Translate this page into:
Pilomyxoid Astrocytoma Occurring in the Third Ventricle
Address for correspondence: Dr. Sunseob Choi, Department of Radiology, Dong-A University Medical Center, 1,3-ga, Dongdaeshin-dong, Seo-gu, Busan - 602-715, Korea. E-mail: sschoi4048@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Pilomyxoid astrocytoma (PMA) is a rare central nervous system tumor that has been included in the 2007 World Health Organization Classification of Tumors of the Central Nervous System. Due to its more aggressive behavior, PMA is classified as Grade II neoplasm by the World Health Organization. PMA predominantly affects the hypothalamic/chiasmatic region and occurs in children (mean age of occurrence = 10 months). We report a case of a 24-year-old man who presented with headache, nausea, and vomiting. Brain CT and MRI revealed a mass occupying only the third ventricle. We performed partial resection. Histological findings, including monophasic growth with a myxoid background, and absence of Rosenthal fibers or eosinophilic granular bodies, as well as the strong positivity for glial fibrillary acidic protein were consistent with PMA.
Keywords
Pilocytic astrocytoma
pilomyxoid astrocytoma
third ventricle

INTRODUCTION
Pilomyxoid astrocytoma (PMA) has been included as a novel clinico-pathological entity in the 2007 World Health Organization Classification of Tumors of the Central Nervous System.[1] PMA has a poor prognosis when compared to pilocytic astrocytoma (PA) because of the propensity of the tumor to metastasize through the cerebrospinal fluid (CSF).[2] Thus, the differentiation between PMA and PA is important. PMA occurs typically in the hypothalamic/chiasmatic region similar to the pilocytic variety, and there have been a few reports regarding the image features that differentiate PMA from PA.[3] We report a rare case of PMA of the third ventricle in a 24-year-old male.
CASE REPORT
A 24-year-old male presented with a 3-month history of dull headache that had progressively worsened over the past 1 week and was associated with nausea and vomiting. On examination, he had no neurological deficit. Noncontrast-enhanced CT scan showed mild hydrocephalus with a uniform hypoattenuated lesion in the third ventricle [Figure 1a]. There was no hemorrhage or calcification. Brain MRI showed a well-defined mass measuring about 2.7 × 2.4 × 2.3 cm in the third ventricle causing hydrocephalus. It showed hypointensity on a T1-weighted image, hyperintensity on a T2-weighted image, and thick peripheral contrast enhancement. There was no dissemination on brain [Figure 1b–d] and spinal MRI [Figure 1e and f]. The radiological differential diagnosis was PMA, PA, germinoma, or craniopharyngioma with difficult differentiation.
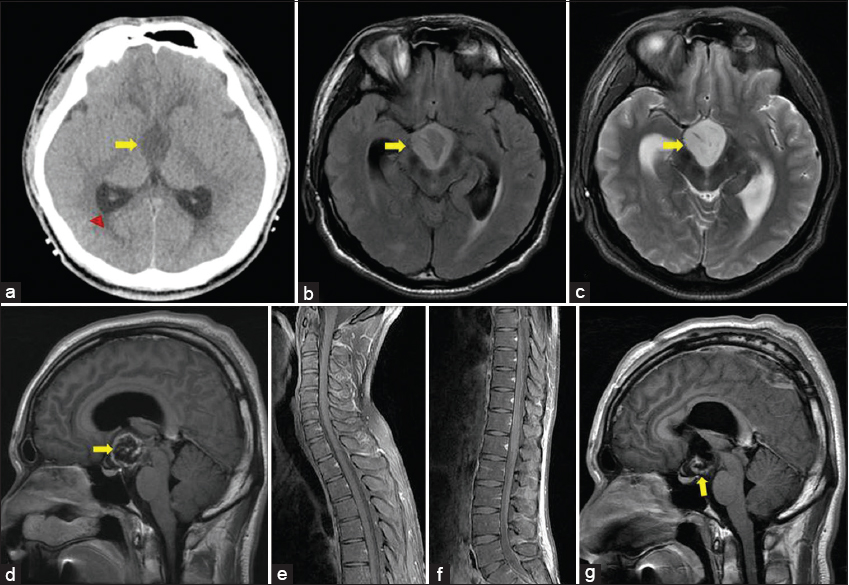
- 24-year-old male presented with a 3-month history of dull headache that had progressively worsened over 1 week with nausea and vomiting. (a) Axial noncontrast CT image shows a homogenously hypoattenuated mass in the third ventricle without hemorrhage or calcification (arrow). Secondary hydrocephalus is revealed (arrowhead). (b) Axial FLAIR image shows a mass of high signal intensity in the third ventricle (arrow). (c) T2-weighted image shows the mass to be hyperintense (arrow). (d) Contrast-enhanced T1-weighted image shows thick peripheral enhancement (arrow). (e, f) Contrast-enhanced T1-weighted images of spine show no cerebrospinal fluid dissemination. (g) Postoperative contrast-enhanced T1-weighted image shows remnant tumor in the floor of the third ventricle (arrow).
The patient underwent a navigation-guided craniotomy and partial resection of the tumor. At surgery, most of the lesion was found to be soft and whitish in color. Although it looked well circumscribed initially, the tumor infiltrated the walls of the third ventricle. We left the tumor which was adherent to the floor of the third ventricle to minimize the hypothalamus injury [Figure 1g]. An external ventricular drain was inserted.
Histologically, it consisted of monomorphous and bipolar piloid cells in a substantial amount of myxoid background [Figure 2a]. No Rosenthal fibers or eosinophilic granular bodies were found. Immunohistochemically, the tumor cells were strongly positive for glial fibrillary acidic protein [Figure 2b]. Anti-Ki-67 monoclonal antibody (MIB-1) is one of the most useful markers for evaluating cellular proliferation in human neoplasm, including intracranial tumors. Rathi et al., demonstrated significant relation between tumor grade and MIB-1 labeling index.[4] They found that mean MIB-1 labeling index values of astrocytomas were lower than those of anaplastic astrocytomas and glioblastomas. In our case, the fraction of MIB-1–positive tumor cells (MIB-1 labeling index) was low [Figure 2c]. The pathological diagnosis was a PMA.
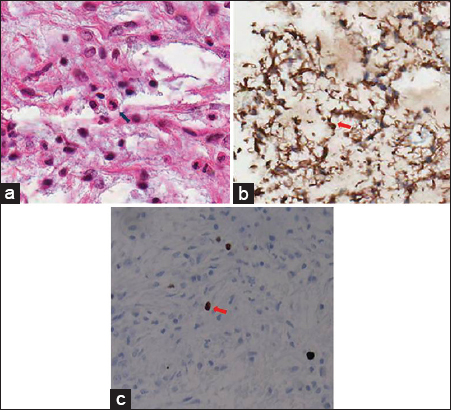
- 24-year-old male presented with a 3-month history of dull headache that had progressively worsened over 1 week with nausea and vomiting. (a) Photomicrograph (hematoxylin–eosin stain, original magnification ×400) shows overall monomorphic tumor cells (arrow) and prominent myxoid background without Rosenthal fibers or eosinophilic granular bodies. (b) Immunohistochemically, the tumor is strongly positive for glial fibrillary acidic protein, confirming its astrocytic origin (glial fibrillary acidic protein, original magnification ×400, arrow). (c) Proliferation of anti-Ki-67 monoclonal antibody (MIB-1)-positive tumor cells is low (MIB-1, original magnification ×400, arrow).
In the postoperative course, the patient clinically improved. Radiotherapy was selected to treat the residual tumor.
DISCUSSION
PMA is a variant of PA with unique histopathologic and clinical characteristics. Unlike PA, PMA shows a prominent mucoid matrix and an angiocentric pattern of monomorphous bipolar tumor cells without eosinophilic granular bodies, Rosenthal fibers, or hyaline droplets.[1] Rosenthal fibers and eosinophilic granular bodies are characteristic of PA, and calcification is more common in PA than PMA.[5] The tumor predominantly occurs in children of different age groups, ranging from the newborn to young adult.[3] Due to the more aggressive behavior, PMA is classified as Grade II neoplasm by the World Health Organization. Komotar et al., reported that the mean duration of progression-free survival for PMA and PA was 26 and 147 months, respectively.[2] PMA is usually found at the hypothalamic/chiasmatic region, but may occur anywhere along the central neuroaxis. However, PMA located only in the third ventricle is extremely rare.
The reported radiologic findings of PMA are a well-defined, predominant solid mass, hypointense on T1-weighted images and hyperintense on T2-weighted images, and homogeneous contrast enhancement.[2345678] Morales et al., reported that unlike PA, PMA is predominantly solid, with only a minimal cystic component.[7] The presence of lesion, mainly solid, with non-enhancing portion of the tumor may be helpful differential features of PMA as compared to PA.[8] Other characteristics on imaging that help in differential diagnosis would be the predilection for suprasellar area, intratumoral hemorrhage, and early leptomeningeal dissemination.[38]
Similarly, T2-weighted image in our case demonstrated a bright hyperintense mass with well-defined margin. Marked hyperintensity on T2-weighted image may reflect the characteristic histopathologic finding of PMA with prominent myxoid component. However, contrast-enhanced T1-weighted image showed a solid mass with central necrosis and thick peripheral enhancement. There was no hemorrhage on noncontrast-enhanced CT scan.
It appears that it is difficult to differentiate PMA from PA on the basis of imaging findings. Linscott et al., reported a much broader imaging spectrum with variable contrast enhancement patterns including rim enhancement but no contrast enhancement, and so they concluded that there were no definitive pathognomonic image findings to distinguish PMA from PA in their study.[3] Moreover, Lee et al., reported that the two tumors could not be diff erentiated based on the presence of hemorrhage.[8]
The differential diagnosis of PMA in the third ventricle also includes meningioma, craniopharyngioma, or germ cell tumor. The mass in our case showed no fat or calcification, but demonstrated thick peripheral enhancement.
Several studies have reported advanced MRI findings of PMA. Horger et al., reported that apparent diffusion coefficient values and T2 signal intensity are generally higher in the latter, reflecting the proportion of myxoid matrix in these tumors.[9] Preliminary investigations with the use of MR spectroscopy in two cases of pediatric optic-chiasmatic PMA revealed an increase in choline concentration in the intratumoral region of PA in comparison with PMA.[10] They attributed this elevation to the more cellular components in PA in comparison with the myxoid background of PMA. Morales et al., reported that elevated choline/creatine outside PMA enhancing margin is usually present in high-grade tumors.[7]
Currently, there is no standard guideline on the management of patients with PMA. Whenever possible, gross total surgical resection remains the primary treatment. Adjuvant chemotherapy or radiotherapy may be considered in cases with subtotal excision or recurrence. Non-enhancing solid tumors give rise to diagnostic problem when they disseminate along the CSF space. Non-enhancing CSF dissemination is more apparent on a Fluid Attenuation Inversion Recovery (FLAIR) image.[8] Careful evaluation of signal changes of CSF to detect CSF dissemination on FLAIR images may be helpful for surgical planning.
CONCLUSION
We have reported a case of a 24-year-old male with a pilomyxoid astrocytoma (PMA) arising in the third ventricle. PMA is associated with a worse prognosis due to early recurrence and cerebrospinal dissemination. Recognition of this tumor may be of help to make an early diagnosis and appropriate treatment plan.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2015/5/1/41/161853
REFERENCES
- WHO Classification of Tumours of the Central Nervous System. (4th ed). Lyon: IARC Press; 2007. p. :20-1.
- [Google Scholar]
- Pilomyxoid astrocytoma: Diagnosis, prognosis, and management. Neurosurg Focus. 2005;18:E7.
- [Google Scholar]
- Pilomyxoid astrocytoma: Expanding the imaging spectrum. AJNR Am J Neuroradiol. 2008;29:1861-6.
- [Google Scholar]
- Proliferative index in astrocytic tumours. Indian J Pathol Microbiol. 2007;50:754-8.
- [Google Scholar]
- Pilocytic and pilomyxoid hypothalamic/chiasmatic astrocytomas. Neurosurgery. 2004;54:72-80.
- [Google Scholar]
- MR imaging characteristics of pilomyxoid astrocytomas. AJNR Am J Neuroradiol. 2003;24:1906-8.
- [Google Scholar]
- Magnetic resonance imaging and spectroscopy of pilomyxoid astrocytomas: Case reports and comparison with pilocytic astrocytomas. J Comput Assist Tomogr. 2007;31:682-7.
- [Google Scholar]
- Imaging characteristics of pilomyxoid astrocytomas in comparison with pilocytic astrocytomas. Eur J Radiol. 2011;79:311-6.
- [Google Scholar]
- T2 and DWI in pilocytic and pilomyxoid astrocytoma with pathologic correlation. Can J Neurol Sci. 2012;39:491-8.
- [Google Scholar]
- Proton magnetic resonance spectroscopic imaging in pediatric pilomyxoid astrocytoma. Childs Nerv Syst. 2005;21:404-9.
- [Google Scholar]






