Translate this page into:
Rare Malignant Tumors of the Breast
Address for correspondence: Dr. Trevor Miller, Department of Radiology, Memorial Sloan Kettering Cancer Center, 1275 York Avenue New York, NY 10065, USA. E-mail: millert1@mskcc.org
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
While the more common forms of breast cancer are well understood and recognized, there are many important rare malignancies that are less appreciated. Many of these cancers have imaging findings that, when understood, help to formulate a more educated differential diagnosis. In this article, the clinical features, imaging, and pathologic findings of rare breast malignancies will be discussed.
Keywords
Breast
breast imaging
cancer
concordance
rad-path correlation
rare breast malignancy

INTRODUCTION
While the more common forms of breast cancer are well understood and recognized, there are many important rare malignancies that are less appreciated. Many of these cancers have imaging findings that, when understood, help to formulate a more educated differential diagnosis. In this article, the clinical features, imaging, and pathologic findings of rare breast malignancies will be discussed.
ADENOID CYSTIC CARCINOMA
Adenoid cystic carcinoma (AdCC) of the breast is rare, comprising approximately 0.1% of all breast malignancies.[1] Similar to AdCC of the salivary gland, this cancer has a low malignant potential.[2] The mean age of diagnosis is 60 years, and AdCC may present with a palpable mass and/or breast tenderness.[34] In regards to imaging features, many are nonspecific, but there are common findings seen with AdCC. The typical mammographic appearance of AdCC is of an irregular or lobular mass with indistinct margins. Even though these tumors are not typically associated with calcifications, microcalcifications may occasionally be present. The ultrasound features of AdCC are variable and nonspecific with studies describing both well-circumscribed and poorly circumscribed masses with peripheral vascularity and heterogeneous echotexture [Figure 1].[1] A periareolar location is favored, but like other breast malignancies, involvement of upper outer breast is also common.[14] Differential diagnosis of this tumor includes both benign and malignant etiologies, including fibroadenomas, medullary/mucinous carcinomas, and invasive ductal carcinomas of no specific type (NST).[4] Pathology demonstrates three typical architectural patterns: Tubular, cribriform, and solid.[25] There are two pathologic features that are characteristic of AdCC. Thefirst of these is a true glandular space which contains periodic acid Schiff (PAS) positive material. The second is a pseudolumina which results from invagination of stroma surrounded by basal-myoepithelial cells.[2] Axillary metastases are rare; however, patients can present with distant metastatic disease. The most common site of metastatic disease is the lung.[46] Treatment is typically mastectomy or partial mastectomy with local radiation.[1] With appropriate definitive therapy, recurrent disease is rare.
![55-year-old female with adenoid cystic carcinoma. The patient initially presented for evaluation of a palpable abnormality in the right breast. (a) Spot compression craniocaudal (CC) view of the right breast demonstrates a dense irregular mass with a few coarse appearing calcifications (arrows), corresponding to the palpable finding. (b) Gray-scale and (c) color Doppler images demonstrate a heterogeneous mass with indistinct margins (arrows) and internal vascularity (arrow) measuring up to 5 cm. The mass contains a few anechoic cystic regions and no posterior acoustic features. (d) Photomicrograph [hematoxylin and eosin stain (H and E), 20×] shows typical cribriform pattern and epithelial cells lining periodic acid Schiff (PAS) positive material (arrow).](/content/12/2015/5/1/img/JCIS-5-58-g002.png)
- 55-year-old female with adenoid cystic carcinoma. The patient initially presented for evaluation of a palpable abnormality in the right breast. (a) Spot compression craniocaudal (CC) view of the right breast demonstrates a dense irregular mass with a few coarse appearing calcifications (arrows), corresponding to the palpable finding. (b) Gray-scale and (c) color Doppler images demonstrate a heterogeneous mass with indistinct margins (arrows) and internal vascularity (arrow) measuring up to 5 cm. The mass contains a few anechoic cystic regions and no posterior acoustic features. (d) Photomicrograph [hematoxylin and eosin stain (H and E), 20×] shows typical cribriform pattern and epithelial cells lining periodic acid Schiff (PAS) positive material (arrow).
APOCRINE CARCINOMA
Apocrine carcinoma is a rare breast malignancy seen most commonly in women 40–50 years of age. The most common presenting clinical features are palpable breast masses and bloody nipple discharge.[7] Mammographically, apocrine carcinomas present as single or multiple irregular masses, oftentimes associated with microcalcifications. Ultrasound features of apocrine carcinoma are variable with descriptions of both solid masses with spiculated margins and complex cystic and solid masses [Figure 2].[8] A frequently described imaging feature of apocrine carcinoma is unilateral multicentric disease.[89] The pathologic classification of apocrine carcinoma as a distinct type of breast cancer has been long debated. The most recent World Health Organization classification of tumors of the breast describes apocrine carcinoma as carcinoma with apocrine differentiation.[2] Many breast cancers have focal apocrine differentiation, but the amount of differentiation dictates classification into the distinct subtype of apocrine carcinoma.[2] Histologically, apocrine differentiation is defined as the presence of large cells with prominent eosinophilic cytoplasm, sharply defined cell borders, and large nuclei.[10] Furthermore, a pure apocrine immunophenotype is estrogen receptor (ER)/progesterone receptor (PR) negative and androgen receptor (AR) positive.[910] The prognosis for apocrine carcinoma is similar to that of invasive ductal carcinoma NST. There have been studies demonstrating no significant difference in survival between apocrine carcinoma and invasive ductal carcinoma NST, as well as studies showing similar recurrence rates, with the time to recurrence longer in patients with apocrine carcinoma.[1112] Treatment considerations for apocrine carcinoma depend on specific immunophenotype. This is similar to treatment for invasive ductal carcinoma NST and continues to evolve.[9]
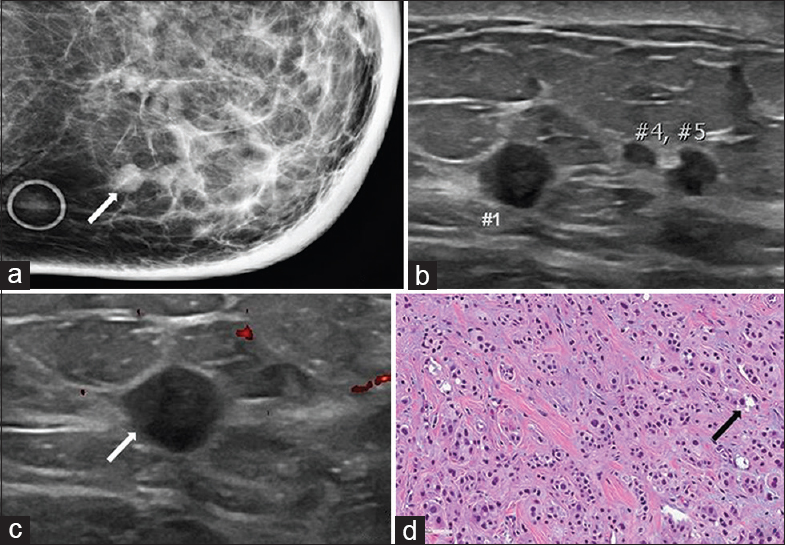
- 72-year-old female with apocrine carcinoma. The cancer was initially found in the left axillary region. The patient subsequently developed local recurrence in both the left axilla and the left breast. (a) Spot compression CC view of the left breast demonstrates multiple partially obscured masses in the inner breast (arrow). Note is made of diffuse skin and trabecular thickening. (b) Gray-scale and (c) color Doppler images of the left breast demonstrate multiple circumscribed hypoechoic masses correlating with the findings seen on mammography (numbers and arrow). (d) Photomicrograph of the surgical specimen (H and E stain, 20×) demonstrates the typical pattern of granular eosinophilic cytoplasm, round vesicular nuclei, and scattered regions of cytoplasmic vacuolization (arrow).
LYMPHOMA
Both primary and secondary involvement of the breast can occur with lymphoma. Lymphoma involving the breast can be unilateral or bilateral. Lymphoma is a rare cancer of the breast, comprising approximately 0.5% of breast malignancies. A large series of breast lymphoma cases described primary breast lymphoma in 61% of patients and secondary breast lymphoma in 39% of patients.[13] Patients with lymphoma most commonly present with painless palpable abnormalities, prompting imaging work-up.[1415] Imaging features of lymphoma involving the breast are similar to lymphoma affecting other sites and include circumscribed oval or round masses without associated calcifications or spiculated margins. These masses can be multiple and can mimic benign breast disease.[13] Patients can also present with global asymmetry and trabecular and/or skin thickening. Sonographic features of lymphoma include heterogeneous or hypoechoic round and/or oval masses with circumscribed or indistinct margins [Figure 3].[13] Differential diagnosis includes metastatic disease, and there are no reliable imaging features which can distinguish between lymphoma and metastatic disease involving the breast. Clinical history is very important in lymphoma cases. The diagnosis of lymphoma may be made with fine needle aspiration or core needle biopsy. Diffuse large B cell lymphoma is the most common histologic type involving the breast, with T cell rarely seen. On histology, tumors are composed of large cells with round-to-irregular nuclei and variably prominent nucleoli.[15] Treatment is systemic therapy, similar to that of lymphoma involving other parts of the body. Surgical excision is not performed, and the prognosis is similar to that of non-breast lymphoma.
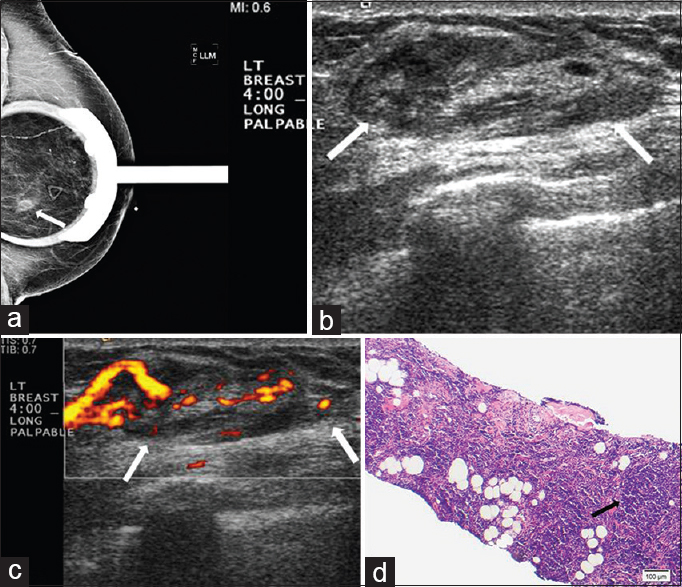
- Extranodal marginal zone lymphoma. (a) Spot magnification mediolateral (ML) view of the left breast demonstrates a focal asymmetry in the lower breast, posterior depth (arrow). This corresponds to a palpable finding. (b) Gray-scale and (c) color Doppler images demonstrate a superficial oval vascular mass in the 4:00 position (arrows) corresponding to the mammographic and palpable findings. (d) Photomicrograph of the surgical specimen (H and E stain, 10×) shows small lymphocytes with dense nuclear chromatin infiltrating extensively into fibroadipose tissue and forming a vaguely nodular pattern (arrow).
NEUROENDOCRINE CARCINOMA
Carcinomas with neuroendocrine differentiation are rare and comprise <1% of all breast cancers. As all breast tumors are not tested for neuroendocrine markers, the incidence of neuroendocrine carcinoma may be underestimated.[2] Most patients diagnosed with neuroendocrine carcinoma present in the 60–70 year age range.[1617] Due to low number of cases, clinical features are not well documented. Patients with neuroendocrine carcinoma have been described as presenting with palpable masses and nipple discharge.[18] Mammographic features include oval, round, or lobular mass without associated calcifications.[1719] Masses are usually circumscribed with a minority of cases having spiculated margins [Figure 4]. Neuroendocrine carcinomas are commonly ER positive, but imaging features are closer to those of triple-negative cancers.[17] Histology varies depending on the degree of differentiation, ranging from well-differentiated low-grade tumors to poorly differentiated high-grade tumors indistinguishable from small cell cancer of the lung.[2] The prognosis for neuroendocrine carcinoma has been described as similar to that of invasive ductal carcinoma-NST; however, a recent larger case series showed a poorer outcome for neuroendocrine carcinoma compared to invasive ductal carcinoma-NST. The degree of neuroendocrine differentiation may be an independent risk factor for poor clinical outcome.[20] A combination of chemotherapy, radiation therapy, and surgery is a typical treatment approach.
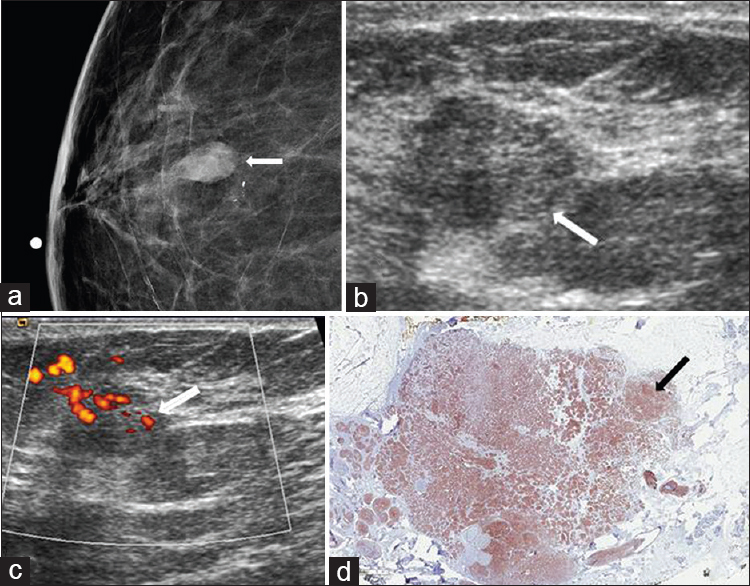
- Neuroendocrine carcinoma. (a) Spot compression CC view of the right breast shows an oval mass (arrow) with associated amorphous calcifications in the lower breast, anterior depth. (b) Gray-scale and (c) color Doppler ultrasound images demonstrate a vascular hypoechoic mass with indistinct margins (arrows) corresponding to the mammographic abnormality. (d) Immunohistochemical stain for neuroendocrine marker synaptophysin (1×) shows diffuse staining of the tumor cells (arrow).
METAPLASTIC CARCINOMA
Metaplastic carcinoma includes a number of cancers which are characterized by dedifferentiation of cells into squamous or mesenchymal elements.[2] These cancers are uncommonly seen and comprise less than 1% of all breast cancers, typically affecting women above 60 years of age.[21] The most common mammographic finding in metaplastic carcinoma is a circumscribed high-density oval mass.[222324] Sonographic findings include oval or irregular shape, heterogeneous echogenicity with cystic elements, and posterior acoustic enhancement, with posterior shadowing less common than in invasive ductal carcinoma NST [Figure 5].[2225] These differences between metaplastic carcinoma and invasive ductal carcinoma NST are noted by the fact that in some tumors with both metaplastic and invasive ductal components, the metaplastic component corresponds to the more lobular and/or circumscribed portion of the mass.[23] Differential diagnosis includes breast sarcoma, phyllodes tumor, invasive ductal carcinoma NST, and mucinous carcinoma. Many different components can be present in metaplastic carcinomas, including but not limited to, squamous cells, matrix-producing cells, spindle cells, and a true mesenchymal component. When evaluating metaplastic carcinomas, there is need for extensive sampling as metaplastic carcinoma needs to be differentiated from sarcoma due to treatment implications.[23] Treatment for metaplastic carcinoma includes surgical excision with radiation therapy. Currently, there is a limited role for chemotherapy.[2627]
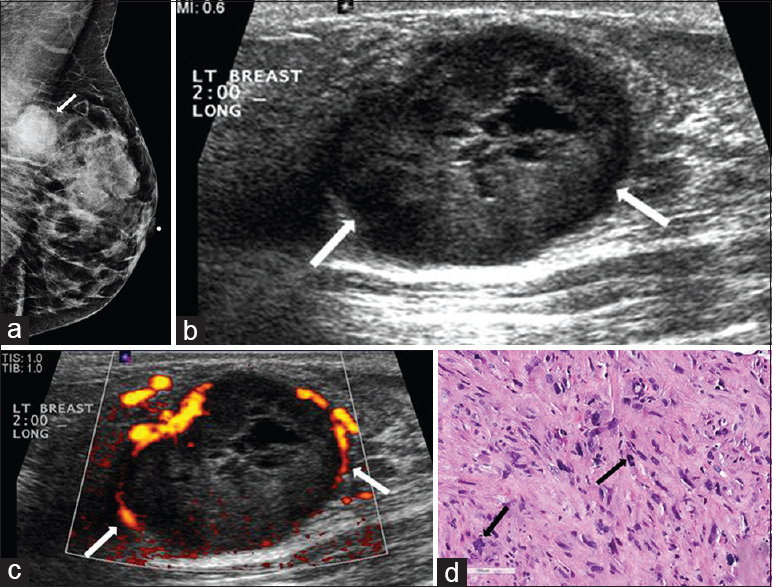
- Metaplastic breast carcinoma. (a) Mediolateral oblique (MLO) view of the left breast demonstrates a partially circumscribed mass in the upper position of the posterior breast (arrow). (b) Gray-scale and (c) color Doppler images of the left breast demonstrate an ovoid, predominantly hypoechoic circumscribed mass with cystic components and vessels in rim in the 2:00 position (arrows). (d) Photomicrograph of the surgical specimen (H and E stain, 20×) shows a spindle cell proliferation with multinucleated giant cells and atypical mitosis (arrows).
ANGIOSARCOMA
Angiosarcoma of the breast is a rare malignancy which can be either primary or secondary. Primary cases arise in the breast parenchyma and comprise approximately 0.04–0.05% of primary breast malignancies.[228] Primary angiosarcomas tend to present in younger patients compared to more common breast cancers, typically at an age of 30–50 years. Patients commonly present with palpable masses, sometimes having associated bluish skin discoloration due to the vascular nature of the tumor.[29] The most common mammographic appearance is a non-calcified, ill-defined mass or a focal asymmetry.[2829] As these tumors present in younger patients, these findings may be partially obscured due to underlying dense breast tissue.[29] On ultrasound, the imaging features include hypervascular masses with mixed echogenicity [Figure 6].[29]
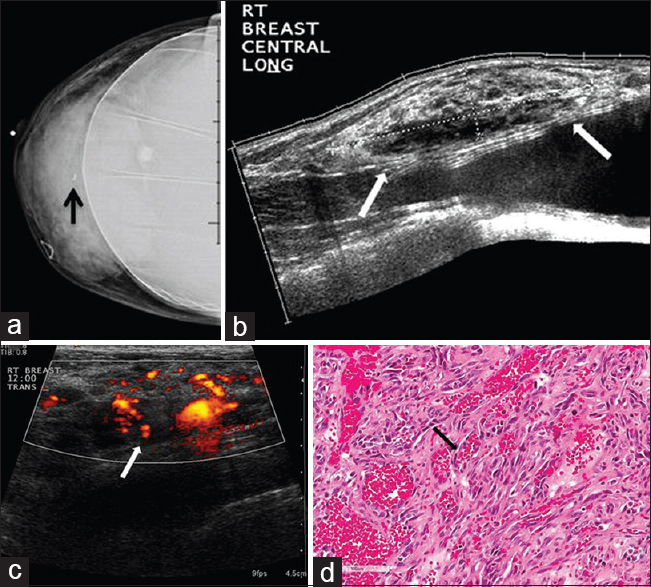
- 42-year-old female with angiosarcoma of the breast. The patient presented with a right breast mass. (a) CC implant view of the right breast demonstrates a mass in the central right breast with a biopsy clip in place (arrow). (b) Gray-scale and (c) color Doppler images of the right breast show a large heterogeneous mass with internal vascularity (arrows) corresponding to mammographic abnormality. (d) Photomicrograph of the surgical specimen (H and E stain, 20×) shows a portion of the tumor composed of solid and spindle cell foci with multiple blood “lakes” (arrow), indicating the vascular nature of tumor.
Secondary angiosarcoma is different in that it is typically seen in patients with a history of breast conservation surgery and radiation for breast cancer. Patients are older than those with primary angiosarcoma, with the typical age of presentation being between 60 and 70 years of age. Also, in contradistinction to primary angiosarcoma, the secondary form almost always affects the dermis of the breast.[29] Post-radiation angiosarcoma is rare, occurring in less than 0.5% of treated patients.[29] Patients most commonly present with palpable masses, usually not painful.[28] There is a latency period between the time of radiation therapy and the time of presentation, averaging around 6 years. The presentation tends to be skin discoloration with nodules or plaques. On mammography, the secondary form of angiosarcoma can appear as asymmetric skin thickening and/or trabecular thickening, or it can present with a mass if the cancer is parenchymal in origin.[29] Ultrasound often shows findings that can be confused with post-radiation change. Therefore, clinical examination findings and history are very important. Treatment typically involves mastectomy with chemotherapy. There is a limited role for radiation. The prognosis is variable and depends on the grade of the tumor. Also, patients with secondary angiosarcoma tend to have a poorer clinical outcome compared to patients with primary angiosarcoma.[29]
METASTATIC DISEASE
Metastatic disease to the breast is rare and comprises approximately 1.7–6.6% of all breast malignancies. The reason for this large variance is due to the inclusion or exclusion of lymphoid malignancies as true metastatic disease.[3031] The most common type of tumor to metastasize to the breast is melanoma. Patients with metastatic disease to the breast tend to be premenopausal and female. This is felt likely to be a result of the effect of circulating estrogen on breast vascularity.[31] Patients commonly present with palpable lumps, thought to be due to the relatively superficial subcutaneous location of the metastases.[32] Imaging features of metastatic disease are variable given the wide variety of primary tumors, but there are still findings which can be helpful in the differential diagnosis. On mammography, patients can present with single or multiple unilateral or bilateral masses. Typically these masses are small in size, measuring less than 2 cm. An important feature that is not normally seen with metastases is calcification. The presence of calcifications in a lesion strongly argues against metastatic disease with a few exceptions, including ovarian, thyroid, and mucin-producing primary cancers.[3033] Sonographic findings in metastatic disease are variable with the most common feature being a hypoechoic irregular mass [Figure 7]. Clinical history is very important in these patients, as several low-grade or benign processes in the breast can have similar findings.[34] A second imaging pattern that can be seen with metastases is a diffusely infiltrating mass that can mimic inflammatory breast carcinoma. This can be seen with metastatic gastric cancer and contralateral breast cancer.[30] Ancillary imaging tests can also be beneficial. For example, nuclear medicine octreotide scans, which utilize a somatostatin analog, can help diagnose metastatic carcinoid tumors. Treatment of metastatic disease involving the breast depends on the primary tumor, and the prognosis is generally poor when the primary lesion is a solid tumor.
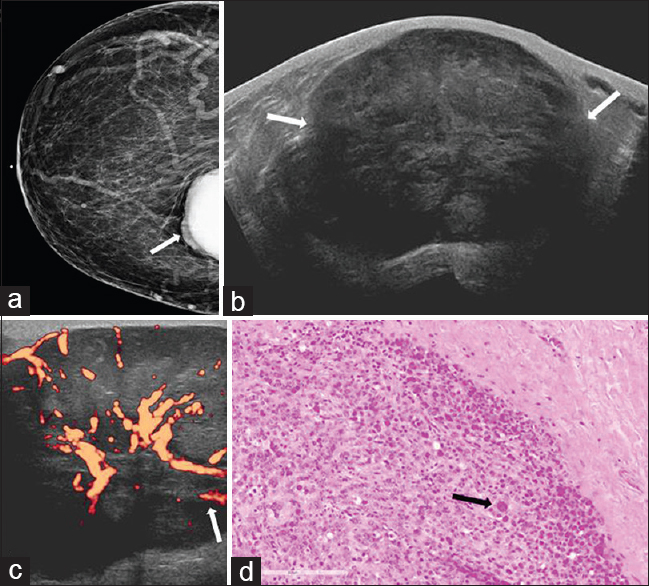
- 50-year-old female with history of uterine leiomyosarcoma, status post hysterectomy presented for evaluation of a palpable abnormality in the right breast. (a) CC view of the right breast demonstrates a partially visualized dense mass in the inner breast, posterior depth (arrow). (b) Gray-scale and (c) power Doppler images show a large circumscribed hypoechoic mass with internal vascularity (arrows) corresponding to mammographic abnormality. (d) Photomicrograph of the surgical specimen (H and E stain, 20×) demonstrates markedly enlarged cells with marked cytologic atypia and abundant cytoplasm (arrow). Increased mitotic activity and the presence of atypical mitotic figures are also identified in other areas.
CONCLUSION
Through this pictorial essay, several rare malignancies of the breast have been described. Understanding the varied imaging and pathologic features of these tumors will help to formulate a differential diagnosis and establish imaging–histopathologic concordance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2015/5/1/58/168711
REFERENCES
- Adenoid cystic carcinoma of the breast: Review of the literature and report of two cases. Oncol Lett. 2012;4:701-4.
- [Google Scholar]
- Adenoid cystic carcinoma of the breast: Mammographic appearance and pathologic correlation. AJR Am J Roentgenol. 1998;171:1679-83.
- [Google Scholar]
- Salivary gland-like tumours of the breast: Surgical and molecular pathology. J Clin Pathol. 2003;56:497-506.
- [Google Scholar]
- Adenoid cystic carcinoma of the breast. Data from the Connecticut Tumor Registry and a review of the literature. Ann Surg. 1987;205:295-301.
- [Google Scholar]
- Apocrine carcinoma of the breast: Mammography and ultrasound findings. Eur J Radiol. 2006;60:55-9.
- [Google Scholar]
- Management of unusual histological types of breast cancer. Oncologist. 2012;17:1135-45.
- [Google Scholar]
- EGFR and HER-2/neu expression in invasive apocrine carcinoma of the breast. Mod Pathol. 2010;23:644-53.
- [Google Scholar]
- Apocrine mammary carcinoma. A clinicopathologic study of 72 cases. Am J Clin Pathol. 1990;94:371-7.
- [Google Scholar]
- ‘Pure’ invasive apocrine carcinoma of the breast: A new clinicopathological entity? Breast. 2005;14:3-10.
- [Google Scholar]
- Primary and secondary breast lymphoma: Prevalence, clinical signs and radiological features. Br J Radiol. 2012;85:e195-205.
- [Google Scholar]
- Lymphoma affecting the breast: A pictorial review of multimodal imaging findings. J Breast Cancer. 2013;16:254-65.
- [Google Scholar]
- Expression of apocrine differentiation markers in neuroendocrine breast carcinomas of aged women. Mod Pathol. 2001;14:768-76.
- [Google Scholar]
- Neuroendocrine carcinoma of the breast: Mammographic features correlated with sonography and histopathological findings. Zhonghua Zhong Liu Za Zhi. 2012;34:917-22.
- [Google Scholar]
- Clinical and radiologic features of neuroendocrine breast carcinomas. J Ultrasound Med. 2014;33:1511-8.
- [Google Scholar]
- Primary neuroendocrine carcinoma of the breast: Clinical, imaging, and histologic features. AJR Am J Roentgenol. 2014;203:W221-30.
- [Google Scholar]
- Invasive neuroendocrine carcinoma of the breast: A population-based study from the surveillance, epidemiology and end results (SEER) database. BMC Cancer. 2014;14:147.
- [Google Scholar]
- Metaplastic carcinoma of the breast: Multimodality imaging and histopathologic assessment. Acta Radiol. 2012;53:5-11.
- [Google Scholar]
- Metaplastic carcinoma of the breast: Mammographic appearance with pathologic correlation. AJR Am J Roentgenol. 1997;169:709-12.
- [Google Scholar]
- Metaplastic carcinoma of the breast: Clinical, mammographic, and sonographic findings with histopathologic correlation. AJR Am J Roentgenol. 2002;178:1421-5.
- [Google Scholar]
- Imaging differences in metaplastic and invasive ductal carcinomas of the breast. AJR Am J Roentgenol. 2007;189:1288-93.
- [Google Scholar]
- Current progress in the treatment of metaplastic breast carcinoma. Asian Pac J Cancer Prev. 2013;14:6221-5.
- [Google Scholar]
- Mammary angiosarcomas: Imaging findings in 24 patients. Radiology. 2007;242:725-34.
- [Google Scholar]
- Metastatic tumors to the breast: Mammographic and ultrasonographic findings. J Ultrasound Med. 2000;19:257-62.
- [Google Scholar]
- Breast metastasis in adolescents with alveolar rhabdomyosarcoma of the extremities: Report of two cases. Pediatr Hematol Oncol. 1996;13:277-85.
- [Google Scholar]
- Imaging findings of metastatic disease to the breast. Yonsei Med J. 2001;42:497-502.
- [Google Scholar]
- Metastatic disease to the breast: The Washington University experience. World J Surg Oncol. 2007;5:74.
- [Google Scholar]






