Translate this page into:
Pulmonary Tumor Thrombotic Microangiopathy: Clinical, Radiologic, and Histologic Correlation
Address for correspondence: Prof. Nader Kamangar, Division of Pulmonary and Critical Care Medicine, UCLA – Olive View Medical Center, David Geffen School of Medicine at UCLA, 14445 Olive View Drive, Room 2B-182, Sylmar, California 91342, USA. E-mail: kamangar@ucla.edu
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Pulmonary tumor thrombotic microangiopathy (PTTM) is a clinicopathologic disease entity in which the tumor cells embolize to the pulmonary vasculature leading to a series of maladaptive reactions including the activation of coagulation and fibrocellular intimal thickening. The resultant stenosis of blood vessels leads to pulmonary hypertension and eventual death from cor pulmonale. In this report, we present a case of PTTM presenting as the initial manifestation of metastatic gastric carcinoma in a young man. Although unusual in its occurrence as the initial manifestation of gastric carcinoma, the case is illustrative in its clinical, radiological and histological presentation.
Keywords
Cancer
embolism
pulmonary hypertension

INTRODUCTION
Pulmonary tumor thrombotic microangiopathy (PTTM) is a clinicopathologic disease entity formally described in 1990 by von Herbay et al.[1] The pathophysiology starts with the embolization of tumor cells to pulmonary vasculature, followed by a series of maladaptive reactions including the activation of coagulation and vessel fibrocellular intimal thickening. The subsequent stenosis of blood vessels leads to pulmonary hypertension (PH) and eventual death from cor pulmonale. Gastric cancer is the most commonly associated malignancy. PTTM leads to death within a few weeks of admission and antemortem diagnosis has been the central challenge. The true prevalence of the disease is unknown and there is information to suggest that it may be higher than the current estimates. A significant amount of the current literature originates in Japan where the incidence of gastric cancer is high. We report a biopsy-proven case of a Latino patient with a severe presentation of PTTM to highlight the clinical, radiologic, and pathologic features of this rare but fatal disease.
A 42-year-old previously healthy man presented to the hospital with 6 weeks of progressively worsening exertional dyspnea and non-productive cough. On review of systems, he noted a gnawing epigastric pain, a 10-pound weight loss, and subjective fevers in the same time period. Prior to the onset of his symptoms, he had a normal exercise tolerance and was able to perform tasks at work, including lifting heavy machinery. His past medical history was non-significant and his medications included ibuprofen infrequently taken for back pain. There was no history of tobacco use, significant alcohol consumption, or any illicit drug use. He denied any recent travel or prior exposure to tuberculosis.
The patient appeared to be in mild distress, but was able to speak in full sentences. His vital signs were normal except for a respiratory rate of 24 breaths per minute and oxygen saturation of 86% on room air. Cardiovascular examination was notable for jugular venous distension to the angle of the mandible. A parasternal heave, an accentuated P2 component of the S2, and an S3 were appreciated. Lung auscultation revealed fine bibasilar rales. The remainder of the examination was unremarkable.
Transthoracic echocardiography revealed severe right ventricular dilatation with flattening of the interventricular septum and an estimated pulmonary artery systolic pressure (PASP) of 75 mmHg.
Chest radiograph demonstrated diffuse fine reticulonodular opacities as well as prominent hila [Figure 1]. Contrast-enhanced chest computed tomography (CT) revealed ill-defined centrilobular micronodules, diffuse smooth interlobular septal thickening, and patchy peribronchovascular ground glass opacities. Given the imaging findings, the decision was made for the patient to undergo a surgical lung biopsy. Histologic analysis was consistent with pulmonary tumor thrombotic microangiopathy (PTTM) secondary to gastric adenocarcinoma.
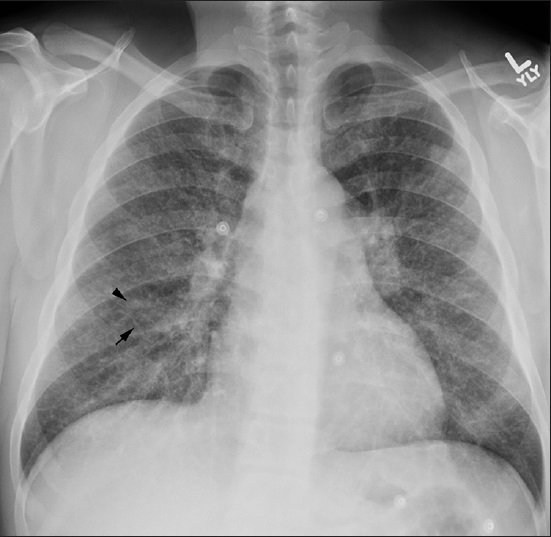
- 42-year-old previously healthy man presented to the hospital with 6 weeks of progressively worsening exertional dyspnea and non-productive cough diagnosed with pulmonary tumor thrombotic microangiopathy (PTTM) secondary to gastric adenocarcinoma. Chest X-ray posterioanterior (PA) view shows diffuse fine reticular (arrow) and nodular (arrowhead) opacifications.
Two weeks after admission, the patient developed worsening hypoxemic respiratory failure requiring transfer to the intensive care unit where he was found to have progressive and refractory obstructive shock. Despite numerous interventions, including systemic corticosteroids, anticoagulation, and application of inhaled nitric oxide (iNO), the patient succumbed to cardiac arrest and died.
RADIOLOGIC FEATURES
Contrast-enhanced chest CT revealed diffuse ill-defined centrilobular micronodules, diffuse smooth interlobular septal thickening, and patchy peribronchovascular ground glass opacities [Figures 2 and 3]. Mediastinal views were most notable for an enlarged main pulmonary artery, diffuse mediastinal and hilar lymphadenopathy, and evidence of right heart dysfunction as manifested by flattening of the interventricular septum and contrast reflux into the inferior vena cava and dilated hepatic veins. Abdominal CT scan showed diffuse gastric wall thickening and enlarged perigastric and para-aortic lymph nodes.
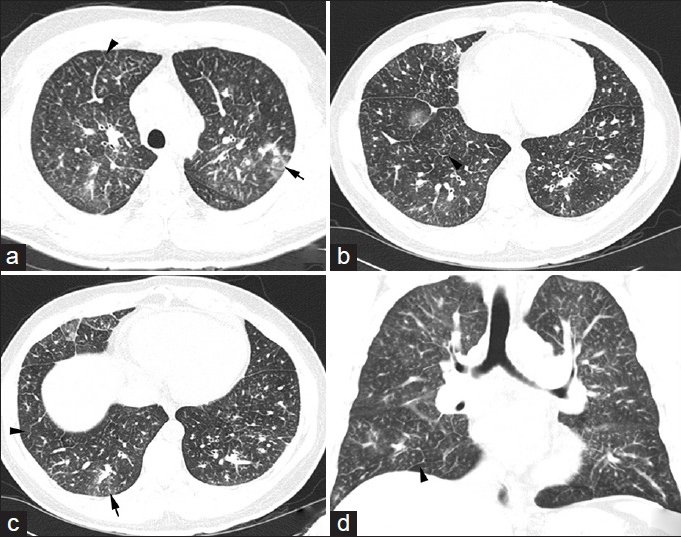
- 42-year-old previously healthy man presented to the hospital with 6 weeks of progressively worsening exertional dyspnea and non-productive cough diagnosed with pulmonary tumor thrombotic microangiopathy (PTTM) secondary to gastric adenocarcinoma. CT chest, lung window, (a-c) Axial slices show interlobular septal thickening (arrowheads) and centrilobular ground glass opacities (arrows), (d) Coronal view shows the same findings described above.
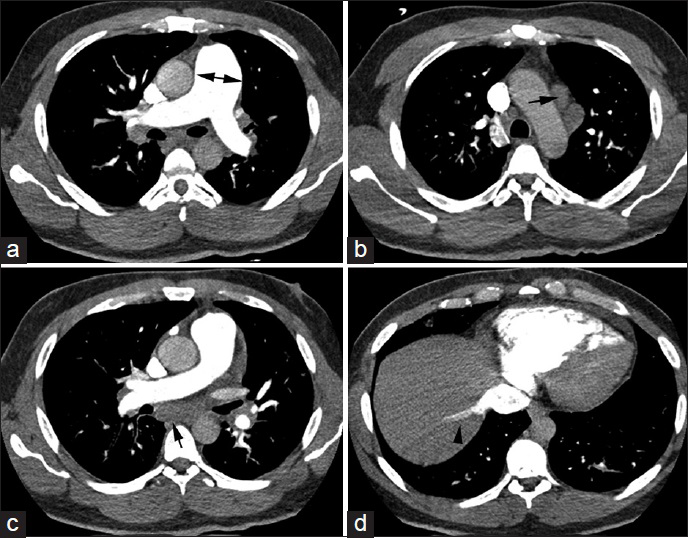
- 42-year-old previously healthy man presented to the hospital with 6 weeks of progressively worsening exertional dyspnea and non-productive cough diagnosed with pulmonary tumor thrombotic microangiopathy (PTTM) secondary to gastric adenocarcinoma. CT chest, soft tissue window, axial slices in all panels, (a) shows an enlarged pulmonary artery (double-headed arrow), (b and c) reveal mediastinal lymphadenopathy (arrows), (d) shows reflux of contrast into the inferior vena cava (arrowhead).
PATHOLOGIC FEATURES
Histopathologic examination demonstrated extensive lymphovascular involvement with poorly differentiated adenocarcinoma and evidence of proliferation of intimal fibromuscular cells and extensive intraluminal tumor [Figure 4]. The neoplastic cells were immunopositive for cytokeratin AE1/AE3, cytokeratin 7 (CK7), cytokeratin 19 (CK19), and cytokeratin 20 (CK20) stains, while they were negative for thyroid transcription factor 1 (TTF1), paired box 8 (PAX8), and prostate specific antigen. Postmortem microscopic examination revealed invasive, poorly differentiated adenocarcinoma with signet ring cells extending through the wall into the perigastric fat, along with extensive lymphovascular involvement. Periodic acid-Schiff and mucicarmine stains were positive for mucin within the tumor cells [Figure 5].
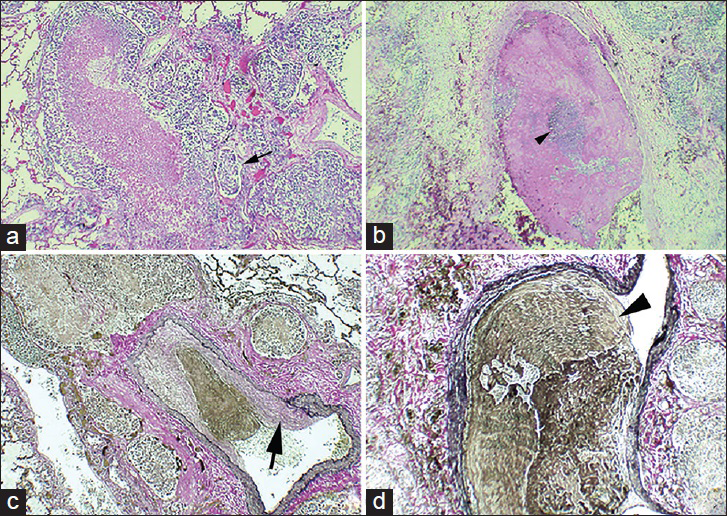
- 42-year-old previously healthy man presented to the hospital with 6 weeks of progressively worsening exertional dyspnea and non-productive cough diagnosed with pulmonary tumor thrombotic microangiopathy (PTTM) secondary to gastric adenocarcinoma. (a) Hematoxylin and eosin stained tissue (10×) shows medium sized blood vessels (small arrow) containing tumor emboli. (b), Hematoxylin and eosin stained tissue (20×) shows a singular blood vessel containing nests of tumor cells (small arrowhead) surrounded by fibrin debris. (c) Verhoeff's elastic stained tissue shows tumor thrombus with fibrocellular intimal proliferation (large arrow) within blood vessel (20×). (d) shows the same blood vessel shown in (b) stained with Verhoeff's elastic stain to highlight vessel wall (large arrowhead) (40×).
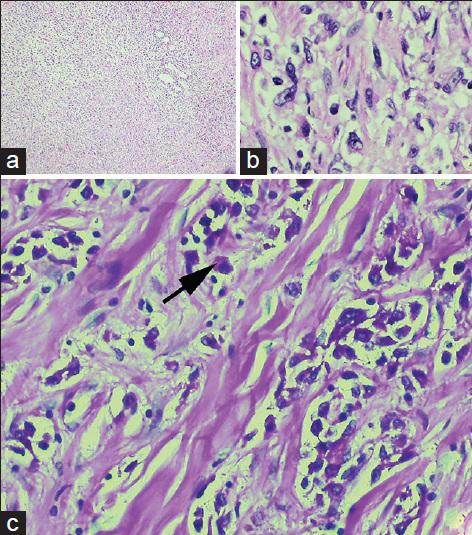
- 42-year-old previously healthy man presented to the hospital with 6 weeks of progressively worsening exertional dyspnea and non-productive cough diagnosed with pulmonary tumor thrombotic microangiopathy (PTTM) secondary to gastric adenocarcinoma. (a) Hematoxylin and eosin (H and E) stain stained tissue (20×) shows sheets of tumor cells seen at low power. (b) H and E stain (40×) reveals tumor cells of gastric origin. (c) Periodic acid-Schiff (PAS) stained gastric tissue with digestion at high power (40×) reveals intracytoplasmic mucin positivity (arrow).
DISCUSSION
PTTM is a clinical, radiographic, and pathologic entity, suspected in the appropriate clinical setting and confirmed by lung biopsy showing pulmonary tumor emboli within pulmonary vasculature surrounded by fibrin deposition and fibrocellular intimal proliferation. The term PTTM was originally coined by von Herbay et al., in 1989 after a review of 630 consecutive autopsy cases of carcinoma. The prevalence of PTTM was found to be 3.3% (total 21 cases). The most common malignancy associated with PTTM is gastric adenocarcinoma, with an estimated prevalence of 16.7% and 26.8% in two well-cited autopsy series.[12] Other commonly associated malignancies include those of the lung, breast, colon, and pancreas, among others. The second most commonly associated malignancy by absolute number was lung adenocarcinoma.[1] Commonly reported symptoms of PTTM are dyspnea on exertion and a non-productive cough. Symptoms begin anywhere from 3 weeks to 6 months prior to presentation.[123]
Due to the rapidity of progression, the diagnosis of PTTM is often made postmortem. Only a few case reports describe the antemortem diagnosis and attempts at treatment.[34] In one case report, the diagnosis of PTTM was made antemortem by video-assisted thoracoscopic surgery (VATS).[3] Miyano et al., reported an antemortem diagnosis of PTTM due to recurrent gastric cancer, which responded to treatment with systemic corticosteroids, anticoagulation, and antiplatelet therapy.[4] PTTM remains a nearly universally fatal disease process and almost all patients rapidly succumb to death due to severe hypoxia within 1–2 weeks of the onset of dyspnea.[12]
The diagnosis of PTTM cannot be established by radiography alone owing to the non-specific features observed with the commonly available radiologic studies.[5] Patients are generally evaluated with a chest radiograph and CT scan. Chest radiography often shows diffuse reticular and nodular opacities. Kerley B lines, representing edema of the interlobular septa and pleural effusions, have also been observed.[567] Chest CT commonly shows ground glass centrilobular micronodules representing fibrocellular intimal hyperplasia of the pulmonary arteries in response to embolized tumor cells. Also, intra- and interlobular septal thickening, as well as mediastinal and hilar lymphadenopathy signifying malignant spread are commonly seen. Fibrocellular intimal proliferation within the small arteries and arterioles may also give rise to a tree-in-bud centrilobular pattern, given that the pulmonary arteries and small airways travel together and, therefore, share the same branching morphology.[567] Chest CT findings of PH and right heart failure are evidenced by prominent pulmonary artery trunk, right ventricular enlargement, deviation of the interventricular septum, and reflux of contrast into the inferior vena cava. Other diagnostic modalities such as positron emission tomography with 18-fluorodeoxyglucose-positron emission tomography (FDG-PET) scanning have been used; however, the data are limited. We found one case report in which the scan revealed evidence of diffuse metastatic disease but failed to identify the primary tumor, a gastric adenocarcinoma, which was later discovered on postmortem examination.[7] Despite the pathophysiologic distinction between PTTM and lymphangitic carcinomatosis, the radiologic correlates of the latter entity, which include diffuse micronodular disease, can be similar to those of the former. In fact, 18 out of 21 cases of PTTM in the von Herbay autopsy series also showed evidence of lymphangitic carcinomatosis, suggesting a relation between the two disease entities.[1] Despite the aforementioned radiographic features, there are no findings that are diagnostic of PTTM.
The pathogenesis of PTTM starts with the formation of microscopic tumor cell emboli, which cause small pulmonary vessel injury and induce local activation of coagulation and recruitment of inflammatory markers, eventually leading to fibrocellular intimal proliferation.[1489] The resultant stenosis of the pulmonary capillaries and arterioles increases pulmonary vascular resistance causing PH. The activation of coagulation by tumor emboli and maladaptive inflammatory response is the defining feature of PTTM. Also, it is evident that the tumor emboli increase the expression of vascular endothelial growth factor (VEGF), tissue factor (TF), and platelet-derived growth factor (PDGF) on the tumor cells.[48] The clinical significance of these findings is incompletely understood. The histopathologic diagnosis to date is the most specific means of diagnosis and may be established by performing a surgical biopsy with a wedge resection, VATS lung biopsy,[3] or a transbronchial biopsy, if safely permissible depending on the pulmonary pressures.[410]
CONCLUSIONS
PTTM should be suspected in cancer patients with acute or subacute onset of respiratory insufficiency and severe pulmonary hypertension in the absence of pulmonary embolism. Although extremely challenging, early diagnosis may allow time for initiation of interventions that may alleviate symptoms and potentially improve outcomes. Chest radiography and CT scan are appropriate initial studies followed by diagnosis of the primary malignancy or surgical lung biopsy, if feasible. Vascular endothelial growth factor (VEGF), tissue factor (TF), and platelet-derived growth factor (PDGF) may play a role in delineating future treatments. There seems to be a variation in the clinical expression of this disease and though patients may have the characteristic pathologic findings, they may not always exhibit the symptomatology. The determinants of the severity of illness are unknown. No treatment has been found to be of significant benefit. The disease progresses rapidly and is often fatal within a few weeks of presentation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2015/5/1/44/161978
REFERENCES
- Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990;66:587-92.
- [Google Scholar]
- Pulmonary tumor thrombotic microangiopathy in patients with gastric carcinoma: An analysis of 6 autopsy cases and review of the literature. Pathol Res Pract. 2010;206:682-9.
- [Google Scholar]
- Pulmonary tumor thrombotic microangiopathy diagnosed antemortem and treated with combination chemotherapy. Intern Med. 2012;51:2767-70.
- [Google Scholar]
- Thrombotic microangiopathy of pulmonary tumors: A vascular cause of tree-in-bud pattern on CT. AJR Am J Roentgenol. 2002;179:897-9.
- [Google Scholar]
- CT findings in lymphangitic carcinomatosis of the lung: Correlation with histologic findings and pulmonary function tests. AJR Am J Roentgenol. 1992;158:1217-22.
- [Google Scholar]
- Pulmonary tumor thrombotic microangiopathy: FDG-PET/CT findings. Clin Nucl Med. 2009;34:175-7.
- [Google Scholar]
- Pulmonary tumor thrombotic microangiopathy caused by a gastric carcinoma expressing vascular endothelial growth factor and tissue factor. Pathol Int. 2005;55:27-31.
- [Google Scholar]
- Pulmonary tumor thrombotic microangiopathy induced by gastric carcinoma: Morphometric and immunohistochemical analysis of six autopsy cases. Diagn Pathol. 2011;6:27.
- [Google Scholar]
- A case of pulmonary tumor thrombotic microangiopathy diagnosed by transbronchial lung biopsy and treated with chemotherapy and long-term oxygen and anticoagulation therapies. Case Rep Pulmonol. 2013;2013:259080.
- [Google Scholar]






