Translate this page into:
Renal Epithelioid Angiomyolipoma Associated with Pulmonary Lymphangioleiomyomatosis: Imaging Findings
Address for correspondence: Dr. Athina C Tsili, Department of Clinical Radiology, Medical School, University of Ioannina, University Campus, Ioannina 45110, Greece. E-mail: a_tsili@yahoo.gr, atsili@cc.uoi.gr
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Renal angiomyolipomas (AMLs) and pulmonary lymphangioleiomyomatosis (LAM) are the most common tumors of the perivascular epithelioid cell (PEComa) family. Both may be associated with tuberous sclerosis (TS) complex. Epithelioid AML (EAML) is a rare variety of AMLs, with a potential aggressive behavior. There are few reports in the English literature addressing on the imaging findings of renal EAMLs, which are considered nonspecific. We present the sonographic, computed tomographic, and magnetic resonance imaging findings of a renal EAML in a pregnant woman with concomitant pulmonary lesions indicative of LAM, without stigmata of TS. We conclude the importance of considering EAML as a possible diagnosis in the presence of a large renal mass with high cellular content and small amounts of fat in the coexistence of pulmonary LAM.
Keywords
Perivascular epithelioid cell tumors
pulmonary lymphangioleiomatosis
renal angiomyolipoma
renal epithelioid angiomyolipoma

Introduction
Neoplasms of the perivascular epithelioid cell (PEComas) represent a heterogeneous group of mesenchymal tumors, including angiomyolipomas (AMLs), clear cell tumors, and lymphangioleiomyomatosis (LAM).[1] PEComa is mainly characterized by epithelioid morphology and perivascular distribution, with immunoreactivity to melanocytic and smooth muscle markers, including HMB-45 and actin.[1] PEComas are known to be related to the tuberous sclerosis (TS) complex sharing similar genetic alterations.[1]
Epithelioid AML (EAML) is a recently described rare variant of AML with potentially aggressive behavior.[123] It has also been referred as atypical or malignant AML.[2345678] Only approximately 160 cases of EAMLs have been reported up to now.[8] There are few series in English literature regarding the imaging features of EAML.[45678]
Pulmonary LAM is a rare interstitial lung disease.[9] Abdominal manifestations may be seen in 50% of patients, including renal AMLs.[9]
We report a case of a young pregnant woman with a surgically confirmed renal EAML coexisting with pulmonary LAM, without stigmata of TS. We present the sonographic, computed tomography (CT) and magnetic resonance imaging (MRI) findings of this tumor and review of the literature.
Case Report
A 41-year-old woman, in the 7th month of pregnancy, was referred for fetal MRI due to right-sided abdominal pain and the sonographic diagnosis of polyhydramnios. Laboratory analysis was unremarkable. The patient had no clinical symptoms or signs of TS. There was no family history of neoplasms or known genetic conditions.
An ill-defined upper pole right renal mass was detected on MRI [Figure 1], with effacement of the ipsilateral adrenal gland. The lesion was heterogeneous, mainly solid, with signal intensity similar and lower than that of normal renal parenchyma on T1-weighted (T1WI) and T2-WI, respectively, and restricted diffusion [Figure 1a, b, and d]. Small amounts of fat were revealed within the mass [Figure 1b and c]. No fetal malformations were detected. Subsequent ultrasound examination showed hypoechoic, vascular right renal mass lesion, with lobulated contour, extending into the renal sinus [Figure 2].
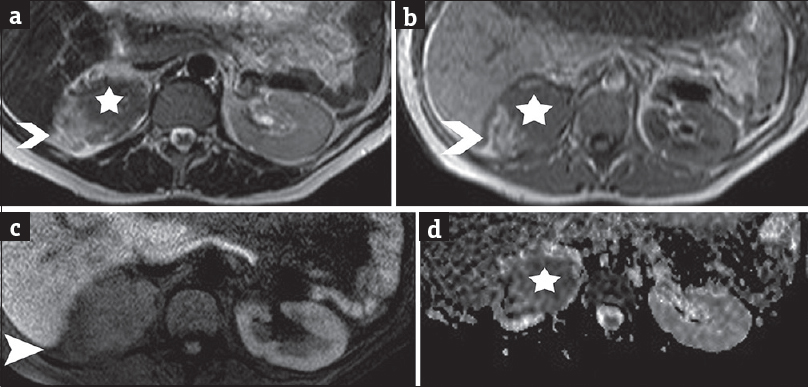
- A 41-year-old pregnant woman in the 7th month of pregnancy referred for right-sided abdominal pain and the sonographic diagnosis of polyhydramnios and diagnosed with renal epithelioid angiomyolipoma associated with pulmonary lymphangioleiomyomatosis. (a) Transverse T2-weighted image depicts right renal heterogeneous mass lesion with slightly hyperintense parts (arrowhead), corresponding to areas of fat and solid components (asterisk), of low-signal intensity when compared to normal renal parenchyma. (b) Transverse T1-weighted image shows lesion heterogeneity, with areas of fat detected hyperintense (arrowhead) and solid parts (asterisk), isointense to normal renal medulla. (c) Transverse fat-suppressed T1-weighted image demonstrates saturation of the hyperintense T1 components (arrowhead) of the lesion, findings compatible with the presence of fat. (d) Transverse apparent diffusion coefficient map derived from source image with b value of 700 s/mm2 shows areas of restricted diffusion (asterisk) within the lesion. The apparent diffusion coefficient values were 0.92 × 10−3 mm2 s−1, lower than that of the normal contralateral kidney (2.30 × 10−3 mm2 s−1).
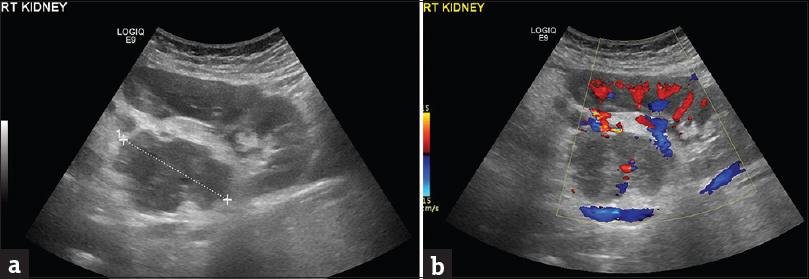
- A 41-year-old pregnant woman diagnosed with renal epithelioid angiomyolipoma associated with pulmonary lymphangioleiomyomatosis. (a) Sagittal sonographic image of the right hypochondrium depicts the presence of a heterogeneous, ill-defined mass (cursors) in the interpolar region of the right kidney. The lesion is mainly hypoechoic, extending into the renal sinus. (b) The presence of vascularity is detected within the mass lesion on Doppler examination.
A few days later, the patient had a premature birth due to fetal complications. A CT study of the chest and abdomen was followed. The presence of a heterogeneous right renal mass was confirmed, with small areas of macroscopic fat [Figure 3]. The solid parts of the tumor were hyperdense on unenhanced images, relative to normal renal parenchyma [Figure 3a]. After intravenous contrast medium administration, the mass enhanced moderately and heterogeneously, with slow wash-out [Figure 3b–d]. The dimensions of the lesion were 12 cm × 8 cm × 4.5 cm. Mild stranding of right perinephric fat was observed. The right renal vein and the inferior vena cava were patent. Neither retroperitoneal lymphadenopathy nor distant metastases were detected.
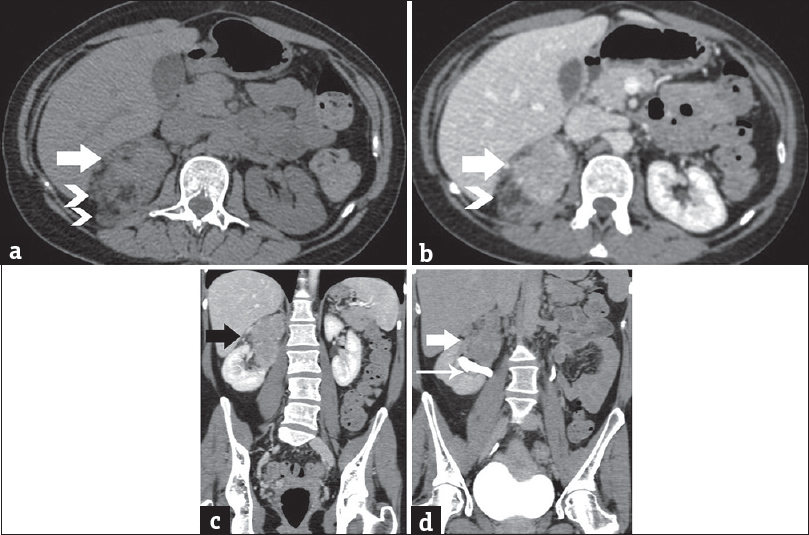
- A 41-year-old woman diagnosed with renal epithelioid angiomyolipoma associated with pulmonary lymphangioleiomyomatosis. (a) Transverse unenhanced computed tomography image depicts lesion heterogeneity, with small areas of fat (arrowheads) and solid parts (arrow), slightly hyperdense (mean computed tomography density: 45 HU) relative to the normal renal parenchyma. Contrast-enhanced computed tomography scan, (b) transverse, and (c) coronal reformations during the portal phase depict moderate, heterogeneous lesion enhancement (mean computed tomography density: 95 HU, arrow). Note areas of fat (arrowhead). (d) Contrast-enhanced computed tomography scan, coronal reformation, delayed phase. The mass (arrow) is seen in proximity to the pelvicaliceal system (long arrow), without obvious signs of invasion. No significant wash-out is detected (mean computed tomography density: 65 HU).
CT of the thorax showed multiple thin-walled cysts, of variable size, measuring from 5 to 15 mm [Figure 4]. Cysts were diffusely distributed throughout both lungs, with no zonal predominance. No pleural/pericardial effusion or mediastinal lymphadenopathy was detected. CT findings were strongly suggestive for pulmonary LAM.
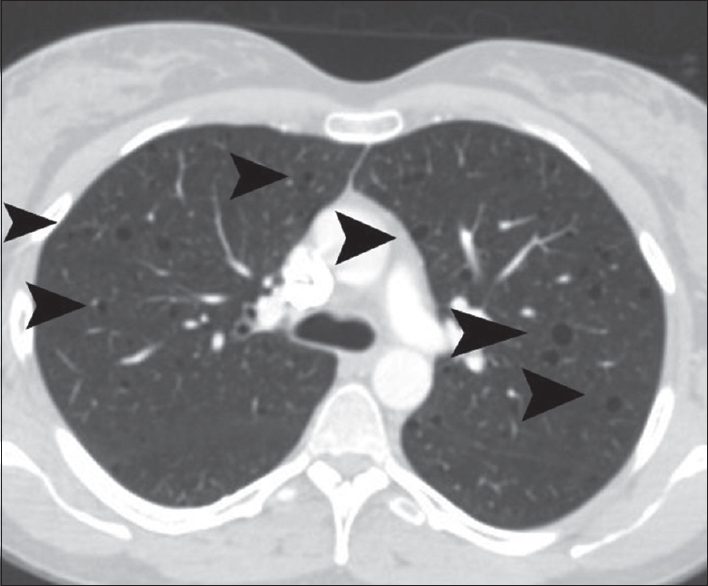
- A 41-year-old woman diagnosed with renal epithelioid angiomyolipoma associated with pulmonary lymphangioleiomyomatosis. Transverse chest computed tomography reveals multiple diffuse pulmonary thin-walled cysts (arrowheads).
Right radical nephrectomy and adrenalectomy were followed. Histology reported the presence of a large highly cellular tumor, predominantly composed of epithelioid cells with perivascular distribution, smooth muscle-like cells, adipocytes, and thick-walled blood vessels. Marked nuclear atypia and/or pleomorphism were seen, involving approximately 20% of the neoplasm. Rare mitoses were revealed (<2 per high-powered field). Epithelioid cells were positive for melanocytic markers, myoid markers, and CD68. Areas of necrosis were detected within the mass. Invasion of the renal parenchyma, renal capsule, perinephric fat, and renal sinus fat was reported on histology. The adrenal gland was free of invasion. Based on histopathologic characteristics, the diagnosis of EAML with uncertain biologic behavior was made.
MRI examination of the brain was subsequently performed and was negative for the presence of TS stigmata. The patient is now well and free of recurrence, based on follow-up CT studies 5 years after surgery.
Discussion
EAMLs described by Pea et al., in 1998, as a rare variant of AMLs are characterized by the presence of predominantly epithelioid cells in perivascular distribution and the absence of both adipocytes and abnormal vessels.[123] However, the degree of epithelioid tumor component that suggests classification as an EAML is unclear, ranging from 5% to 100%.[123] The 2004 World Health Organization classification of renal neoplasms classifies EAMLs as tumors of uncertain malignant potential that may exhibit aggressive behavior, including local invasiveness, recurrence, and metastases.[123] Histological features and morphologic criteria cannot predict clinical course, since the majority of these tumors have an entirely benign course, even in the presence of cytologic atypia.[123] Recently, Brimo et al., developed a predictive model of four atypical histologic features that can be used to distinguish the “aggressive” from the more “benign” EAMLs, including the following: (1) ≥70% of atypical epithelioid cells, (2) ≥2 mitotic figures per high-powered field, (3) atypical mitotic figures, and (4) necrosis.[3] The presence of three or all of these features was considered as highly predictive of malignant behavior.[3] Surgical resection is a beneficial treatment for EAML. A close follow-up similar to renal cell carcinoma (RCC) rather than classical AML may be justified.[345678]
Renal EAMLs have a broad-spectrum of imaging features, reflecting their wide histological diversity.[45678] Tsai et al., reported the absence of macroscopic fat and calcifications on CT examination in five cases of EAMLs.[4] All tumors showed early contrast enhancement in this study. The same authors also performed an analysis of the literature and found no gross fat component for a total of twenty cases of EAMLs.[4] Froemming et al., reported the imaging findings in nine cases of histologically confirmed EAMLs.[5] The size of the tumors ranged from 1.4 to 22 cm and the mean diameter was 7.8 cm. Imaging findings were variable, from small, well-defined, homogeneous tumors to large, markedly heterogeneous masses and the presence of minor amounts of fat in five cases. The authors suggested that EAML should be considered in the differential diagnosis of a renal mass with small foci of macroscopic fat without calcifications or when acute hemorrhage occurs within a renal mass.[5] Bharwani et al., reported the CT and MRI features of two highly cellular EAMLs.[6] These tumors appeared as hyperattenuating masses on CT, variably enhancing, without fat on both imaging examinations.[6] Tirumani et al., in a retrospective study of 36 histologically confirmed malignant PEComas, including eight renal tumors, concluded that malignant PEComas should be considered in the differential diagnosis of large, well-demarcated renal masses, without renal vein involvement or metastatic lymphadenopathy.[7] The most frequent sites of metastatic disease were the lung, liver, and peritoneum in the same report.[7] Imaging findings considered as more suggestive of the diagnosis of this rare tumor include the following: hyperdense mass on unenhanced CT images when compared to normal renal parenchyma, of low T2 signal, both mainly due to lesion hypercellularity, lack of fat, large size, distinct margins, bulging contour of the affected kidney, marked heterogeneous contrast enhancement, with rapid wash-in and slow wash-out.[8] However, most studies conclude that renal EAML can have a range of imaging characteristics and can be indistinguishable from RCC and AML with minimal fat.[45678]
In our case, EAML was detected as a large renal mass with high cellular content, seen hypoechoic on sonographic examination, hyperdense on unenhanced CT, and hypointense on T2-WI. Low T2 signal apart from hypercellularity may be related to the presence of epithelioid and muscular components within the mass. High cellularity was confirmed by tumor's restricted diffusion on MRI. The mass had small amounts of macroscopic fat on both CT and MRI and enhanced moderately and heterogeneously, with early wash-in and slow wash-out.
Pulmonary LAM is a well-recognized clinical entity, considered as a benign mesenchymal neoplasm with PEComas differentiation.[9] It is a rare idiopathic disease affecting almost exclusively young women in childbearing age that was first described by von Stossel in 1937.[9] In the course of the disease, a nonneoplastic interstitial proliferation of atypical smooth muscle occurs, resulting in progressive obstruction of airways, blood and lymphatic pulmonary vessels.[9] The detection of multiple thin-walled cysts throughout the lung parenchyma without zonal predominance is considered the imaging hallmark of LAM on CT.[9] Renal AML represents the most frequent extrapulmonary manifestation of pulmonary LAM and both clinical entities are well known to be encountered sporadically and at much higher frequency in patients with TS (up to 93%).[9] To our knowledge, the coexistence of a malignant renal EAML in patients with sporadic pulmonary LAM has been limited reported in the literature.[10] Herein, we present a case of a pregnant woman with histologically confirmed renal EAML displaying pulmonary lesions compatible with LAM on CT, in the absence of clinical findings of TS.
Conclusion
Renal EAMLs have variable imaging characteristics, rendering their preoperative characterization difficult. However, in the presence of a large renal mass with high cellular content and small amounts of fat, coexisting with pulmonary LAM, the diagnosis of this rare tumor should be suggested. Patterns of contrast enhancement on dynamic imaging, that is, early wash-in and slow wash-out, may be used as ancillary findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2017/7/1/18/206659
References
- Neoplasms of the perivascular epithelioid cell involving the abdomen and the pelvis: Cross-sectional imaging findings. J Comput Assist Tomogr. 2007;31:688-96.
- [Google Scholar]
- Pure epithelioid PEComas (so-called epithelioid angiomyolipoma) of the kidney: A clinicopathologic study of 41 cases: Detailed assessment of morphology and risk stratification. Am J Surg Pathol. 2011;35:161-76.
- [Google Scholar]
- Renal epithelioid angiomyolipoma with atypia: A series of 40 cases with emphasis on clinicopathologic prognostic indicators of malignancy. Am J Surg Pathol. 2010;34:715-22.
- [Google Scholar]
- Epithelioid angiomyolipoma of the kidney mimicking renal cell carcinoma: A clinicopathologic analysis of cases and literature review. Kaohsiung J Med Sci. 2009;25:133-40.
- [Google Scholar]
- Renal epithelioid angiomyolipoma: Imaging characteristics in nine cases with radiologic-pathologic correlation and review of the literature. AJR Am J Roentgenol. 2013;200:W178-86.
- [Google Scholar]
- Imaging features of primary and metastatic malignant perivascular epithelioid cell tumors. AJR Am J Roentgenol. 2014;202:252-8.
- [Google Scholar]
- Imaging appearance of renal epithelioid angiomyolipomas. J Comput Assist Tomogr. 2013;37:957-61.
- [Google Scholar]
- Renal angiomyolipoma in association with pulmonary lymphangioleiomyomatosis. Am J Kidney Dis. 2003;41:877-83.
- [Google Scholar]
- Malignant epithelioid angiomyolipoma in the kidney and liver of a patient with pulmonary lymphangioleiomyomatosis: Lack of response to sirolimus. Intern Med. 2009;48:1821-5.
- [Google Scholar]






