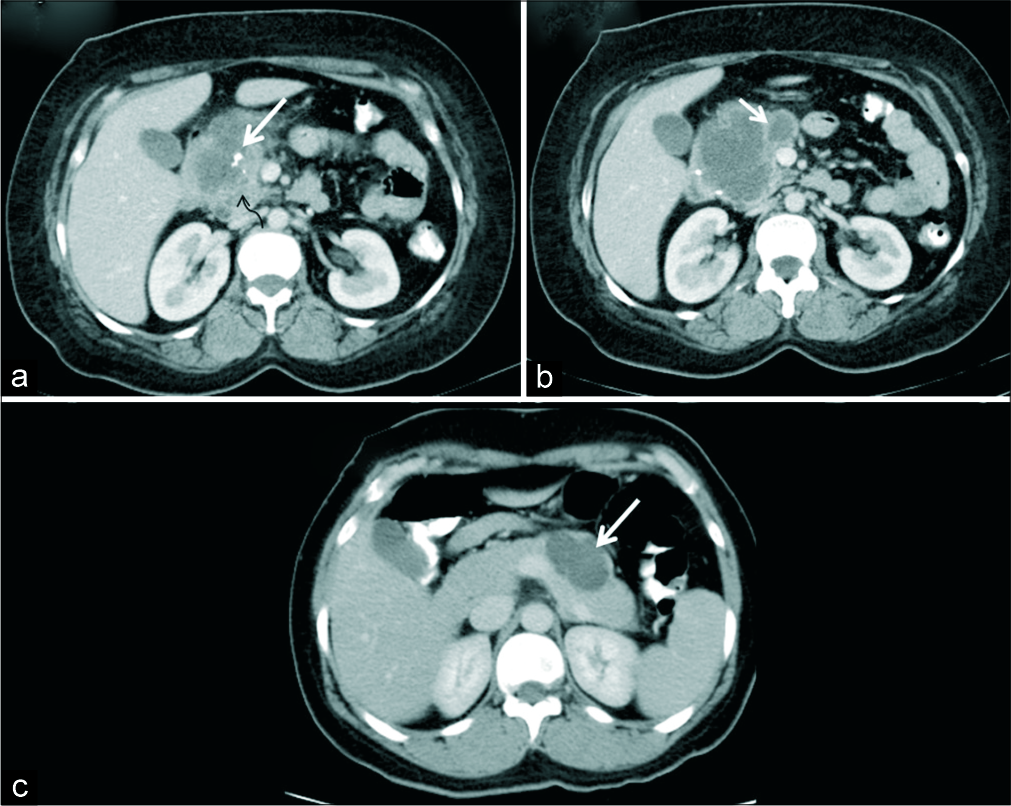
- A 39-year-old woman with upper abdominal pain. (a) Axial IV contrast-enhanced CT demonstrates a macrocystic lesion involving the head and uncinate process of pancreas with coarse calcification (white arrow) and solid appearing areas (black arrow). (b) CT sections at a caudal level show thick septations (arrow) mimicking mucinous cystic neoplasm. HPE revealed macrocystic variant of serous cystadenoma. (c) Another 39-year- old woman with incidentally detected pancreatic lesion. (c) Axial IV contrast-enhanced CT demonstrates a macro-oligocystic lesion in the body of pancreas (arrow) which was also a macrocystic variant of serous cystadenoma on HPE.
The most frequently encountered morphology in our series was solid cystic morphology and majority of these lesions were SPN [Figure 4]. Although strictly not cystic neoplasms, many authors include them in this category as many of these lesions can have cystic component of varying sizes.[22] There was female preponderance as is described in the literature.[22,23] Majority of SPNs had debris/hemorrhage within (80.7%) and 34.6% had calcification. Debris/fluid level was observed in up to 18% cases and calcification in up to approximately 29% cases of SPN in the study by Buetow et al.[23] Pathological correlation of their cases revealed hemorrhagic degeneration in almost all their cases of SPN which can be visualized as cystic areas or fluid level on CT. If present this is a useful feature to make a diagnosis of SPN.[23] Mucinous cystic neoplasm is a close differential especially when the lesion has a large cystic component and solid cystic morphology.[1] We encountered this dilemma in our series too.
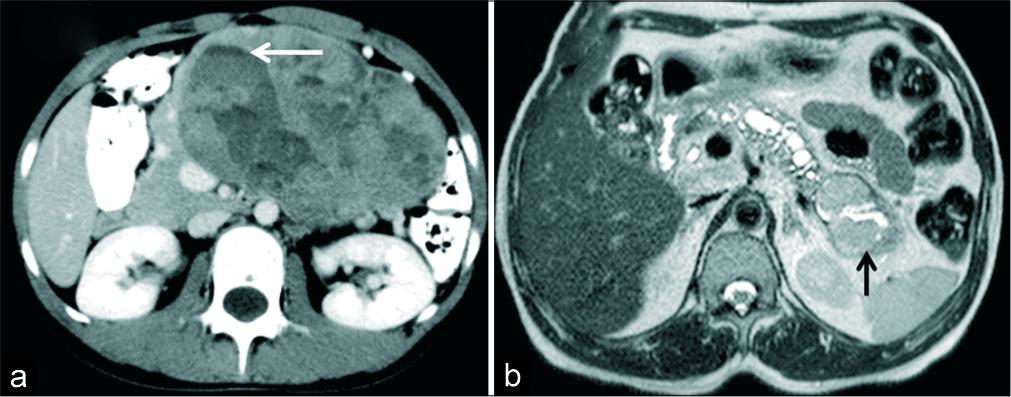
- (a) A 18-year-old female with a palpable abdominal mass. (a) Axial IV contrast-enhanced CT demonstrates a large mass involving the body and tail of pancreas with solid cystic morphology. Fluid level is seen within the cystic component (arrow). Imaging features consistent with solid pseudopapillary neoplasm (SPN) was also confirmed on HPE. (b) A 48-year-old man with suspected pancreatic mass. MRI T2 axial images show dilated MPD and lobulated cystic lesion with T2 intermediate signal intensity solid component (black arrow) in the tail communicating with MPD in keeping with the diagnosis of IPMN. HPE showed high grade IPMN, pancreaticoduodenal type with foci of invasive carcinoma.
During evaluation of unilocular cysts in our study, wall thickness alone was not a useful feature in characterizing lesions into benign and malignant. Many inflammatory lesions of the pancreas had thick wall (taken as >3 mm in our study). Thick wall in inflammatory lesions like pseudocyst correspond to the thick granulation tissue and fibrosis.[19] This is yet another reason why mucinous cystic neoplasm is a close differential for pseudocyst. Peripheral rim like calcification which has been associated with mucinous cystic neoplasm [1,24] was found in 16.6% of mucinous cystadenomas and half of mucinous cystadenocarcinoma in our series whereas it was seen in about 25% cases of mucinous cystic neoplasm in the study by Curry et al.[15] Mucinous cystadenoma is mostly a disease of women and histologically contains ovarian like stroma in the lesion.[24] All cases of mucinous cystadenoma in our series were females.
IPMN was the most frequent diagnosis in lesions with morphology of cyst with mural nodule [Figure 5]. Main pancreatic duct (MPD) dilatation was present in all cases of IPMN; however, in one case the communication with MPD could not be well demonstrated. Duct communication is an important feature that helps to differentiate IPMN from other cystic neoplasms of pancreas. However, this finding is sometimes difficult to demonstrate on cross-sectional imaging.[24] The size of the MPD in our cases of IPMN ranged from 4.1 to 14 mm. Some studies have assessed the risk of malignancy in patients with IPMN based on the MPD size. In main duct IPMN, duct size >10 mm was associated with higher risk for malignancy in the study by Tanaka et al. (11) whereas Abdeljawad et al. in their study observed a higher chance of malignancy in cases with MPD size >8 mm.[25]
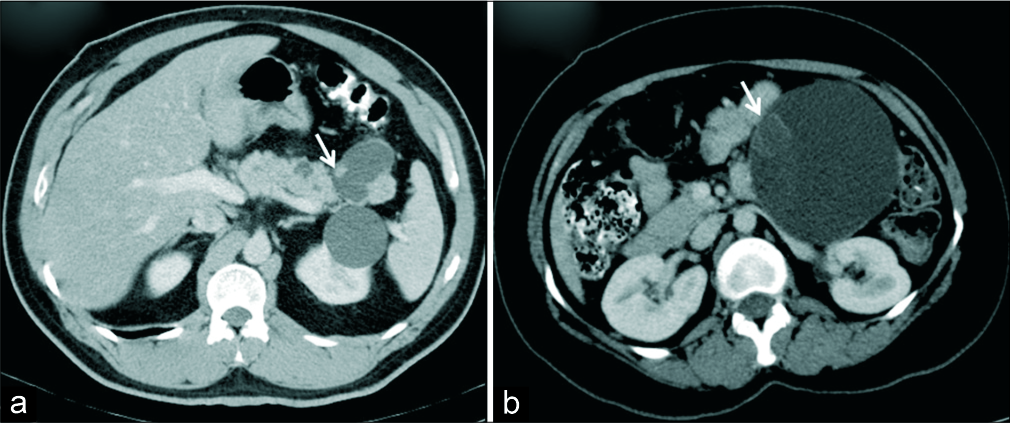
- (a) A 51-year-old man with history of pancreatitis in the past. (a) Axial IV contrast-enhanced CT demonstrates cystic lesion in the tail of pancreas with suspected communication with MPD and an eccentric mural nodule (arrow). Diagnosis of intraductal papillary mucinous neoplasm was made. HPE showed IPMN with low grade dysplasia. (b) A 35-year-old woman with abdominal pain. (b) Axial IV contrast-enhanced CT demonstrates a macrocystic lesion suggestive of mucinous cystic neoplasm with a probable mural nodule (arrow) which was concerning for malignancy, HPE revealed mucinous cystadenoma with no evidence of malignancy.
Multivariate logistic regression was performed to identify morphological features and other clinical parameters that best predicted premalignancy/malignancy in cystic lesions of pancreas. Solid cystic morphology, cyst with mural nodule and female gender showed statistically significant association with premalignancy/malignancy in our study. Salla et al. found that the presence of mural nodule was associated with increased risk of high grade dysplasia/carcinoma in patients with branch duct IPMN.[26] In a recent multicenter study by Postelwait et al.; male sex, pancreatic head, and neck location, increased size of the lesion, presence of solid component, or mural nodule, and duct dilatation were independently associated with malignancy in patients with pancreatic mucinous cystic neoplasms.[27]
Several societies and associations have formulated guidelines for evaluation, management and follow-up of cystic pancreatic lesions with some differences and similarities between them.[28] For incidentally detected, asymptomatic cystic lesions of pancreas, the American College of Radiology (ACR) recommends a surveillance protocol based on the patient characteristics (particularly age) and imaging features.[29]
The limitations of the study include relatively small number of cases in the individual histopathology groups and referral bias.
CONCLUSION
Cross-sectional imaging is a valuable tool for characterization of cystic lesions of pancreas within its limitations. This study highlights few atypical imaging features of pancreatic cystic lesions, awareness of which is crucial during pre-operative evaluation. Indeterminate lesions are not infrequent and ancillary tools such as high resolution imaging with EUS and cyst fluid aspiration help in clinical decision-making.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Cystic pancreatic lesions: A simple imaging-based classification system for guiding management. Radiographics. 2005;25:1471-84.
- [CrossRef] [PubMed] [Google Scholar]
- Macrocystic neoplasms of the pancreas: CT differentiation of serous oligocystic adenoma from mucinous cystadenoma and intraductal papillary mucinous tumor. AJR Am J Roentgenol. 2006;187:1192-8.
- [CrossRef] [PubMed] [Google Scholar]
- The 'extracapsular cystic' sign in pancreatic serous cystic neoplasms: A clinicopathologic study of 177 patients with cystic pancreatic lesions. Eur J Radiol. 2018;106:167-72.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of cystic pancreatic masses: Relative accuracy of CT and MRI. Am J Roentgenol. 2007;189:648-56.
- [CrossRef] [PubMed] [Google Scholar]
- Incidental pancreatic cysts: Clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427-3.
- [CrossRef] [PubMed] [Google Scholar]
- Intraductal papillary mucinous tumors of the pancreas. Comparison of helical CT and MR imaging. Acta Radiol. 2003;44:464-71.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079-84.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of cystic tumors of the pancreas: CT accuracy. J Comput Assist Tomogr. 1999;23:906.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy of CT in predicting malignant potential of cystic pancreatic neoplasms. HPB (Oxford). 2008;10:483-90.
- [CrossRef] [PubMed] [Google Scholar]
- International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-97.
- [CrossRef] [PubMed] [Google Scholar]
- The Differentiation between pancreatic neoplastic cysts and pancreatic pseudocyst. Scand J Surg. 2005;94:161-4.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic cystic neoplasm diagnosis: Role of imaging. Endosc Ultrasound. 2018;7:297-300.
- [CrossRef] [PubMed] [Google Scholar]
- The role of endoscopic ultrasound in cystic pancreatic tumor treatment. Gastroenterol Hepatol (NY). 2006;2:578-83.
- [Google Scholar]
- CT of primary cystic pancreatic neoplasms: Can CT be used for patient triage and treatment? AJR Am J Roentgenol. 2000;175:99-103.
- [CrossRef] [PubMed] [Google Scholar]
- Cystic pancreatic tumors: CT and sonographic assessment. Am J Roentgenol. 1988;151:1133-8.
- [CrossRef] [PubMed] [Google Scholar]
- Discrimination of unilocular macrocystic serous cystadenoma from pancreatic pseudocyst and mucinous cystadenoma with CT: Initial observations. Radiology. 2003;228:727-33.
- [CrossRef] [PubMed] [Google Scholar]
- Mucinous cystic neoplasms and serous cystadenomas arising in the body-tail of the pancreas: MR imaging characterization. Eur Radiol. 2015;25:940-9.
- [CrossRef] [PubMed] [Google Scholar]
- MR imaging of cystic lesions of the pancreas. Radiographics. 2009;29:1749-65.
- [CrossRef] [PubMed] [Google Scholar]
- The incidental cystic pancreas mass: A practical approach. Cancer Imaging. 2012;12:414-21.
- [CrossRef] [PubMed] [Google Scholar]
- Serous cystadenoma of the pancreas: Tumor growth rates and recommendations for treatment. Ann Surg. 2005;242:413-21.
- [CrossRef] [PubMed] [Google Scholar]
- Solid pseudopapillary tumours of the pancreas: Spectrum of imaging findings with histopathological correlation. Br J Radiol. 2012;85:e1140-4.
- [CrossRef] [PubMed] [Google Scholar]
- Solid and papillary epithelial neoplasm of the pancreas: Imaging-pathologic correlation on 56 cases. Radiology. 1996;199:707-11.
- [CrossRef] [PubMed] [Google Scholar]
- Cystic neoplasms of the pancreas; findings on magnetic resonance imaging with pathological, surgical, and clinical correlation. Abdom Imaging. 2014;39:1088-101.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of malignancy in patients with pure main duct intraductal papillary mucinous neoplasms. Gastrointest Endosc. 2014;79:623-9.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging and cytopathological criteria indicating malignancy in mucin-producing pancreatic neoplasms: A series of 68 histopathologically confirmed cases. Pancreas. 2018;47:1283-9.
- [CrossRef] [PubMed] [Google Scholar]
- Association of preoperative risk factors with malignancy in pancreatic mucinous cystic neoplasms: A multicenter study. JAMA Surg. 2017;152:19-25.
- [CrossRef] [PubMed] [Google Scholar]
- Overview and comparison of guidelines for management of pancreatic cystic neoplasms. World J Gastroenterol. 2019;25:4405-13.
- [CrossRef] [PubMed] [Google Scholar]
- Management of incidental pancreatic cysts: A white paper of the ACR incidental findings committee. J Am Coll Radiol. 2017;14:911-23.
- [CrossRef] [PubMed] [Google Scholar]
APPENDIX 1
Protocol for MRCP:
On the Philips 1.5T machine, MRCP was performed using a torso phased array coil. Heavily T2 weighted turbo spin echo sequence was used (TR 1204 ms;TE 650 ms;flip angle 90 degrees, FOV 260 mm2; slice thickness 0.8 mm; echo train length 105). On 3T Philips Ingenia scanner the parameters were TR 2560 ms; TE 740 ms; flip angle 90 degrees, FOV 250 × 231 mm2; slice thickness 0.47 mm. SENSE (Sensitivity Encoding) parallel imaging technique was employed. A stack of 70-80 slices were obtained which are contiguous and heavily T2 weighted, causing the pancreatico –biliary tree to show high signal intensity with a reduced background intensity. 20 volume data MIP reformats were generated at 9 degree intervals to each other over a radial array of 180 degree.
On 1.5 T Siemens Magnetom Avanto Fit, torso coil with 18 elements was used for acquisition. It uses a 3D volume space technique (TR 2500 ms; TE 701 ms ; FOV read 380 mm; FOV in the phase encoding direction 100mm; acquisition matrix 353 × 384). On Siemens Magnetom Avanto 1.5T scanner parameters were TR 1800 ms; TE 642 ms; FOV 375 × 100 mm2; Slice thickness 1 mm. GRAPPA (Generalised autocalibrating partial parallel acquisition ) also known as I-Pat ( Integrated parallel acquisition technique) was employed with respiratory or navigation triggered gating.






