Peripartum hemorrhage: Two cases of ruptured ovarian artery aneurysms with additional multifocal intact aneurysms

*Corresponding author: Bill S Majdalany, Department of Radiology, Emory University Hospital, Atlanta, Georgia, United States. Bmajdal@emory.edu
-
Received: ,
Accepted: ,
How to cite this article: Arleo TL, Peters GL, Kokabi N, Majdalany BS. Peripartum hemorrhage: Two cases of ruptured ovarian artery aneurysms with additional multifocal intact aneurysms. J Clin Imaging Sci 2022;12:10.
Abstract
We report two cases of peripartum ruptured ovarian artery aneurysms (OAA). One patient was treated through endovascular embolization and the other with percutaneous thrombin injection. Multiple additional unruptured OAAs were incidentally discovered in each patient. We describe the pathophysiologic basis for OAA rupture, approaches to treatment, and suggest management strategies for incidentally discovered ovarian aneurysms.
Keywords
Postpartum hemorrhage
Ovarian artery
Aneurysm
Percutaneous thrombin
Embolization
INTRODUCTION
Although first described in 1963, ovarian artery aneurysm (OAA) ruptures are rare with less than 30 reported cases in the literature.[1-3] Multigravida and the physiologic changes of pregnancy are thought to be major risk factors for OAA development, with most cases occurring near the intrapartum period.[1-5] Herein, two cases of OAA are presented. One patient was treated with percutaneous direct thrombin injection while the other underwent endovascular embolization. Both patients were found to have additional intact OAAs.
CASE REPORTS
Case 1
A 32-year-old woman, gravida 6 para 5, birthed a healthy infant via uncomplicated spontaneous vaginal delivery at an outside hospital. Five hours later she became hypotensive and postpartum hemorrhage was suspected, but exploratory laparotomy was unrevealing. She has transfused 4 units of packed red blood cells (pRBC), 3 units of fresh frozen plasma (FFP), and 2 units of platelets prior transferring to our institution. On arrival, her blood pressure was 119/74 mmHg with a pulse of 77 beats per minute (bpm) and hemoglobin of 8.3 g/dL. Computed tomography (CT) of the abdomen and pelvis revealed a 16.8 × 9.4 × 13.3 cm hematoma, an adjacent OAA, and an arteriovenous fistula to the right ovarian vein [Figure 1a]. A second OAA was present at the origin of the right ovarian artery [Figure 1b].

- Coronal CT of a 32-year-old woman, gravida 6 para 5, with post-partum hypotension, but negative exploratory laparotomy revealed (a) a ruptured right ovarian artery aneurysm (black arrow) and hematoma (white arrow). (b) An additional, unruptured aneurysm measuring 1.2 cm was present at the origin of the right ovarian artery (black arrowhead) with a more posterior view of the hematoma (white arrow).
Interventional radiology (IR) was consulted for embolization. Using a standard 5 French sheath and right common femoral arterial approach, the right ovarian artery was identified and selected with a 5 French catheter. Angiography confirmed both a proximal and a distal right OAA [Figure 2a]. A 2.4 French Progreat microcatheter (Terumo Medical, Tokyo, Japan) was advanced to the distal OAA for repeat angiography which revealed rapid filling of the right ovarian vein, in keeping with an arteriovenous fistula. Despite repeated attempts, the microcatheter could not cross the OAA given the vascular tortuosity. Access into the right internal jugular vein was established in standard fashion and a 7 French Destination sheath (Terumo Medical) was placed. The right ovarian vein was selected using a 5 French MPA catheter (Cook Medical) for venography which demonstrated retrograde flow into the pelvis. A 5.5 French Fogarty balloon catheter (Edwards Lifesciences, Irvine, CA) was advanced into the right ovarian vein cephalad to the OAA [Figure 2b]. Under direct ultrasound guidance, a 21-gauge Chiba needle (Cook Medical) was percutaneously advanced into the right OAA [Figures 2c and d]. The Fogarty balloon was inflated in the right ovarian vein, and 200 units of thrombin were injected into the OAA under direct ultrasound visualization [Figures 2e and f]. Direct ultrasound and right ovarian arteriography demonstrated ovarian artery patency without visualization of the OAA [Figures 2g and h]. Angiography of the left and right internal iliac arteries demonstrated diminutive uterine arteries bilaterally with aneurysms [Figures 3a-d].
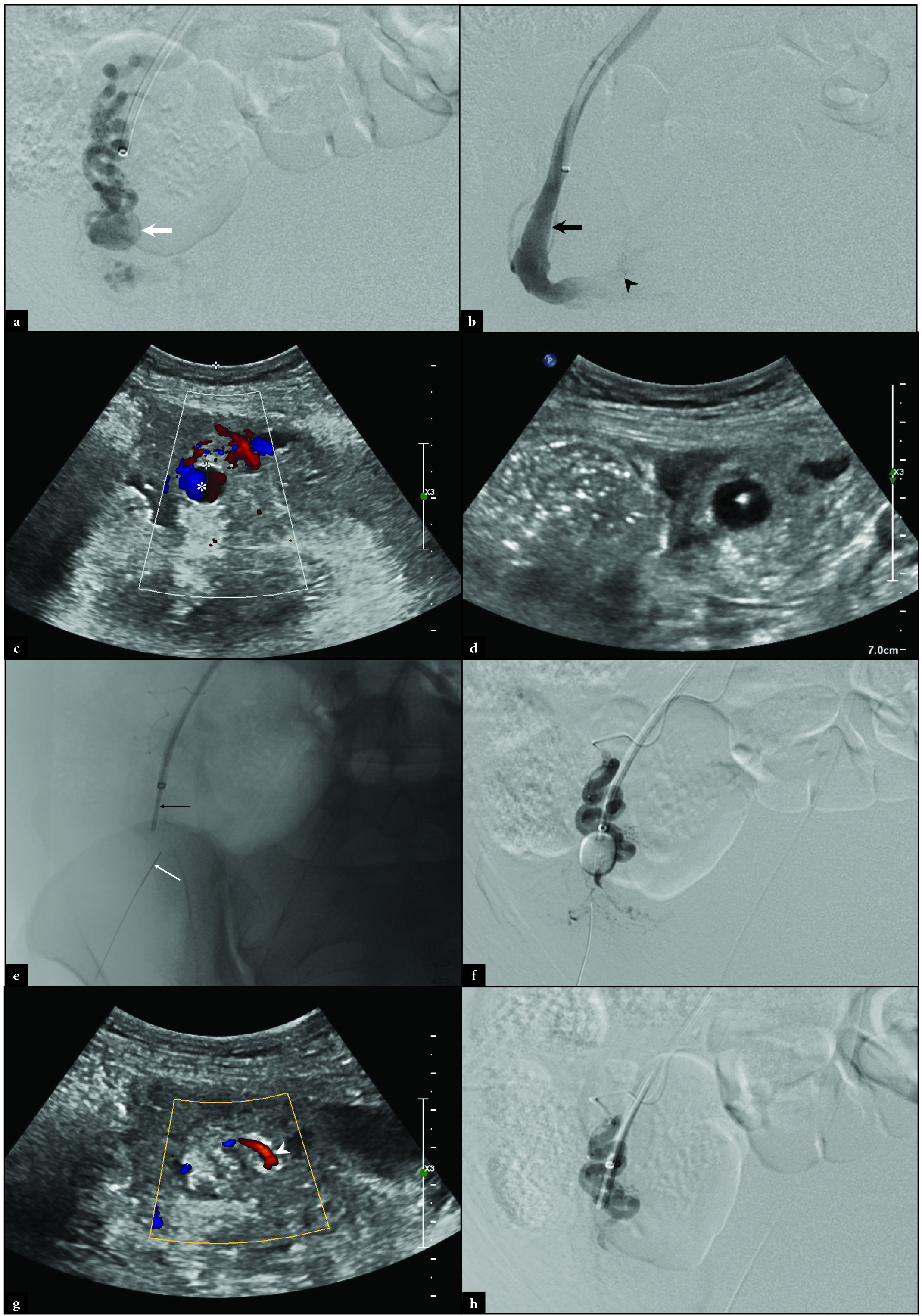
- Intraprocedural right ovarian arteriography revealing (a) a distal OAA (white arrow). Right ovarian venography reveals (b) a patent right ovarian vein (black arrow) with retrograde contrast flowing into the pelvis (black arrowhead). Direct ultrasound of the OAA demonstrates (c) the “ying-yang” sign (white asterisk) and (d) a 21-gauge Chiba needle placed under ultrasonographic guidance. Fluoroscopy demonstrates (e) the Chiba needle (thin white arrow) in the OAA with an uninflated Fogarty balloon (thin black arrow) in the ovarian vein. Digital subtraction right ovarian arteriography demonstrates (f) the inflated Fogarty balloon in the right ovarian vein with the Chiba needle in the OAA. Post-thrombin injection ultrasound shows (g) thrombosis of the right OAA with flow (white arrowhead) in the adjacent ovarian artery. Post-thrombin injection digital subtraction arteriography demonstrates (h) patency of the ovarian artery without visualization of the OAA.

- A 32-year-old woman with successful thrombin injection of a ruptured OAA underwent (a-b) right internal iliac and (c-d) left internal iliac completion angiography demonstrating uterine arteries that are smaller than the ovarian arteries and containing additional aneurysms (white arrows).
The patient was uneventfully discharged the next day. Two weeks later, CT imaging revealed no further hemorrhage but a persistent right OAA at the origin [Figure 4a]. The patient was followed clinically and repeat CT imaging 1 year later demonstrated complete resolution of the right OAA [Figure 4b].

- Follow up coronal CT imaging for a 32-year-old woman who underwent successful treatment for a right ovarian artery pseudoaneurysm rupture was performed. Two-week follow-up CT imaging (a) demonstrates resolution of the ruptured OAA, a residual proximal right ovarian artery aneurysm (black arrow), and an evolving hematoma (white arrow). One year follow-up CT imaging (b) demonstrates resolution of the proximal right ovarian artery aneurysm (black arrowhead) and hematoma.
Case 2
A 28-year-old woman, gravida 7 para 3, with no significant past medical history presented to an outside hospital with severe left flank pain 72 hours after giving birth via a normal spontaneous vaginal delivery. Outside CT imaging revealed an 11.6 × 5.6 × 8.1 cm retroperitoneal hematoma adjacent to a left ovarian artery aneurysm and multifocal, bilateral OAA [Figures 5a-d]. The patient was transferred to our facility with a blood pressure of 125/64 mmHg, the pulse of 65 bpm, and hemoglobin of 9.6 g/dL. IR was consulted for embolization of the ruptured left OAA. Using a standard left radial arterial approach, angiography was performed at the origin of the left and right ovarian arteries confirming proximal aneurysms [Figure 6a]. A 2.8 French Progreat microcatheter was advanced into the right ovarian artery and angiography demonstrated multifocal aneurysmal dilation which was embolized using Gelfoam slurry (Johnson and Johnson, New Brunswick, NJ) [Figure 6b]. A completion angiogram demonstrated nonopacification through the embolized right OAAs [Figure 6c]. Left ovarian arteriography revealed a distal OAA [Figure 6d]. The microcatheter was advanced distal to the OAA and embolized with a combination of Gelfoam slurry and Concerto micro coils (Medtronic, Minneapolis, MN) [Figure 6e]. The microcatheter was retracted proximally to the OAA and additional Concerto coils were placed. Completion of left ovarian angiography confirmed nonopacification of the left OAA [Figure 6f]. A final pelvic angiogram confirmed a congenitally absent right uterine artery and diminutive left uterine artery [Figure 6g].

- Axial CT of a 28-year-old woman, gravida 7 para 3, with severe post-partum left flank pain revealed (a-d) multifocal aneurysmal dilations of the proximal and distal ovarian arteries (black arrowheads) and an enlarged aneurysm (black arrow) at the site of hematoma (white arrows).
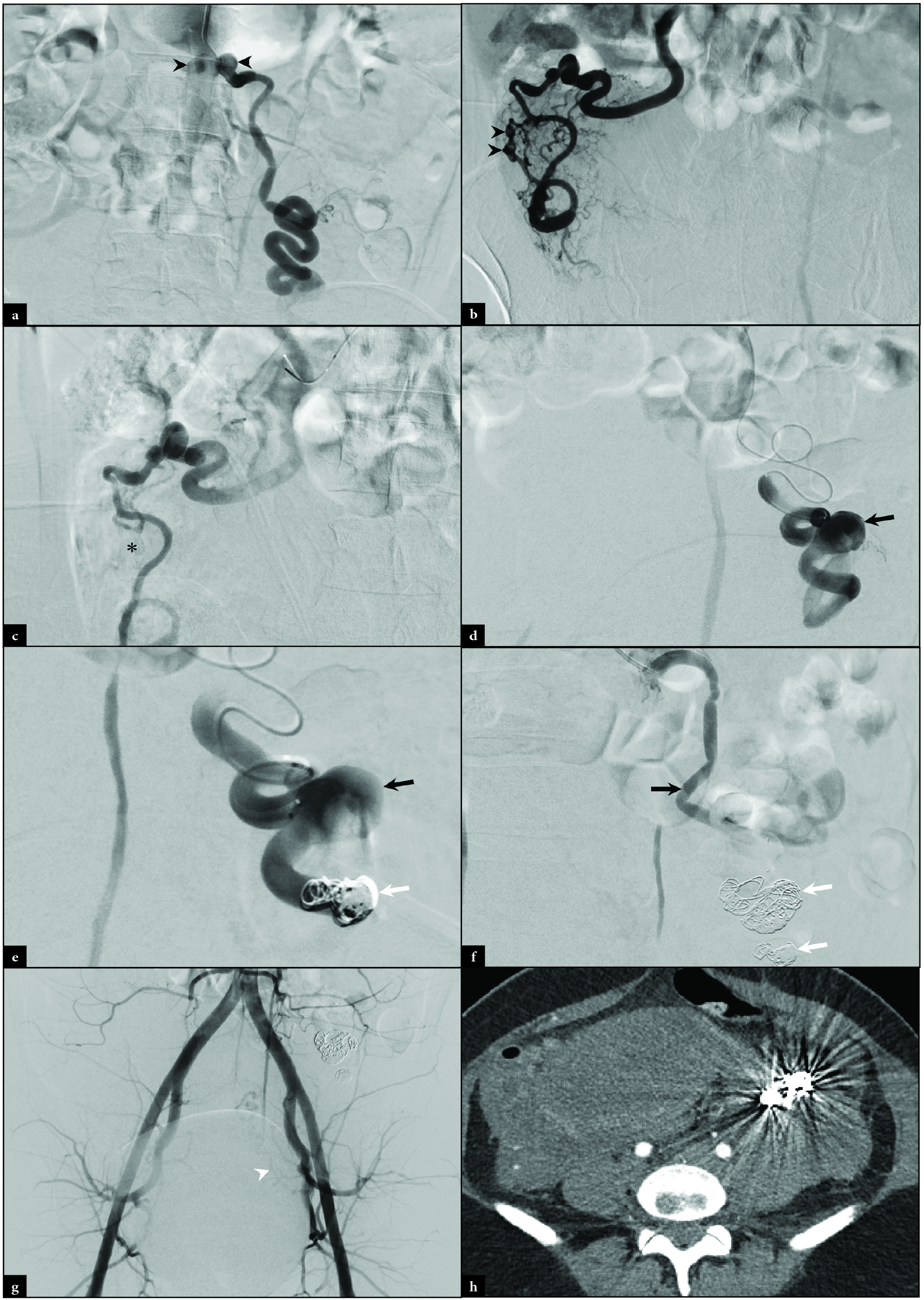
- Intraprocedural angiography of a 28-year-old woman, gravida 7 para 3, with severe post-partum left flank pain revealed at the level of the left and right ovarian arteries demonstrates (a) bilateral proximal aneurysms (black arrowheads). Right ovarian angiography demonstrates (b) multifocal aneurysmal dilation distally (black arrowheads). After Gelfoam embolization, right ovarian angiography demonstrates (c) contrast staining and static flow through the aneurysmal sites (black asterisk). Left ovarian arteriography demonstrates (d) a large aneurysm (black arrow). Left ovarian arteriography was performed after (e) distal Gelfoam and coil (white arrow) embolization. (f) Proximal coiling (white arrows) was also completed. Completion pelvic angiography (g) demonstrates an absent right uterine artery and diminutive left uterine artery (white arrowhead). Follow up axial CT demonstrates (h) artifact from coil embolization.
CT imaging 2 days later demonstrated no active bleeding but the persistence of multifocal OAA with coils in place [Figure 6h]. The patient was uneventfully discharged and despite repeated attempts, the patient could not be reached with the provided contact information for further follow-up.
DISCUSSION
Ovarian artery aneurysms are rare and often asymptomatic until stress such as trauma, increased pressure, or a change in flow dynamics cause rupture and consequentially rapid clinical decline.[1-3] The underlying mechanism of OAA formation is not clear, but a major risk factor appears to be multiparity. As a rare condition, it is unknown if vasculitis or connective tissue disorders predispose patients to OAA, though it would be prudent to monitor those patients closely should they become pregnant. Structurally, the ovarian arteries are lengthy and often tortuous. In pregnancy, total blood volume increases by 50%, increasing utero-ovarian perfusion.[4] Increased female sex hormones that are produced during pregnancy may also cause degeneration of the arterial walls.[5] These additional factors likely explain why the majority of OAA ruptures are reported in multiparous women.[1-4] Further, the first patient in this case series were found to have diminutive uterine arteries [Figure 3] while the second patient was found to have a congenitally absent right uterine artery and diminutive left ovarian artery [Figure 6g]. This suggests that the pregnancies were preferentially supported by the ovarian arteries which would necessarily hypertrophy and be subject to increased flow. Consequently, this increased stress on the ovarian arterial wall could cause weakening and susceptibility to aneurysm formation and rupture.[6] This may also explain the numerous aneurysmal dilations throughout both ovarian arteries in case two.
Multiparous women may also develop uterine artery aneurysms (UAA) in pregnancy, as seen in this series.[7] Both OAAs and UAAs may rupture in the intrapartum period. Because distal uterine artery branches penetrate through the circumference of the myometrium, intrapartum uterine contraction, and involution can reduce excess blood loss in the case of ruptured UAA. Conversely, ovarian arteries course external to the uterus and are not subject to the same protective effect of uterine contractions.
Due to the rarity of this condition, there is no consensus treatment algorithm for OAA rupture. Current treatment modalities include surgical ligation and trans-arterial embolization (TAE). There are 15 reported cases treated surgically and 13 with TAE. TAE has increasingly gained favor over surgical approaches in hemodynamically stable patients due to lower perioperative risk, the potential for fertility preservation, shorter hospital stays, and less invasive nature.[2,4] A variety of embolic have been used successfully in TAE and while thrombin is frequently used to treat peripheral pseudoaneurysms, it has not been previously reported in the treatment of OAAs.[8] Given its favorable safety profile and relative ease of use, thrombin injection should be considered a viable alternative to TAE in cases where OAAs are ultrasonographically visualized and percutaneously accessible.
Additionally, the presence of multiple additional OAA in both patients is uncommon. Neither patient had a prior diagnosis of systemic disease, vasculitis, or connective tissue disorder. A prior case report discusses management strategies of incidental OAAs discovered on intrapartum imaging of ruptured OAAs. In that case, the intact OAA was treated within the following week of the initial OAA rupture.[9] General guidelines for incidentally discovered visceral arterial aneurysms suggest treatment of aneurysms >2 cm in diameter, although the threshold may be lower for nonatherosclerotic aneurysms like those described in peripartum OAA formation.[10] However, as seen in case one, asymptomatic incidental OAAs discovered on peripartum imaging that does not enlarge with follow-up imaging may resolve spontaneously. This may be explained in part by the return to normal physiologic conditions in the postpartum period. While the intact pelvic OAAs in case two were embolized in the same session as the ruptured OAA, the OAA present at the origin remained. Unfortunately, the natural history of those aneurysms remains unknown as the patient could not be reached for follow-up.
CONCLUSION
Utero-ovarian perfusion changes during pregnancy increase the potential for aneurysm formation, possibly more so if the longer and tortuous ovarian artery is the predominant supply to support the pregnancy. Ruptured ovarian artery aneurysms respond to percutaneous and endovascular embolization.
DECLARATION OF PATIENT CONSENT
Patients’ consent not required as patients’ identity is not disclosed or compromised.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
Nima Kokabi has a research grant and serves as a consultant for Sirtex Medical. Bill Majdalany serves on the scientific advisory board for Balt Medical.
References
- Ruptured ovarian artery pseudoaneurysm in a postmenopausal patient treated with transcatheter embolization. Case Rep Radiol. 2020;2020:6728318.
- [CrossRef] [PubMed] [Google Scholar]
- Successful embolization of a ruptured ovarian artery aneurysm in a postmenopausal woman: Case report and literature review of gonadal artery aneurysms. Vasc Endovascular Surg. 2018;52:159-63.
- [CrossRef] [PubMed] [Google Scholar]
- Retroperitoneal hematoma secondary to spontaneous rupture of ovarian artery pseudoaneurysms: Case report and review of literature. J Obstet Gynaecol. 2019;39:422-24.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous retroperitoneal hemorrhage caused by rupture of an ovarian artery aneurysm: A case report and review of the literature. J Med Case Rep. 2015;9:84.
- [CrossRef] [PubMed] [Google Scholar]
- Arterial dissections associated with pregnancy. J Vasc Surg. 1995;21:515-20.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of diameter, wall stress, and rupture potential index for abdominal aortic aneurysm rupture risk prediction. Ann Biomed Eng. 2010;38(10):3124-34.
- [CrossRef] [PubMed] [Google Scholar]
- True aneurysm of ovarian and uterine arteries: A comprehensive review. Ann Vasc Surg. 2021;72:610-16.
- [CrossRef] [PubMed] [Google Scholar]
- Complications of percutaneous thrombin injection in patients with postcatheterization femoral pseudoaneurysm. J Clin Ultrasound. 2016;44:188-95.
- [CrossRef] [PubMed] [Google Scholar]
- Staged endovascular treatment of bilateral ruptured and intact ovarian artery aneurysms in a postmenopausal woman. J Vasc Surg. 2009;49:208-10.
- [CrossRef] [PubMed] [Google Scholar]
- Managing incidental findings on abdominal and pelvic CT and MRI, Part 2: white paper of the ACR incidental findings committee II on vascular findings. J Am Coll Radiol. 2013;10:789-94.
- [CrossRef] [PubMed] [Google Scholar]






