Translate this page into:
Percutaneous microwave ablation for early-stage intrahepatic cholangiocarcinoma: A single-institutional cohort

*Corresponding author: Sophia Humphrey, Department of Radiology, University of Kentucky College of Medicine, Lexington, United States. sbhu224@uky.edu
-
Received: ,
Accepted: ,
How to cite this article: Humphrey S, Newcomer JB, Raissi D, Gabriel G. Percutaneous microwave ablation for early-stage intrahepatic cholangiocarcinoma: A single-institutional cohort. J Clin Imaging Sci 2024;14:4. doi: 10.25259/JCIS_59_2023
Abstract
Objectives:
Cholangiocarcinoma (CCA) is the second-most common primary hepatic malignancy with an increasing incidence over the past two decades. CCA arises from the epithelial cells lining the bile ducts and can be classified as intrahepatic, perihilar, or distal based on the site of origin in the biliary tree. Surgical resection is the definitive curative therapy for early-stage intrahepatic CCA; however, only a minority of patients may be ideal surgical candidates. Percutaneous microwave ablation (MWA) is a minimally invasive procedure widely used for hepatocellular carcinoma and colorectal cancer metastases to the liver. Growing evidence suggests MWA can play a role in the management of patients with early-stage intrahepatic CCA. In this study, we aim to describe the safety and efficacy of MWA for the management of intrahepatic CCA.
Material and Methods:
A retrospective review of patients with intrahepatic CCA treated with MWA at our tertiary referral medical center was performed. Eight patients were treated between 2014 and 2019. Diagnosis of CCA was made based on histopathological studies of samples obtained by surgical resection or percutaneous liver biopsy. All procedures were performed under computed tomography (CT) guidance using a high-power single antenna MWA system. General anesthesia was used for all procedures. Patient medical history, procedural technical information, outcomes, and follow-up data were reviewed. Progression-free survival was estimated with a Kaplan–Meier curve.
Results:
A total of 25 tumors with an average size of 2.2 ± 1.7 cm (range 0.5–7.8) were treated with MWA. Our cohort consisted of eight patients (4 males and 4 females) with an average age of 69.3 ± 5.7 years (range 61–79). Three out of eight (3/8, 37.5%) patients were treated initially with surgical resection. NASH-related cirrhosis was documented in 3/8 (37.5%) patients, while 1/8 (12.5%) had alcoholic cirrhosis; the remaining 4 patients (4/8, 50%) did not have cirrhosis. All patients were discharged within 24 h after ablation. Average total follow-up time was 10.6 ± 11.8 months (range 0–41). The incomplete ablation rate and local recurrence rate were 4% (1/25 lesions) and 12% (3/25 lesions), respectively.
Conclusion:
In patients who do not qualify for surgical resection, MWA is a safe alternative therapy for the treatment of intrahepatic CCA.
Keywords
Liver cancer
Cholangiocarcinoma
Microwave ablation
Survival
Locoregional therapy
INTRODUCTION
Cholangiocarcinoma (CCA) arises from the epithelial cells lining the biliary ducts and represents the second-most common primary hepatic malignancy following hepatocellular carcinoma (HCC).[1] The overall incidence of CCA in the United States has increased exponentially from 2001 to 2015, likely due to improved diagnostic imaging over time.[2] CCA can be classified into three categories based on the origin of malignancy within the biliary tree: Intrahepatic, perihilar, and distal. Intrahepatic cholangiocarcinoma (ICC) is the least common, comprising only 10% of CCA.[3] Despite this, the prognosis of ICC remains dismal as most patients are asymptomatic until the advanced disease stage. As a result, surgical resection remains the only curative therapy for ICC and is usually not an option for most patients. An analysis of the Surveillance Epidemiology and End Results database from 1988 to 2003, Tan et al. found that only 446 of 3756 patients (12%) with ICC underwent cancer-directed surgery.[4] Another multi-center study of 584 ICC patients found that, even after curative-intent resection, overall survival at 5 years was only 22%, and the probability of being cured of ICC was only 9.4%.[5] Given that surgical resection is not an option for most patients with ICC at the time of diagnosis, minimally invasive image-guided procedures such as transarterial chemoembolization (TACE), transarterial radioembolization (TARE), and percutaneous ablative therapies may offer therapeutic alternatives; however, these therapies do not fall under any standardized treatment algorithms.
The best-described percutaneous ablative therapies for ICC include radiofrequency ablation (RFA) and microwave ablation (MWA). RFA, first described as a treatment option for liver tumors in the early 1990s, involves the use of high-frequency alternating electric current to generate heat and induce coagulative necrosis of tissues.[6,7] MWA, the newest thermal ablative therapy, is based on the same underlying principle of generating heat and inducing coagulative necrosis but involves the use of electromagnetic energy instead of electric current. As a result, MWA attenuates the heat-sink effect, allows for more rapid and homogenous tissue heating, produces higher intratumoral temperatures, and generates a larger, more predictable ablation zone compared to RFA.[8,9] Given these advantages, MWA is gradually replacing RFA as the ablative therapy of choice for hepatic tumors. Despite this, recent meta-analyses, although limited by retrospective nature, have shown that both therapies may be safe and effective treatments for ICC in nonsurgical candidates.[10,11]
In this single-center retrospective review, we aim to further describe the safety and efficacy of MWA for ICC in a cohort of non-surgical patients at our tertiary referral medical center.
MATERIAL AND METHODS
Study population
This study was approved by our Institutional Review Board. All study participants provided written consent. We retrospectively reviewed the medical records and imaging studies of all patients who underwent MWA for ICC at our institution from 2014 to 2019. Diagnosis of ICC was confirmed by histopathological studies of samples obtained from surgical resection or percutaneous liver biopsy. Parameters of interest included age, sex, history of cirrhosis, and underlying etiology, biopsy and histopathological results, treatment modalities before ablation, tumor location (liver segment), tumor size, total follow-up time, and presence of residual or recurrent disease post ablation.
Equipment
MWA was performed using the Emprint™ Microwave Ablation System (Covidien, Boulder, CO, USA), which consists of a generator with a frequency of 2450-MHz, a maximum power of 100 W, and a 13-gauge water-cooled dipole antenna.
Pre-procedural planning and MWA technique
Contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI), interpreted by fellowship-trained abdominal radiologists, was used to determine the size and location of liver lesions 2–4 weeks before ablation [Figure 1a]. Our MWA technique has been previously described.[12] All procedures were carried under general anesthesia. After appropriate skin preparation and draping of the procedural site, a microwave antenna was advanced into the liver lesion under CT-guidance (Somatom Aera, Siemens, Erlangen, Germany) [Figure 1b]. Pre-procedural contrast-enhanced CT was used when deemed necessary by the operator. Minor positional adjustments were made to the antenna under CT-fluoroscopic guidance. Adequate positioning of the microwave probe was confirmed with non-contrast CT. MWA was performed with a surgical margin of 1 cm. Ablation time and power were recorded. The antenna was then removed with cauterization of the tract through the liver to the level of the hepatic capsule. Postprocedural CT without contrast was obtained to exclude acute complications [Figure 1c]. All patients were admitted to our inpatient service overnight and discharged on the following day if no complication was recorded.
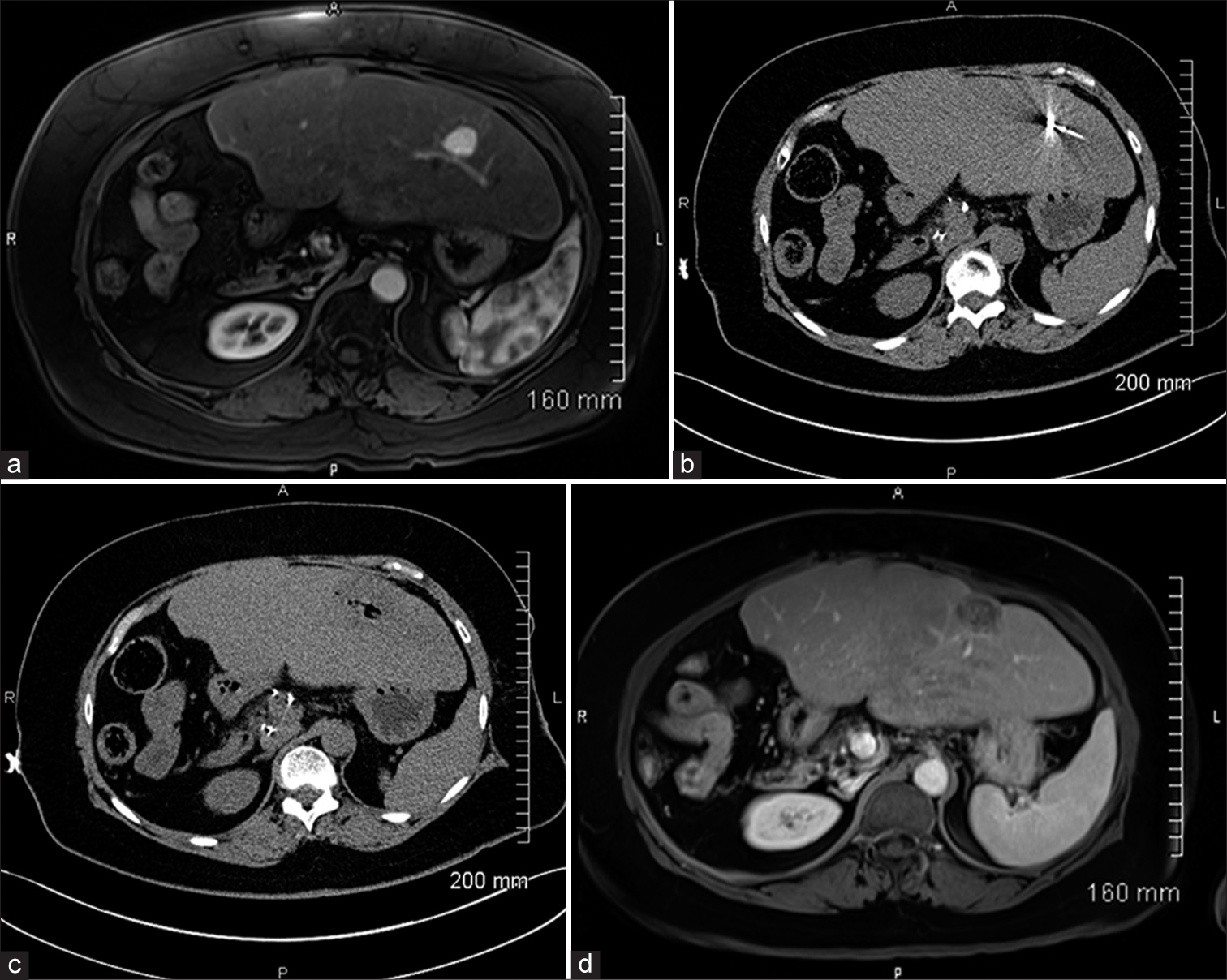
- 65 year-old female with cholangiocarcinoma diagnosed with new liver lesions three years post right hepatectomy and en bloc caudate lobectomy. (a) T1 post-contrast magnetic resonance imaging (MRI) of the abdomen demonstrating an approximately 2 cm left lobe homogeneously enhancing lesion. (b) Intraprocedural computed tomography (CT) demonstrating microwave ablation antenna within the lesion. (c) Post ablation intraprocedural computed tomography (CT) demonstrating ablation zone. (d) T1 post-contrast magnetic resonance imaging (MRI) of the abdomen 15 months post-ablation demonstrating no arterial enhancement, T1 hyperintense signal representing hemorrhagic component.
Follow-up
All patients returned to the clinic within 4–6 weeks post ablation. CT or MRI of the abdomen and chest were performed to assess for ablation efficacy and restaging purposes [Figure 1d]. All local recurrences, incomplete ablations, and de novo tumors were subjected to further treatment. After the initial post ablation encounter, follow-up was scheduled at 3-month intervals in the 1st year and then at 6-month intervals afterward.
Statistical analysis
Statistical analysis was performed with STATA v.16.0 (STATA, StataCorp, College Station, TX, USA). Proportional comparison of variables was performed with Chi-square (n ≥ 5) or Fisher’s exact test (n < 5); P < 0.05 was considered to be significant. Progression-free survival was analyzed using a Kaplan–Meier curve.
RESULTS
Patient characteristics
Our cohort consisted of eight patients (4 males and 4 females) with an average age of 69.3 ± 5.7 years (range 61–79). Three patients (3/8, 37.5%) were treated initially with surgical resection. All patients had received another prior therapeutic modality, such as chemotherapy, radiation therapy, TACE, or TARE. Cirrhosis related to nonalcoholic steatohepatitis was documented in 3/8 (37.5%) patients, while 1/8 (12.5%) had alcoholic cirrhosis; the remaining four patients (4/8, 50%) did not have cirrhosis. Five patients (5/8, 62.5%) had percutaneous biopsy-proven CCA, while the remaining 3 patients (3/8, 37.5%) were diagnosed from a surgical biopsy specimen. One patient (1/8, 12.5%) was diagnosed with mixed-type HCC/CCA and one patient (1/8, 12.5%) had histopathology suggestive of CCA; however, mixed-type HCC/CCA could not be ruled out. A total of 25 tumors with an average size of 2.2 ± 1.7 cm (range 0.5–7.8) were treated with MWA. Patient characteristics, including prior chemotherapy regimens, TNM staging, and the reasoning behind the choice of percutaneous MWA are shown in Table 1.
| Pt# | Age at time of MWA | Sex | Concomitant or adjuvant | Chemotherapy regimen | Reason for unresectability | TNM stage |
|---|---|---|---|---|---|---|
| 1 | 79 | F | N/A | 2018: Monotherapy with 5FU and radiation 2018: Y90 radioembolization with RadOnc 2019: MWA |
High cardiac risk for surgery during pre-op cardiac risk assessment coronary artery disease | T2N0M0 |
| 2 | 61 | F | Adjuvant | 2018: Y-90 with SIR-spheres 2018: Gemzar and oxaliplatin with last dose given November 12, 2018 |
Multiple hepatic lesions Bilobar disease Unfavorable tumor biology |
N/A |
| 3 | 70 | M | N/A | 2019: C4 of Gem (1000 mg/m2) and Oxaliplatin (100 mg/m2) D1, D15 was started on 2/5/19. | Stage IV fibrosis | Stage IV (rcT3, cN0, cM1) |
| 4 | 68 | F | Adjuvant | 2017: Left lobe resection and Gemcitabine 2018: MWA after recurrence and chemotherapy 2019: Recurrence |
Non-Hodgkin’s lymphoma multiple hepatic lesions and recurrence | T2N0M0 |
| 5 | 65 | F | Adjuvant | 2016: Three cycles of gemzar | NASH cirrhosis | Clinical Stage IV (rcTX, cNX, cM1) |
| 6 | 75 | M | N/A | 2018: Cisplatin/gemcitabine 2018: Induction chemotherapy followed by Y90 embolization 2019: Pembro |
Multiple hepatic lesions | N/A |
| 7 | 70 | M | N/A | No chemotherapy TACE, resection | Cardiac risk – Stent placement and coronary artery surgery Multiple hepatic lesions NASH cirrhosis |
T1N0M0 |
| 8 | 65 | F | N/A | No chemotherapy TACE | Multiple hepatic lesions NASH cirrhosis | N/A |
TACE: Transarterial chemoembolization, MWA: Microwave ablation, TMN stage: Tumor, Node, Metastasis staging, 5FU: 5-Fluorouracil, SIR-spheres: Selective Internal Radiation Spheres, NASH cirrhosis: Non-alcoholic steatohepatitis, N/A: Not applicable
Postprocedural complications
All patients were discharged within 24 h following ablation. As defined by the 2017 Society of Interventional Radiology (SIR) adverse event (AE) severity scale, one moderate and three mild AEs occurred.[13] In the single patient who experienced a moderate AE, transpleural probe placement resulted in hemopneumothorax requiring chest tube placement, which was subsequently removed the following day. Hospital stay was not prolonged past 24-h following ablation in this case. Among the three patients that experienced mild AE, one reported subjective fever, one experienced nausea and vomiting, and one developed asymptomatic minor pneumothorax due to transpleural probe placement. All patients experiencing mild AEs had symptoms that were self-limited and required no therapeutic intervention.
Follow-up and recurrence
Average total length of follow-up was 10.6 ± 11.8 months (range 0–41). By the end of the study, one patient (1/8, 12.5%) died from non-hepatic causes. No complications occurred within 30 days after ablation. At 1-month follow-up, incomplete ablation and residual tumor were present in one patient (1/8, 12.5%). Local recurrence within the ablation cavity was observed in 3 patients (3/8, 37.5%) at 6-, 9-, and 10-month, respectively. The incomplete ablation rate and local recurrence rate was 4% (1/25 lesions) and 12% (3/25 lesions), respectively. All residual and recurrent lesions were subsequently treated with repeat ablation. Tumor progression was seen on imaging up to 100% by 18 months after the ablation [Figure 2]. According to Fisher’s exact test, the concurrent existence of multiple liver lesions at 1 time was associated with a higher likelihood of recurrence [Table 2].
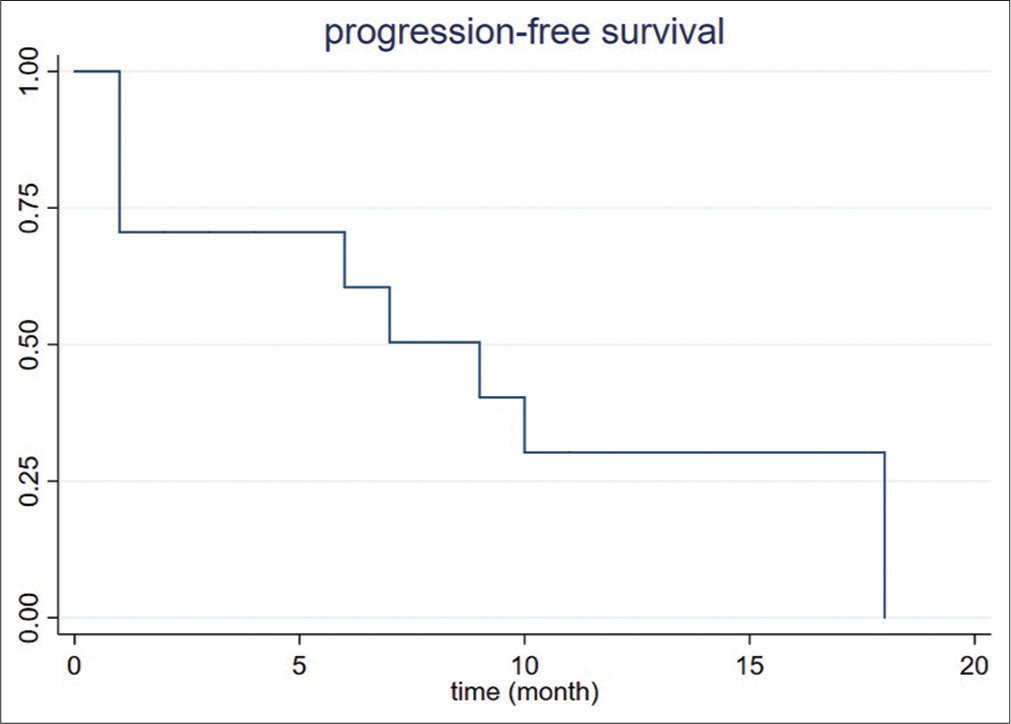
- Progression-free survival in patients with intrahepatic cholangiocarcinoma treated with microwave ablation.
| Gender | Age | Biopsy Modality | Diagnosis | Cirrhosis | Tumor Location | Longest Dimension (cm) | Procedure Specifications (power, duration, ablation zone size) | Prior therapeutic interventions | Follow-up time | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | 79 | Percutaneous Liver Biopsy | CCA | No | Segment 2 | 5.8 | 100 W, 10 min, 4.1 cm (x4 times) |
5-fluorouracil, radiation therapy, TARE | 2mo | No local recurrence, no residual disease |
| Female | 61 | Percutaneous Liver Biopsy | CCA | No | Segment 5 | 4.2 | 100 W, duration not documented, 3.7 cm | Gemcitabine, oxaliplatin, Y-90, radiation | 4mo | No local recurrence, no residual disease |
| Segment 4A | 1.5 | 100 W, 7.5 min, 4.0 cm | 2mo | No local recurrence, no residual disease | ||||||
| Male | 70 | Percutaneous Liver Biopsy | CCA | Alcoholic cirrhosis | Segment 4B & 5 | 7.8 | 100 W, 10 min 4 cm (x3 times); 100 W, 3 min, 3 cm (x1 time); 100 W, 10 min, 4 cm (x2 times) |
TACE, gemcitabine, oxaliplatin | 10mo | Local recurrence at 9 mo |
| Segment 4 (recurrent) | 0.5 | 100 W, 1 min, 2.4 cm (x2 times); 100 W, 1 min, 2.4 cm (x2 times) |
1mo | No local recurrence, no residual disease | ||||||
| Female | 68 | Surgical Biopsy | CCA | No | Segment 6 | 1.7 | 100 W, 6.5 min, 3.5 cm | Gemcitabine, surgical resection, capecitabine | 15mo | No local recurrence, no residual disease |
| Segment 7 | 2.1 | 100 W, 10 min, 4.2 cm | 15mo | Local recurrence at 10 mo |
||||||
| Segment 7 (recurrent) | 1.5 | 100 W, 10 min, 4.1 cm | 4mo | No local recurrence, no residual disease | ||||||
| Female | 65 | Surgical Biopsy | CCA | NASH cirrhosis | Segment 3 | 1.9 | 100 W, 10 min, 4.1 cm | Surgical resection, gemcitabine | No follow-up | N/A |
| Male | 75 | Percutaneous Liver Biopsy | CCA | No | Segment 5 | 3 | 100 W, 10 min, 4 cm (x2 times) |
TARE, gemcitabine, cisplatin | 3mo | No local recurrence, no residual disease |
| MALE | 70 | Surgical Biopsy | Mixed-type HCC/CCA | NASH cirrhosis | Segment 8 | 1.1 | 100 W, 6 min, 3 cm | TACE, surgical resection | 41mo | No local recurrence, no residual disease |
| Segment 5 | 5 | 100 W, 8.5 min, 4 cm (x2 times); 100 W, 6 min, 3.5 cm (x1 time) |
41mo | No local recurrence, no residual disease | ||||||
| Segment 2 | 1.2 | 100 W, 6 min, 3.5 cm; 100 W, 2.5 min, 2.3 cm |
11mo | Residual disease at 1 mo |
||||||
| Segment 2 (residual) | 1.2 | Not documented | 30mo | No local recurrence, no residual disease | ||||||
| Segment 7 | 2 | 100 W, 10 min, 4 cm | 11mo | No local recurrence, no residual disease | ||||||
| Female | 66 | Percutaneous Liver Biopsy | CCA but cannot exclude mixed-type HCC/CCA | NASH cirrhosis | Segment 6 | 1.8 | 100 W, 3.5 min, 3.0 cm | TACE | 20mo | No local recurrence, no residual disease |
| Segment 5 & 8 | 1.5 | 100 W, 3.5 min, 3.0 cm | 20mo | No local recurrence, no residual disease | ||||||
| Segment 6 | 1.4 | 100 W, 3.5 min, 3.0 cm | 11mo | Local recurrence at 6 mo | ||||||
| Segment 7 | 1.6 | 100 W, 3.5 min, 3.0 cm | 6mo | No local recurrence, no residual disease | ||||||
| Superior dome | 1.2 | 100 W, 8.5 min, 4 cm; 100 W, 3.5 min, 3 cm |
6mo | No local recurrence, no residual disease | ||||||
| Segment 7 | 1.4 | 100 W, 8.5 min, 4 cm | 3mo | No local recurrence, no residual disease | ||||||
| Segment 7 | 1 | 100 W, 6 min, 3.5 cm | 3mo | No local recurrence, no residual disease | ||||||
| Segment 7 | 1 | 100 W, 3.5 min, 3 cm | 3mo | No local recurrence, no residual disease | ||||||
| Segment 4 | 1 | 45 W, 1 min, ablation zone not documented | 3mo | No local recurrence, no residual disease | ||||||
| Segment 6 | 1.8 | 100 W, 10 min, 4.2 cm; 100 W, 6 min, 3.5 cm | No follow-up | N/A |
TACE: Transarterial chemoembolization, HCC: Hepatocellular-cholangiocarcinoma, CCA: Cholangiocarcinoma, Y-90: Yttrium-90, N/A: Not applicable, NASH: Nonalcoholic steatohepatitis, TARE: Transarterial radioembolization
DISCUSSION
The management of CCA remains challenging today as most cases are already in an advanced stage at the time of diagnosis, as it can have an insidious disease onset and course without overt clinical symptoms. Many patients with CCA are diagnosed as an incidental finding, although in some cases, the primary tumor has grown large enough to produce a liver mass or cause jaundice due to obstruction of the biliary tree.[14] Additional symptoms that can be present include abdominal pain, night sweats, nausea, and weight loss. Historically, surgical resection has been primarily recommended for debulking, though most patients are not surgical candidates at the time of diagnosis. Even for those who undergo surgical resection, the recurrence rate can be as high as 71%.[15] As more minimally invasive percutaneous therapies continue to evolve, percutaneous thermal ablation of liver lesions has gained popularity among patients with unresectable or recurrent CCA to achieve locoregional control of tumor burden.
Whereas some authors described their experience with cryoablation, there is far more data on RFA in the treatment of CCA. Technical effectiveness, defined as the absence of residual tumor within 1 month after the ablation, ranges from 66% to 100%, according to the previous literature of RFA. The technical effectiveness in our cohort was 93%, which is consistent with the existing literature. Similar to surgical resection, larger tumor size correlates directly with likelihood of incomplete ablation.[16] Only one published study reported on the efficacy of MWA in the management of CCA. In this study with 15 patients and 24 targeted lesions, the complete ablation rate was 91.7%, which is slightly lower than ours.[17] One explanation is that the authors used ultrasound for image guidance. By contrast, CT guidance in our study may have provided better visualization to accomplish larger ablation zones.
CCA is associated with a high recurrence rate. Previous RFA cohorts reported a recurrence rate from 36.3% (4/11 patients) to 100% (20/20), whereas that of the published MWA cohort was 40%. Four of eight of our patients developed new hepatic lesions, and the other four had 4 months or less of follow-up [Table 3]. However, the local tumor recurrence rate, defined by recurrence within the ablation cavity after 1 month postoperatively, was 12.5% in our study, which is significantly lower than the 25% of the previous MWA cohort. Yet, the median progression-free survival of our cohort was <1 year; the longest progression-free survival was 20 months [Figure 2]. In our cohort, new lesions were more likely to arise after the treatment of concurrent multiple lesions than solitary lesions [Table 3]. This observation might be due to the existence of tiny satellites at the time of treatment in patients with multiple hepatic lesions. Future studies may consider performing concomitant biopsies of each ablated lesion to compare the histopathological grading of different lesions. Furthermore, improvement in resolution of cross-sectional imaging and molecular imaging may help improve pre-procedural diagnosis of these satellite lesions. Furthermore, neoadjuvant chemotherapy and immunotherapy regimens are likely a necessity to improve outcomes after percutaneous ablation.
| Progression | No progression | Fisher’s exact test P value | |
|---|---|---|---|
| Solitary lesion | 0 | 5 | P=0.044 |
| Multiple lesions | 6 | 4 |
In terms of short-term clinical outcomes, all our patients were discharged the following day of ablation with zero 30-day re-admission. As per the SIR classification system for AEs, only one major complication (therapy and <48 h hospitalization required) occurred, which was hemopneumothorax resulting from intentional transpleural probe placement.[18] This resolved with chest tube placement for <24 h without affecting the length of hospital stay. In the study of Yu et al.,[17] no major complication occurred within 30 days post ablation, and all minor complications resolved without further intervention.
Percutaneous MWA can offer significant advantages over surgical resection, including but not limited to shorter average hospital stays and lower postoperative complication rates. According to Glassberg et al., “when MWA was combined with HR and compared with either modality alone, complications and blood loss were significantly lower with the combination treatment.”[19] In terms of long-term survival outcomes, only one patient was deceased due to non-hepatic related causes at the end of our study, though no control group was available for comparison. According to a meta-analysis of 84 patients who underwent RFA for CCA, the pooled survival rates at 1-year, 3-year, and 5-year were 82%, 47%, and 24%, respectively.[10] Yet, longer follow-up and a comparative study should be performed before any comparison could be made between RFA and MWA for the treatment of CCA.
Although surgical resection is the optimal therapeutic tool for the treatment of early-stage CCA, few options exist for those with inoperable disease. In addition, many patients with CCA have multiple comorbidities that confer a poor surgical prognosis, making surgery a less favorable option even in patients diagnosed at earlier stages. Percutaneous MWA allows patients who are either poor surgical candidates or present at later stages with unresectable disease a treatment option to improve their survival rates. Percutaneous MWA is a much less invasive option, proven to be effective in the treatment of liver lesions such as HCC and metastatic colorectal carcinoma, allowing for same or next day discharge, shorter recovery times and with overall minimal complications as compared to surgical resection.[20] In many patients, MWA can serve as a definitive treatment option, while in others, it can improve survival by decreasing overall disease burden. Even in patients who develop new or recurrent disease, repeat ablation is an option, which is often not the case with surgical intervention. This is especially critical for patients deemed poor surgical candidates due to multiple comorbidities, making MWA their only potentially curative option.
Limitations of our study include retrospective nature, small sample size, and inconsistent follow-up intervals. This study consisted of eight patients with 25 ablated lesions, some of which were lost to follow-up within 4 months after MWA, limiting longer term outcome conclusions. A study with a larger sample size and longer-term follow-up would increase the validity of our results and provide more information on recurrence rates of CCA and overall survival. In addition, a direct comparison between MWA and RFA would allow for definitive distinctions between the two procedures, as data comparing these two procedures are limited. Finally, quality of life was not assessed in our study, which is an avenue of future research to assess the impact of MWA on overall well-being over time, which is important in our patients in the later stages of their lives. As MWA is not always a definitive treatment option, it is important to assess quality of life measures to determine the value it has been improving the disease burden of patients with CCA.
CONCLUSION
CCA is a disease that is challenging to diagnose and manage effectively. Although surgical resection has long been the gold standard treatment for this disease, many patients diagnosed with CCA are not surgical candidates. Our study illustrates that CT-guided MWA is a safe and effective treatment strategy for patients with CCA, with low complications and recurrence rates. CT-guided MWA should be considered in patients with early-stage CCA who are not ideal surgical candidates.
Ethical approval
Institutional Review Board/Institutional Ethics Committee permission has been obtained for this study. IRB number: 42425 University of Kentucky Office of Research Integrity.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115-25.
- [CrossRef] [PubMed] [Google Scholar]
- Forty-year trends in cholangiocarcinoma incidence in the U.S. Intrahepatic disease on the rise. Oncologist. 2016;21:594-9.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical management of intrahepatic cholangiocarcinoma--a population-based study. Ann Surg Oncol. 2008;15:600-8.
- [CrossRef] [PubMed] [Google Scholar]
- Can hepatic resection provide a long-term cure for patients with intrahepatic cholangiocarcinoma? Cancer. 2015;121:3998-4006.
- [CrossRef] [PubMed] [Google Scholar]
- Radiofrequency ablation: Mechanism of action and devices. J Vasc Interv Radiol. 2010;21(8 Suppl):S179-86.
- [CrossRef] [PubMed] [Google Scholar]
- Ablation of intrahepatic cholangiocarcinoma. Semin Intervent Radiol. 2019;36:298-302.
- [CrossRef] [PubMed] [Google Scholar]
- Microwave ablation: Principles and applications. Radiographics. 2005;25(Suppl 1):S69-83.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1054-63.
- [CrossRef] [PubMed] [Google Scholar]
- Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: Systematic review and meta-analysis. J Vasc Interv Radiol. 2015;26:943-8.
- [CrossRef] [PubMed] [Google Scholar]
- Thermal ablation in the treatment of intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. Eur Radiol. 2021;32:1205-15.
- [CrossRef] [PubMed] [Google Scholar]
- Early outcomes with single-antenna high-powered percutaneous microwave ablation for primary and secondary hepatic malignancies: Safety, effectiveness, and predictors of ablative failure. J Clin Imaging Sci. 2020;10:10.
- [CrossRef] [PubMed] [Google Scholar]
- Proposal of a new adverse event classification by the society of interventional radiology standards of practice committee. J Vasc Interv Radiol. 2017;28:1432-7.e3.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):98-107.
- [CrossRef] [PubMed] [Google Scholar]
- Management and outcomes of patients with recurrent intrahepatic cholangiocarcinoma following previous curative-intent surgical resection. Ann Surg Oncol. 2016;23:235-43.
- [CrossRef] [PubMed] [Google Scholar]
- Incomplete ablation after radiofrequency ablation of hepatocellular carcinoma: Analysis of risk factors and prognostic factors. Ann Surg Oncol. 2008;15:782-90.
- [CrossRef] [PubMed] [Google Scholar]
- Sonography-guided percutaneous microwave ablation of intrahepatic primary cholangiocarcinoma. Eur J Radiol. 2011;80:548-52.
- [CrossRef] [PubMed] [Google Scholar]
- CT-guided microwave ablation of hepatic malignancies via transpulmonary approach without ancillary techniques. J Clin Imaging Sci. 2022;12:2.
- [CrossRef] [PubMed] [Google Scholar]
- Microwave ablation compared with hepatic resection for the treatment of hepatocellular carcinoma and liver metastases: A systematic review and meta-analysis. World J Surg Oncol. 2019;17:98.
- [CrossRef] [PubMed] [Google Scholar]
- Ablative technologies for hepatocellular, cholangiocarcinoma, and metastatic colorectal cancer of the liver. Surg Oncol Clin N Am. 2015;24:97-119.
- [CrossRef] [PubMed] [Google Scholar]







