Translate this page into:
Review of Metaplastic Carcinoma of the Breast: Imaging Findings and Pathologic Features
Address for correspondence: Dr. Rebecca Leddy, Department of Radiology and Radiological Science, Medical University of South Carolina, 96 Jonathan Lucas St. MSC 323, Charleston, SC 29425, USA. E-mail: leddyr@musc.edu
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Metaplastic carcinoma (MPC), an uncommon but often aggressive breast cancer, can be challenging to differentiate from other types of breast cancer and even benign lesions based on the imaging appearance. It has a variable pathology classification system. These types of tumors are generally rapidly growing palpable masses. MPCs on imaging can present with imaging features similar to invasive ductal carcinoma and probably even benign lesions. The purpose of this article is to review MPC of the breast including the pathology subtypes, imaging features, and imaging pathology correlations. By understanding the clinical picture, pathology, and overlap in imaging characteristics of MPC with invasive ductal carcinoma and probably benign lesions can assist in diagnosing these difficult malignancies.
Keywords
Metaplastic carcinoma
spindle cell
invasive ductal carcinoma
INTRODUCTION

Metaplastic carcinoma (MPC) of the breast is an uncommon form of breast cancer comprising less than 5% of breast cancer patients.[1] It consists of a heterogenous group of malignant neoplasms containing both glandular and non-glandular components with mixed epithelial and mesenchymal differentiation.[2] The classification of MPCs according to the various systems can be confusing. The World Health Organization classifies MPC into (1) epithelial type and (2) mixed type with further differentiation into subtypes.[3] The more popular Wargotz and Norris classification differentiates MPCs into 5 subtypes: spindle cell, squamous cell, carcinosarcoma, matrix-producing, and MPC with osteoclastic giant cells.[24–6] The differential diagnosis between invasive ductal carcinoma, MPC, and breast sarcoma is important for management and prognosis.
CLINICOPATHOLOGICAL FEATURES
MPCs often present as a palpable breast mass in women more than 50 years of age.[57] The lesions are characterized by larger tumor size and rapid growth.[48] MPCs are often not associated with estrogen and progesterone receptors, or HER2 expression.[4910] The axillary lymph node involvement at the time of presentation is variable with rates ranging from 8% to 40%.[458] There is a high hematogenous metastatic potential to lung and bone rather than lymphatic spread.[146] There is a documented increased risk of tumor recurrence and worse prognosis with MPC compared with invasive ductal or invasive lobular carcinoma.[347] Several predictors affecting a poorer outcome are presence of skin invasion, younger age (less than 39 years of age) at presentation, and appearance of squamous cell carcinoma in the lymph nodes.[3] The subtype of MPC has no effect on outcome.[3]
PATHOLOGIC SUBTYPES AND FEATURES
Most cases of MPC are sporadic. The spindle cell subtype is the most common of the 5 subtypes classified by Wargotz and Norris. The spindle cell subtype demonstrates cells forming poorly cohesive sheets or predominant spindle cell morphology [Figure 1].[249] The spindle cell component often resembles a low-grade sarcoma or reactive process such as granulation tissue which can be challenging to differentiate.[4] The squamous cell carcinoma subtype demonstrates infiltrating squamous carcinoma with polygonal cells, eosinophilic cytoplasm, and possible keratin pearl formation [Figure 2].[49] The carcinosarcoma contains both malignant epithelium and malignant stroma.[24] The matrix-producing subtype contains overt carcinoma with transition to cartilaginous and/or osseous stromal matrix without a spindle component.[210] The MPC with osteoclastic giant cells subtype shows intraductal or infiltrating carcinoma contiguous or mixed with spindle cell or sarcomatous stroma plus osteoclastic cells [Figure 3].[4]
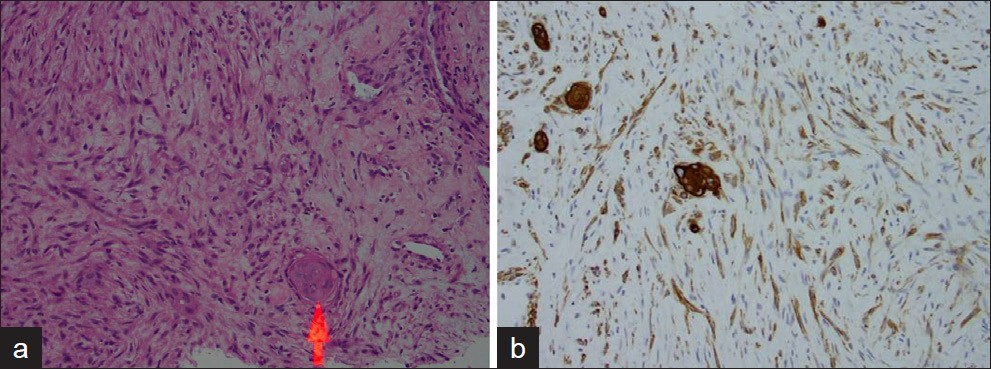
- A 58-year-old female with palpable mass in the right breast diagnosed as spindle cell type metaplastic carcinoma: (a) Photomicrograph (H and E, high-power magnification) shows a moderately cellular spindle lesion with elongated spindle cells, bland cytologic features, and squamous cell nest (red arrow)indicating differentiation. (b) Photomicrograph (immunoperoxidase AE1/AE3).
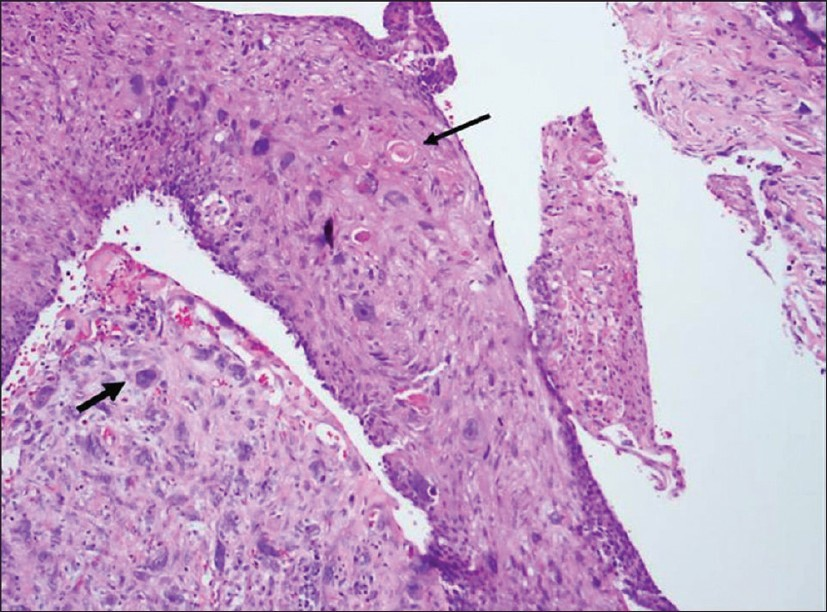
- A 64-year-old patient with palpable left breast mass diagnosed as squamous cell type metaplastic carcinoma: Photomicrograph (H and E, high-power magnification) shows large pleomorphic cells (short arrow) with squamous differentiation and keratin pearls (long arrow).
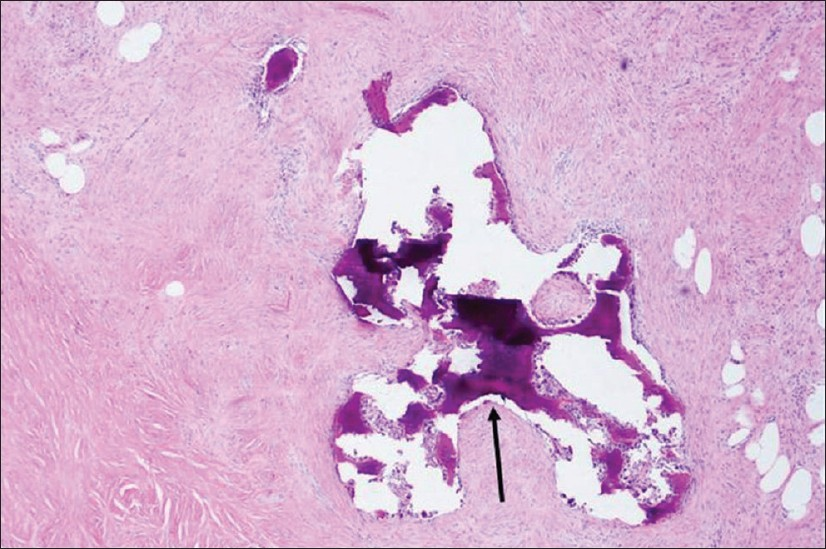
- A 51-year-old female with palpable mass in the left breast with pathology of metaplastic carcinoma, with osseous metaplasia. Photomicrograph (H and E, low-power magnification) shows spindle cells with osteoid formation (arrow).
IMAGING FEATURES OF METAPLASTIC CARCINOMA
The mammographic appearance of MPC has been described as a high-density mass with either circumscribed, obscured, irregular, spiculated, or circumscribed with partially spiculated margins [Figure 4].[15–71112] Yang et al., reported more benign appearance on mammogram including round or oval shape and circumscribed margins of a mass compared with invasive ductal carcinoma.[6] The lesions are often non-calcified.[5] If calcifications are present, the pattern is amorphous, coarse, punctate, or pleomorphic [Figure 5].[711] Park et al., described a high rate of architectural distortion associated with MPC.[11]

- Mammographic appearance of metaplastic carcinoma in two patients, both having metaplastic carcinoma mixed with invasive carcinoma. (a) A 49-year-old female called back from screening mammogram shows a circumscribed mass with partially defined (thin arrow) and partially spiculated (thick arrows) border on spot compression craniocaudal mammogram view of her right breast. (b) A 51-year-old female called back from screening shows a circumscribed mass with partially spiculated border (thin arrows) with adjacent circumscribed satellite mass (thick arrow) on spot compression mediolateral oblique mammogram view.
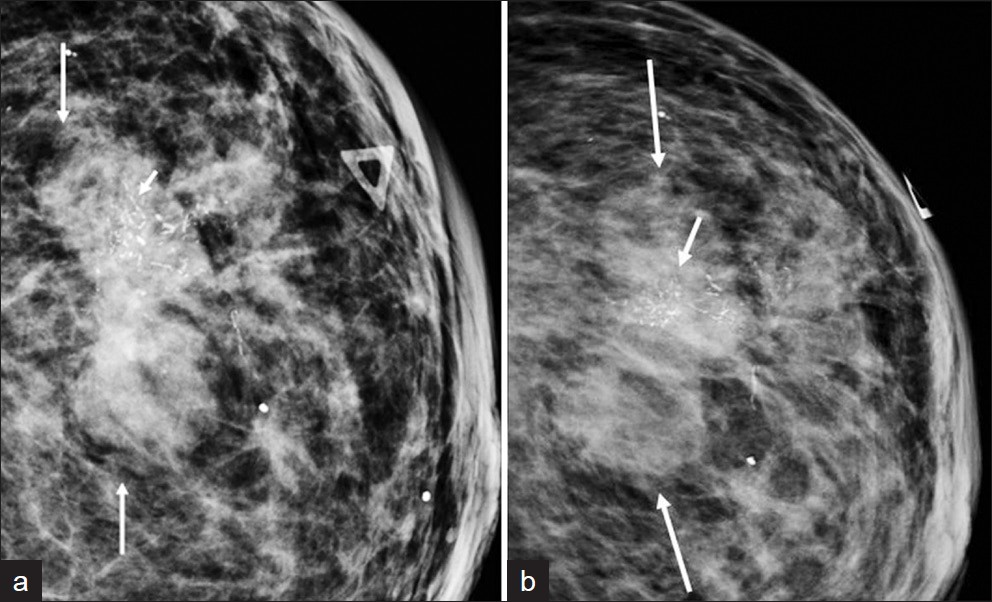
- A 64-year-old female presented with a palpable mass in her left breast. (a) mediolateral oblique mammogram view and (b) craniocaudal mammogram view show an irregular mass (long arrows) with pleomorphic calcifications (short arrows). Final histopathologic diagnosis was metaplastic carcinoma, squamous subtype.
The sonographic appearance of MPCs have been previously described as a heterogenous or hypoechoic solid mass or mixed cystic and solid mass [Figures 6–8].[5–711] The mass is oval, round, lobular, or irregular shaped with circumscribed, microlobulated, irregular, or indistinct margins [Figures 6–8].[5–711] Yang et al., reported more benign features of oval, round, or lobular solid hypoechoic mass with circumscribed or indistinct margins in contrast to invasive ductal carcinoma [Figure 7].[6] MPCs often demonstrate posterior acoustic enhancement compared with posterior shadowing most often seen with invasive ductal carcinoma [Figures 6 and 8].[67]
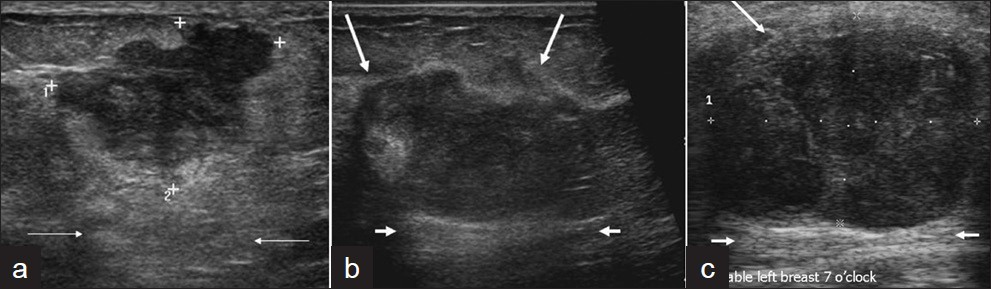
- Ultrasound images of three patients with metaplastic carcinoma, all show posterior acoustic enhancement. (a) A 49–year-old female with a palpable mass in her right breast. Transverse gray-scale imaging shows an irregular hypoechoic mass with posterior acoustic enhancement (arrows). (b) A 49-year-old female called back from screening for a mass in her right breast. Transverse gray-scale imaging demonstrates a hypoechoic mass with irregular margins (long arrows) and posterior acoustic enhancement (short arrows). (c) A 52-year-old female who presented with a palpable mass in her right breast. Ultrasound gray-scale imaging shows a large mass with partially circumscribed margins (long arrows) in her area of palpable concern and posterior acoustic enhancement (short arrows).

- A 40-year-old patient who presented with palpable left breast: Ultrasound image shows hypoechoic microlobulated mass with parallel orientation (wider than tall) (arrows) which can be seen with probably benign features. This lesion was biopsied due to the palpable presentation and microlobulated borders and pathology diagnosis showed metaplastic carcinoma.

- A 57-year-old female with a palpable mass in her right breast: Sagittal and transverse gray-scale ultrasound images show a solid hypoechoic mass with internal cystic component (thin arrow), partially defined and partially irregular margins, and posterior acoustic enhancement (think short arrows). Pathology showed metaplastic carcinoma, spindle type with osseous metaplasia.
The MRI features described for MPC are an irregular mass with spiculated margins, often intermediate to increased T2 signal intensity and isointense or hypointense on T1-weighted imaging [Figures 9 and 10].[712] Velasco et al., reported an increase in T2 hyperintensity in cancers of 11 of 12 patients (91%) with MPC.[12] Although T2 hyperintensity is often associated with benign lesions, it can be secondary to necrosis, or mucoid production in cancer. The T2 hyperintensity with MPC is reported secondary to necrosis with differential considerations including invasive ductal carcinoma with abundant necrosis and mucinous carcinoma.[12] The documented enhancement characteristics of these lesions include ring-like, homogenous, heterogeneous, or containing non-enhancing internal components [Figures 9 and 10].[712] The kinetic pattern may be variable but more often reported as early enhancement with delayed washout or plateau pattern.[712]
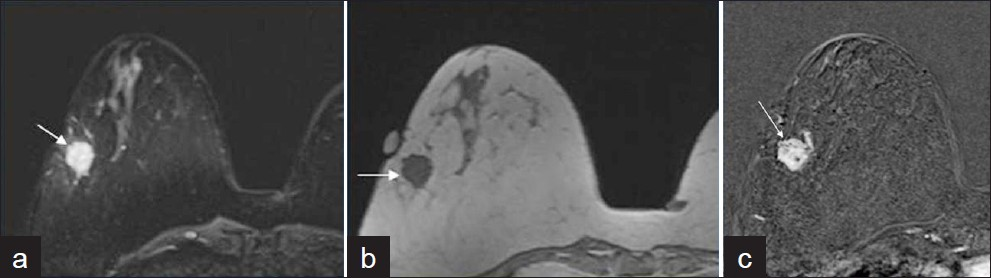
- A 49-year-old female with newly diagnosed metaplastic carcinoma, matrix-producing subtype mixed with invasive ductal carcinoma in her right breast: (a) Axial T2-weighted MR image shows a hyperintense mass (arrow), (b) Axial T1-weighted MR image shows the mass is hypointense (arrow), (c) Axial T1-weighted fat suppressed subtracted post-contrast MR imaging shows the mass is heterogeneously enhancing mass with irregular margin (arrow).

- A 57-year-old female with newly diagnosed metaplastic carcinoma, spindle cell subtype with osseous metaplasia in her right breast: (a) Axial fat suppressed T2-weighted MRI MRI shows hyperintense region with central cystic necrosis (arrow), (b) Axial T1-weighted MRI shows hypotense regions, and (c) T1-weighted fat suppressed subtracted post-contrast image shows heterogeneously enhancing regions with irregular margins and central cystic necrosis.
IMAGING PATHOLOGY CORRELATION
Spindle cell subtype of MPC is more likely to have circumscribed margins.[5] A circumscribed margin with a spiculated portion is often seen in MPC with a mixture of metaplastic and invasive carcinoma not otherwise specified [Figure 4].[5] Solid and cystic components correlated on pathology with MPCs containing necrosis, hemorrhage, cystic degeneration, and matrix formation [Figures 8, 10 and 11].[511] The lesions with more cystic components were associated with squamous components [Figure 11].[11]
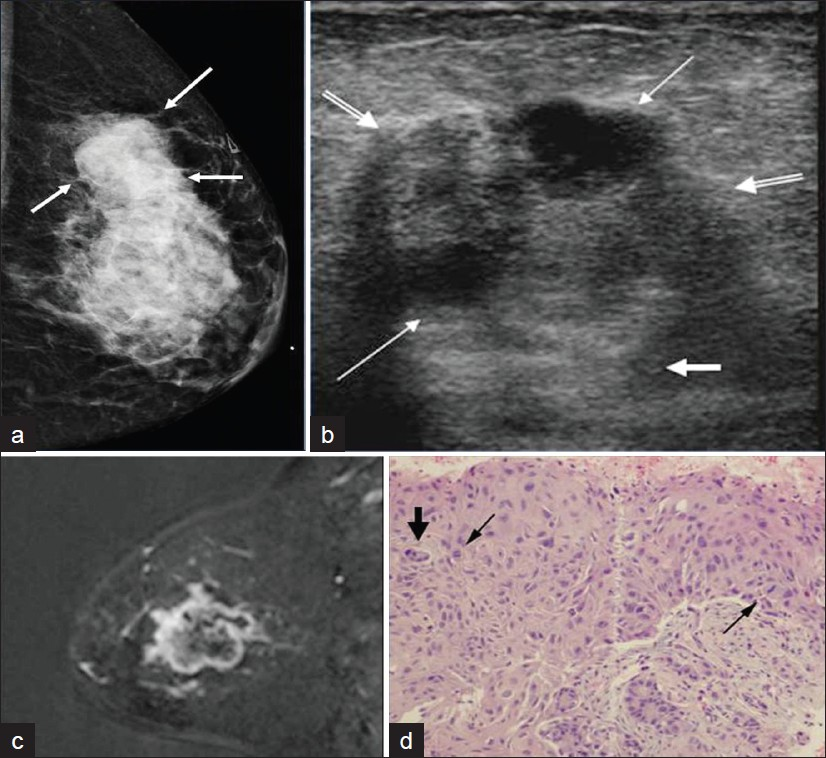
- A 47-year-old female who presented with a palpable lump in her left breast (a) Mediolateral oblique mammogram shows an irregular large mass in the left upper breast (arrow). (b) Transverse gray-scale image shows an irregular hypoechoic mass (double lined arrows) with internal anechoic cystic components (thin arrows) and posterior acoustic enhancement (short thick arrows). (c) Sagittal fat suppressed subtracted post-contrast T1-weighted MR image shows irregular peripheral rim enhancing large mass in the left breast. (d) Photomicrograph (H and E, high-power magnification) shows abnormal cells with increased cytoplasm, cells undergoing mitosis (arrows), and larger pleomorphic cells (short thick arrow). Final histopathologic diagnosis was metaplastic carcinoma, squamous subtype which often correlates with metaplastic carcinoma containing more cystic components.
CONCLUSION
MPC should be differentiated from invasive ductal or invasive lobular carcinoma and primary breast sarcoma in order to determine appropriate treatment options, management considerations, and patient outcome. The pathology classification of MPCs is often variable. The mammographic, sonographic, and MRI imaging characteristics of MPCs can be similar to invasive ductal carcinoma as well as probably benign features. The imaging appearance reported similar to invasive ductal carcinoma includes an irregular or circumscribed palpable mass with spiculated portion on mammography and solid irregular vs. mixed cystic and solid mass on ultrasound. Prior papers have also reported presentations with imaging features more suggestive of benign masses including circumscribed round or oval masses on mammogram, oval, round, or lobular circumscribed hypoechoic solid mass with posterior acoustic enhancement on ultrasound, and T2 hyperintensity on MRI. By understanding the clinical picture, pathology, and overlap in imaging characteristics of MPC with invasive ductal carcinoma and probably benign lesions can assist in diagnosing these difficult malignancies.
Source of Support: Nil
Conflict of Interest: None declared.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2012/2/1/21/95435
REFERENCES
- Metaplastic carcinoma of the breast: Report of three cases. Am Cancer Soc. 1998;82:1082-87.
- [Google Scholar]
- Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: The experience of the European Institute of Oncology and review of the literature. Breast Cancer Res Treat. 2007;101:349-53.
- [Google Scholar]
- Metaplastic carcinoma of the breast: Clinical, mammographic, and sonographic findings with histopathologic correlation. AJR Am J Roentgenol. 2002;178:1421-5.
- [Google Scholar]
- Imaging differences in metaplastic and invasive ductal carcinomas of the breast. AJR Am J Roentgenol. 2007;189:1288-92.
- [Google Scholar]
- Imaging features of metaplastic carcinoma with chondroid differentiation of the breast. AJR Am J Roentgenol. 2007;188:691-6.
- [Google Scholar]
- Clincopathologic features and outcomes of metaplastic breast carcinoma: Comparison with invasive ductal carcinoma of the breast. Yonsei Med J. 2010;51:864-9.
- [Google Scholar]
- Metaplastic carcinoma of the breast: A clinicopathological review. J Clin Pathol. 2006;59:1079-83.
- [Google Scholar]
- Matrix-producing carcinoma: A rare variant of metaplastic breast carcinoma with heterologous elements. Breast J. 2010;16:420-3.
- [Google Scholar]
- Metaplastic carcinoma of the breast: Mammographic and sonographic findings. J Clin Ultrasound. 2000;28:179-86.
- [Google Scholar]






