Translate this page into:
Radiographic Features of a Benign Mixed Brenner Tumor and Mucinous Cystadenoma: A Rarely Identified Ovarian Neoplasm on Imaging

*Corresponding author: Mitchell P. Wilson, Department of Radiology and Diagnostic Imaging, University of Alberta, Edmonton, Alberta, Canada, USA. mitch.wilson@ualberta.ca
-
Received: ,
Accepted: ,
How to cite this article: Wilson MP, Katlariwala P, Hwang J, Low G. Radiographic features of a benign mixed brenner tumor and mucinous cystadenoma: A rarely identified ovarian neoplasm on imaging. J Clin Imaging Sci 2020;10:22
Abstract
Imaging features of benign mixed Brenner tumor and mucinous cystadenomas are rarely reported. This report aims to describe the case of a benign mixed Brenner tumor and mucinous cystadenoma with a dominant Brenner tumor component and to review the typical imaging features of this ovarian neoplasm.
Keywords
Ovary
Neoplasm
Brenner
Mucinous
Cystadenoma
INTRODUCTION
Benign mixed Brenner tumor and mucinous cystadenomas have been recognized in the pathology literature for over a century, although imaging appearances of these combined lesions are rarely reported.[1] In part, this may relate to the mucinous neoplasm components often representing the dominant lesion identified on imaging with the Brenner lesion component identified incidentally at pathology.[1-3] In some cases, the dominant mucinous component of the lesion can be as large as 50 cm.[4] This report aims to describe the case of a benign mixed Brenner tumor and mucinous cystadenoma with a dominant Brenner tumor component and to review the imaging features of a combined ovarian lesion rarely identified on imaging.
CASE REPORT
A 40-year-old female underwent an ultrasound for abnormal uterine bleeding incidentally identifying a left adnexal mass. She described an intermittent bloating sensation but no focal tenderness in the left lower quadrant or pelvis. She had a surgical history of two prior caesarian sections and a previous tubal ligation performed in the Philippines. She had no other relevant medical history and denied any family history of malignancy.
On clinical examination, she had a healed caesarean section abdominal scar and elicited minor discomfort to the left adnexa with bimanual examination, where a lesion could be manually palpated. Her relevant biochemical examination was normal and reported as follows: Hemoglobin 131 g/L (n 120–160); beta-hCG <5 (n < 5); cancer antigen 125 (CA-125) 31 kU/L (n < 35); alpha-fetoprotein 2 ug/L (n < 9); carcinoembryonic antigen 2.1 ug/L (n < 5); and lactate dehydrogenase 4 U/L (n 100–225).
Initial ultrasound imaging identified a complex solid and cystic 5 cm left adnexal mass with the solid component representing the dominant portion of the lesion [Figure 1]. The cysts were thinly septated and adjacent to the solid component. The solid component was hypoechoic with posterior shadowing demonstrating small echogenic foci suggestive of internal calcifications. The right adnexa and uterus were unremarkable.
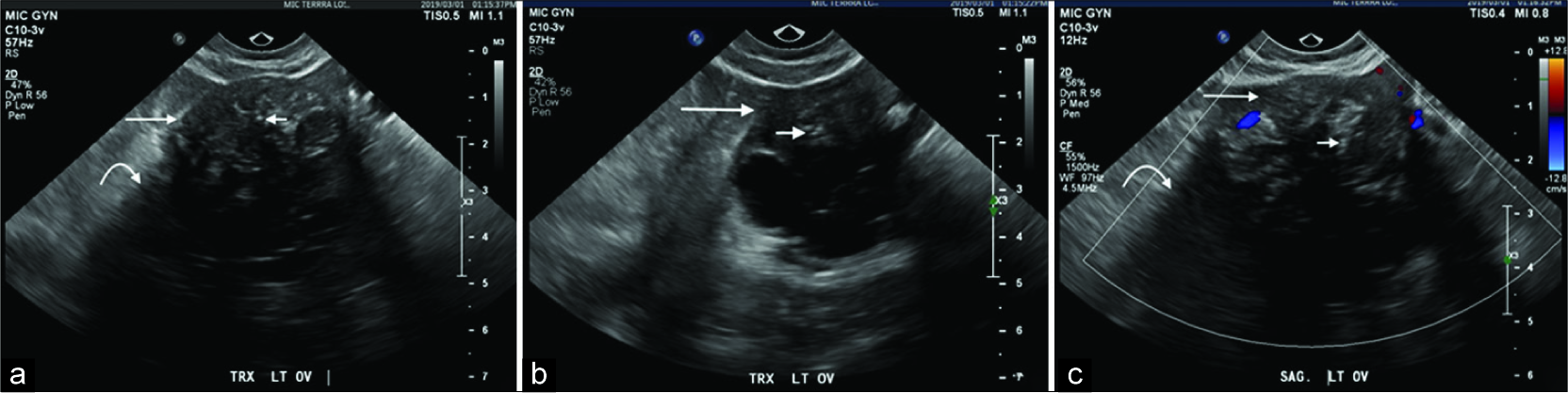
- A 40-year-old female underwent a pelvic ultrasound for abnormal uterine bleeding. Axial (a and b) and sagittal (c) ultrasound images demonstrate an incidental complex left adnexal lesion with a solid component demonstrating diffuse hypoechogenicity (long arrow) and posterior shadowing (curved arrow) in addition to punctate echogenic calcifications (short arrow) with internal Doppler enhancement.
A subsequent computed tomography confirmed the findings of an enhancing solid and cystic mass in the left adnexa [Figure 2]. The cystic component was again shown to be thinly septated. The solid component demonstrated enhancement with small punctate and amorphous calcifications. Following this, an magnetic resonance imaging (MRI) was performed [Figure 3]. This demonstrated subtle T1 hyperintensity within the septated fluid component best appreciated on fat saturation images and consistent with hemorrhage or proteinaceous/mucinous content. The solid component was T1 and markedly T2 hypointense (isointense to skeletal muscle) and homogenously enhancing, consistent with fibrous stromal tissue. No central necrosis was present.
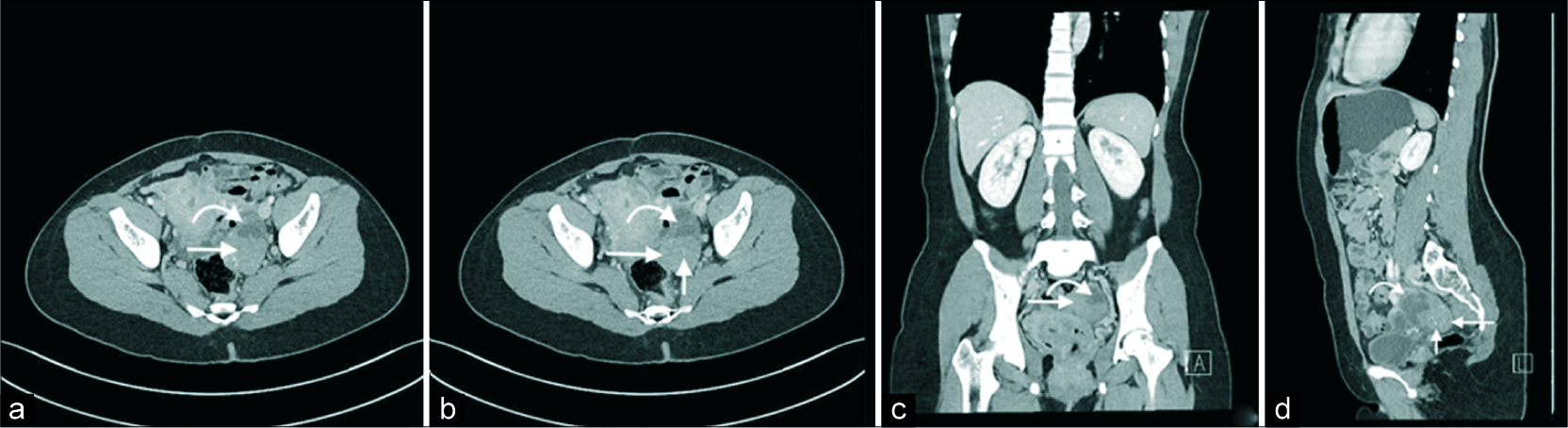
- A 40-year-old female with an incidental left adnexal lesion on ultrasound underwent subsequent computed tomography (CT) imaging. Axial (a and b), coronal (c), and sagittal (d) CT images demonstrate an enhancing solid (long arrow) and cystic (curved arrow) left adnexal mass with thinly septated amorphous and punctate calcifications (short arrow).
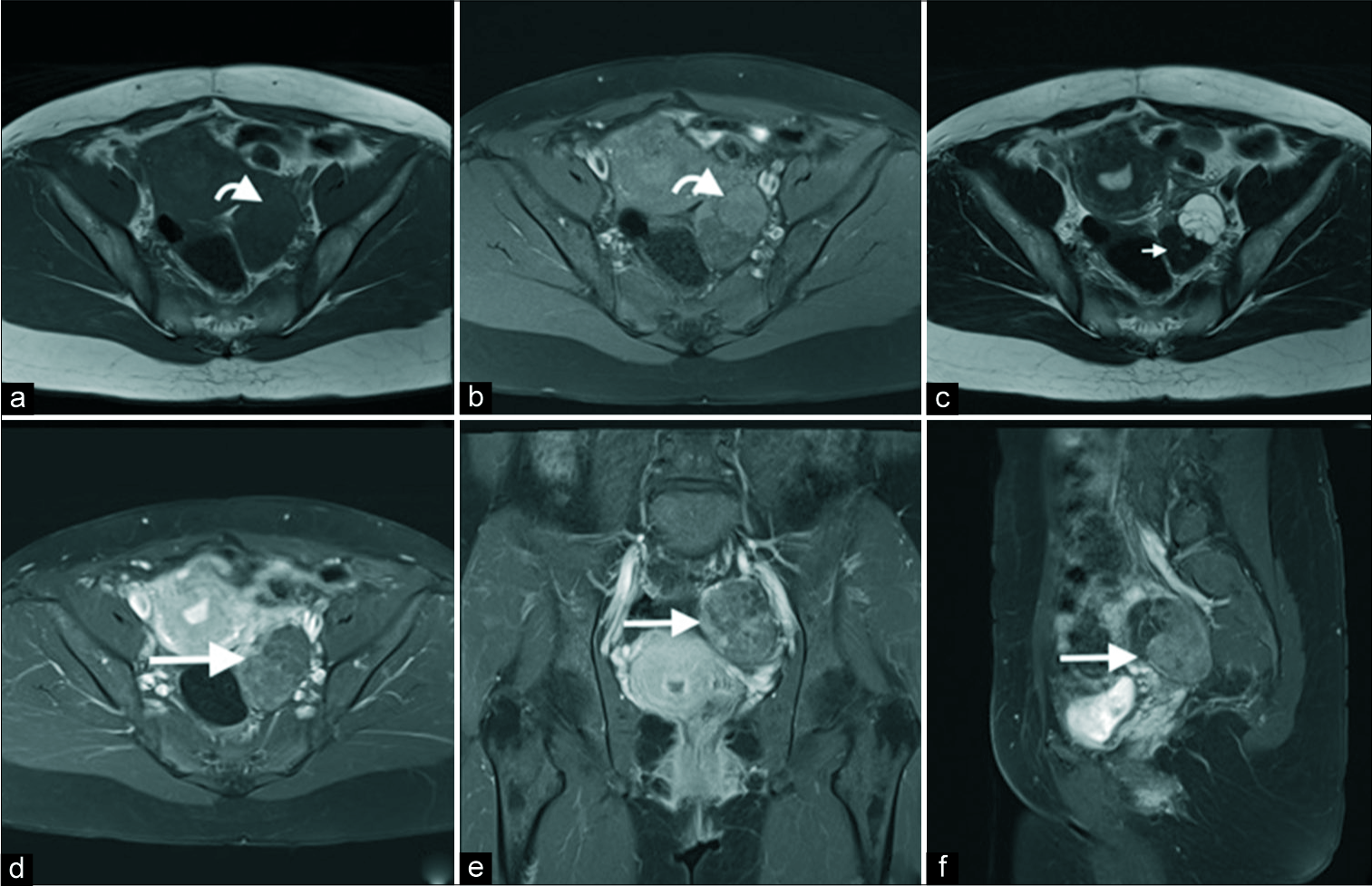
- A 40-year-old female with an incidental left adnexal lesion on ultrasound underwent subsequent magnetic resonance imaging (MRI). Axial T1 (a), T1 fat saturation (b), T2 (c), axial (d), coronal (e), and sagittal post-gadolinium MRI sequences demonstrate an enhancing cystic (anterior) and solid (posterior) lesion (d-f, long arrow) with mild T1 hyperintensity of the cystic component (a and b, curved arrow) and markedly T2 hypointensity of the dominant solid fibrous component (c, short arrow). No central necrosis was present.
The patient was referred to gynecology, where she underwent a total abdominal hysterectomy and left salpingo-oophorectomy with complete excision of the left adnexal lesion. Gross pathology confirmed a 7 × 5 × 4 cm mass with a solid tan-yellow component comprising 75% of the mass and a mucin containing cystic component representing the other 25% of the lesion. No hemorrhage or necrosis was identified. Corresponding histopathology confirmed the solid component consisted of transitional-type epithelium within a fibrous stroma characteristic of Brenner tumor and the cystic tumor showed a single row of uniform mucin-rich columnar cells with basal nuclei lining glands and cysts characteristic for mucinous cystadenoma [Figure 4]. The final diagnosis was benign mixed Brenner tumor and mucinous cystadenoma.
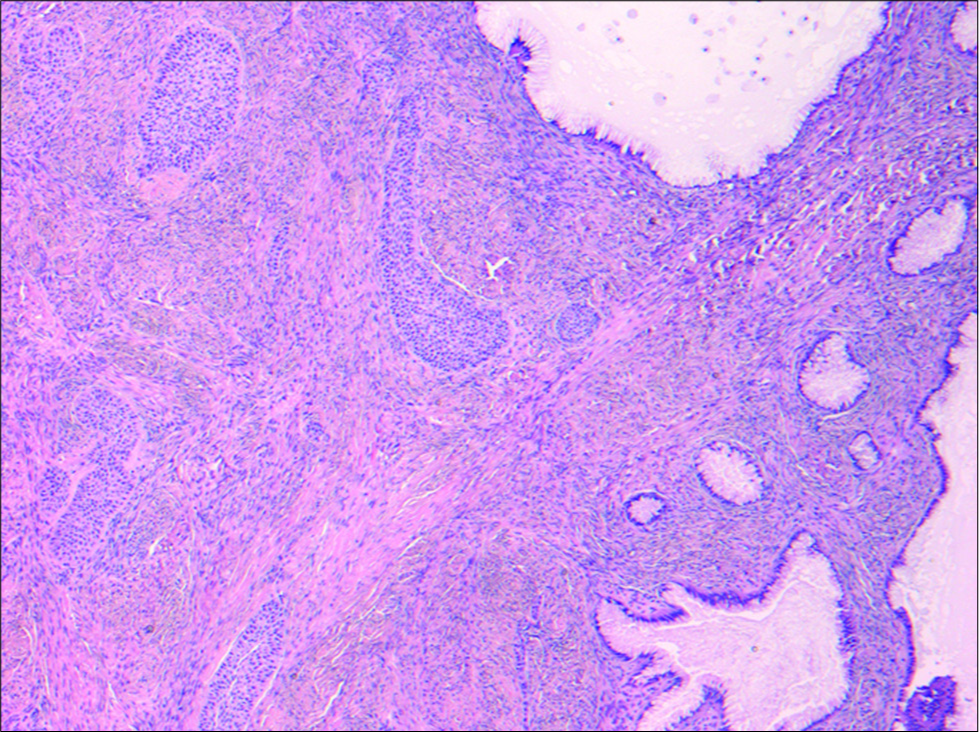
- A 40-year-old female underwent surgical excision of an incidental left adnexal lesion. Hematoxylin and eosin stain (50×) of the surgical specimen is demonstrated. Nests of transitional- type epithelium within a fibrous stroma characteristic for Brenner tumor were present (left) in association with a cystic tumor showing a single row of uniform mucin-rich columnar cells with basal nuclei lining glands and cysts characteristic for mucinous cystadenoma (right). The microscopic appearance correlated with macroscopic features of both solid and cystic areas within the tumor.
The patient had recovered completely by the 2-month clinical follow-up and has not received subsequent post-operative imaging. On clinical examination, the patient was noted to have a cervical polyp which was resected with a separate operation and the patient has had no ongoing abnormal bleeding. An endometrial biopsy was negative for malignancy.
DISCUSSION
Ovarian mucinous neoplasms consist of a lower percentage of epithelial tumors than serious neoplasms, representing approximately 10–15% of all ovarian tumors.[2] Approximately 80% of these lesions are benign. Brenner tumors are rare epithelial tumors accounting for around 2–3% of ovarian tumors.[5] The tumor consists of transitional cell epithelium surrounded by dense fibrous stroma and are rarely malignant.[2,5] Brenner tumors are frequently identified incidentally following resection of other lesions, with as many as 30% of Brenner tumors identified as combined lesions.[5] The lesions frequently present in postmenopausal patients with a mean age around 60 years.[6]
Three imaging patterns were identified by Kato et al. in a series of five benign mixed Brenner tumors and mucinous cystadenomas including: (1) A bulky solid mass adjacent to the mucinous cystic component; (2) a mural nodule at the peripheral of the cystic component; and (3) a cystic component without detectable solid component.[2] Our case demonstrates the uncommon presentation, in which the Brenner tumor is the dominant component and follows the first of the imaging patterns described by Kato et al.
The mucinous component frequently presents as a complex cystic mass on imaging. Mucinous neoplasms tend to be larger and more frequently multiseptated than simple serous neoplasms. These epithelial lesions can also have multiloculated cysts of varying signal depending on mucin concentration (“stained glass” appearance), with the subtle T1 hyperintensity well-demonstrated on the T1 fat saturation images in our study. MR subtraction imaging may also be helpful in cases, where proteinaceous content is suspected but not confirmed with pre-gadolinium imaging alone.[7] Features of the cystic component such as necrotic soft tissue, thickened mural nodularity, papillary projections within the cyst, associated peritoneal ascites, and peritoneal implants, wall invasion, or adenopathy favor a mucinous cystadenocarcinoma rather than an cystadenoma.[5]
The Brenner tumor component may have varying presentations from imperceptible to dominant, but are most frequently small (<2 cm).[5] In a series of 17 Brenner tumors, nearly half (8/17) were not seen sonographically.[3] Amorphous and/or punctate calcification occurs more frequently in Brenner tumors than other fibrous lesions.[3,5] The dense fibrous stroma of the Brenner tumor appears hypoechoic with shadowing on ultrasound and uniformly T2-hypointense on MRI, a feature which is well demonstrated in our lesion. Post-contrast imaging most often demonstrates mild-to-moderate homogenous enhancement, another feature well-demonstrated in our case.[5]
The differential diagnosis for a mixed Brenner tumor and mucinous cystadenoma would include an ovarian cystadenofibroma and fibroma-thecoma with internal cystic degeneration. These neoplasms can demonstrate both solid and cystic components, with the solid components showing similarly low T2 signal intensity (isointense to skeletal muscle) due to fibrous stromal tissue. These lesions are challenging to confidently differentiate on imaging alone. Gynecology consultation with view for surgical resection is appropriate, particularly if complex components are identified within the cystic portion of the lesion.[8,9]
CONCLUSION
Benign mixed Brenner tumor and mucinous cystadenomas are uncommon lesions which are rarely described in the radiology literature. The lesions present as one of three lesion types with varying degrees of solid and cystic components. The solid component often demonstrates imaging features typical of fibrous stroma including hypoechogenicity with shadowing on ultrasound and marked T2 hypointensity on MRI. Gynecology referral with view for surgical resection is clinically appropriate.
Declaration of patient consent
Patient consent is not required as patient identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Exploring the histogenesis of ovarian mucinous and transitional cell (Brenner) neoplasms and their relationship with walthard cell nests: A study of 120 tumors. Arch Pathol Lab Med. 2008;132:1753-60.
- [Google Scholar]
- Ovarian mucinous cystadenoma coexisting with benign Brenner tumor: MR imaging findings. Abdom Imaging. 2013;38:412-6.
- [CrossRef] [PubMed] [Google Scholar]
- Brenner tumors of the ovary: Sonographic and computed tomographic imaging features. J Ultrasound Med. 2006;25:1245-51.
- [CrossRef] [PubMed] [Google Scholar]
- Brenner's tumor associated with ovarian mucinous cystadenoma reaching a huge size in postmenopausal woman. J Cancer Res Ther. 2015;11:1030.
- [CrossRef] [PubMed] [Google Scholar]
- CT and MR imaging of ovarian tumors with emphasis on differential diagnosis. Radiographics. 2002;22:1305-25.
- [CrossRef] [PubMed] [Google Scholar]
- Brenner tumor of the ovary: CT and MR findings. J Comput Assist Tomogr. 2000;24:72-6.
- [CrossRef] [PubMed] [Google Scholar]
- Deep infiltrating endometriosis: Role of magnetic resonance subtraction imaging. Quant Imaging Med Surg. 2018;8:722-3.
- [CrossRef] [PubMed] [Google Scholar]
- Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of radiologists in ultrasound consensus conference statement. Radiology. 2010;256:943-54.
- [CrossRef] [PubMed] [Google Scholar]
- O-RADS US risk stratification and management system: A consensus guideline from the ACR ovarian-adnexal reporting and data system committee. Radiology. 2020;294:168-85.
- [CrossRef] [PubMed] [Google Scholar]






