Translate this page into:
Normalized Subtraction of Serial Brain Magnetic Resonance Images and Fludeoxyglucose-Positron Emission Tomography Images for Tumor Treatment Monitoring: Case Report and Method Description
Address for correspondence: Dr. Nghi C Nguyen, Department of Radiology, University of Pittsburgh/UPMC Presbyterian, 200 Lothrop Street, East Wing, Suite 200, Pittsburgh, PA, USA. E-mail: nncc.nguyen@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A 60-year-old Caucasian male with a long history of cigarette smoking was diagnosed with epidermal growth factor receptor-mutation negative lung adenocarcinoma. The single cerebral metastasis in the right frontal lobe was treated with stereotactic radiosurgery and systemic chemotherapies. Normalized subtraction (NS) method was used to evaluate the serial brain magnetic resonance (MR) and fludeoxyglucose-positron emission tomography (FDG-PET) findings retrospectively, and the potential benefit of concurrent NS of serial MR images (MRIs) and PET images was demonstrated. MIM 4.1 (MIM Software Inc., Cleveland, OH) was used to co-register MRI with PET data and to perform NS on the serial MRI and PET data. MIM 4.1 provides fully automated alignment of imaging data by maximization of mutual information. Cortical regions distant from the brain lesion were used to adjust for the intensity differences between scans, so the voxel differences in normal brain regions were near zero in the NS images. A difference of 15% or greater in voxel densities was used for both MRI and PET, above or below which a change in MR signal intensity and FDG avidity was considered significant. The use of NS, in this case, allowed for an enhanced correlation of morphologic and functional information, which may have added value in the early treatment monitoring of brain tumors and help distinguish recurrent tumor from postradiation changes.
Keywords
Brain tumor
fludeoxyglucose positron emission tomography
magnetic resonance imaging
normalized subtraction
treatment monitoring

INTRODUCTION
Current standard imaging procedures – computed tomography (CT) and magnetic resonance imaging (MRI) with or without magnetic resonance spectroscopy – provide adequate anatomic localization and characterization of metastatic and primary brain tumors. However, treatment effects such as postsurgical inflammation, radiation fibrosis, and necrosis are difficult to distinguish from residual and recurrent tumor.[1] Surgical biopsy of the brain carries significant risks and may not be clinically warranted. Thus, it is desirable to have enhanced analytic tools for noninvasive imaging that can help distinguish benign from malignant processes. Positron emission tomography (PET) with 18F-fludeoxyglucose (FDG) has proven utility in the diagnosis, staging, and restaging of various cancers and can play an important role in the treatment monitoring of brain tumors.[2] We present this case to demonstrate the potential benefit of concurrent normalized subtraction (NS) of serial brain MR and PET images for the treatment monitoring and differentiation of recurrent tumor from radiation change.
CASE REPORT
A 60-year-old Caucasian male presented with a left-sided weakness including dragging his left foot, and the inability to write for about 6 weeks. Brain MRI showed a solitary 2.3 cm brain lesion in the right frontal lobe. Whole-body F-18 FDG PET/CT scanning including the brain demonstrated multiple bilateral FDG avid pulmonary nodules, and mediastinal adenopathy, as well as FDG avidity of the MRI-described brain lesion. A mediastinal lymph node biopsy revealed metastatic adenocarcinoma, negative for epidermal growth factor receptor mutation. The patient underwent stereotactic radiosurgery (1st SRS) to the frontal lobe lesion with 18 Gy in one fraction (CyberKnife System, Accuray Incorporated), and four cycles of carboplatin and paclitaxel, and additional four cycles of pemetrexed. He improved in symptoms and continued with short-term steroids and antiseizure medication. However, follow-up imaging with brain MRI and whole-body FDG PET/CT 17 months later showed worsening findings of right frontal lobe lesion and progressive extracranial metastases, which lead to the treatment with four cycles of the single-agent docetaxel.
Brain MRI and FDG PET images before his 1st SRS and chemotherapy showed a ring-enhancing brain lesion with intense FDG avidity [Figure 1 a and d]. One month after SRS, MRI images showed small areas of both interval decrease and increase in contrast enhancement as well as other areas with no interval change, as seen on the NS images [Figure 1 b and c]. In contrast, NS images of the PET images of the same period demonstrated interval decrease in lesional FDG uptake visually and semi-quantitatively with NS, consistent with partial treatment response [Figure 1e and f].
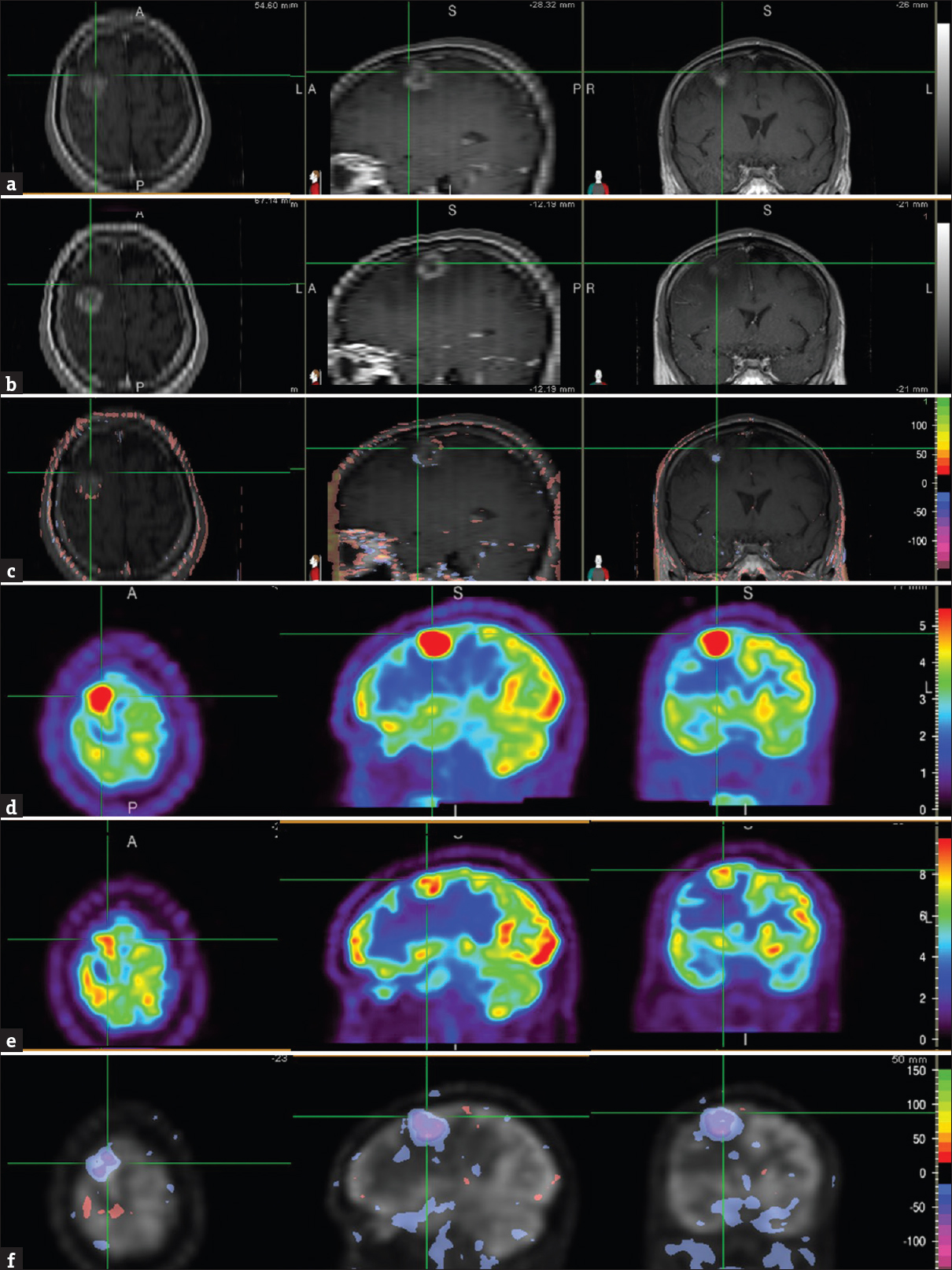
- A 60-year-old Caucasian male presented with a left-sided weakness including dragging his left foot, and the inability to write for about 6 weeks. Brain magnetic resonance imaging showed a solitary 2.3 cm brain lesion in the right frontal lobe, and subsequent positron emission tomography/computed tomography study showed multiple fludeoxyglucose avid bilateral pulmonary nodules and mediastinal adenopathy. Biopsy of a mediastinal lymph node revealed metastatic adenocarcinoma, negative for epidermal growth factor receptor mutation. Brain magnetic resonance images, T1-weighted contrast-enhanced (axial = left image; sagittal = middle image; coronal = right image), before (a) and 1 month after stereotactic radiosurgery (b) showed the right frontal lobe metastasis (cross-hair). Normalized subtraction result of A and B overlaid with the magnetic resonance image (c), demonstrated small areas of mixed interval decrease (purple) and increase (orange-red) of contrast enhancement, and other areas with no interval change. Corresponding fludeoxyglucose positron emission tomography scans (axial = left image; sagittal = middle image; coronal = right image) before (d) and 1 month (e) after stereotactic radiosurgery showed the brain metastasis (cross-hair). NS result of D and E, overlaid with the positron emission tomography image (f), showed interval decrease (purple) in lesional fludeoxyglucose uptake. The residual uptake, however, was still greater than that in the surrounding and contralateral brain parenchyma suggestive of partial treatment response.
Brain MRIs at 7 months demonstrated a significant decrease in lesion size and contrast enhancement compared with the scan performed at 1 month after SRS [Figure 2 a, b, and c]. In contrast, the PET scan of the same periods showed small areas of mildly decreased FDG uptake [Figure 2 d, e, and f], but the lesion remained FDG avid suggestive of residual tumor. Because the patient reported no neurologic symptoms and the MR findings showed improvement, the brain lesion was thought to be treated, and the remaining enhancement was favored to be radiation change.
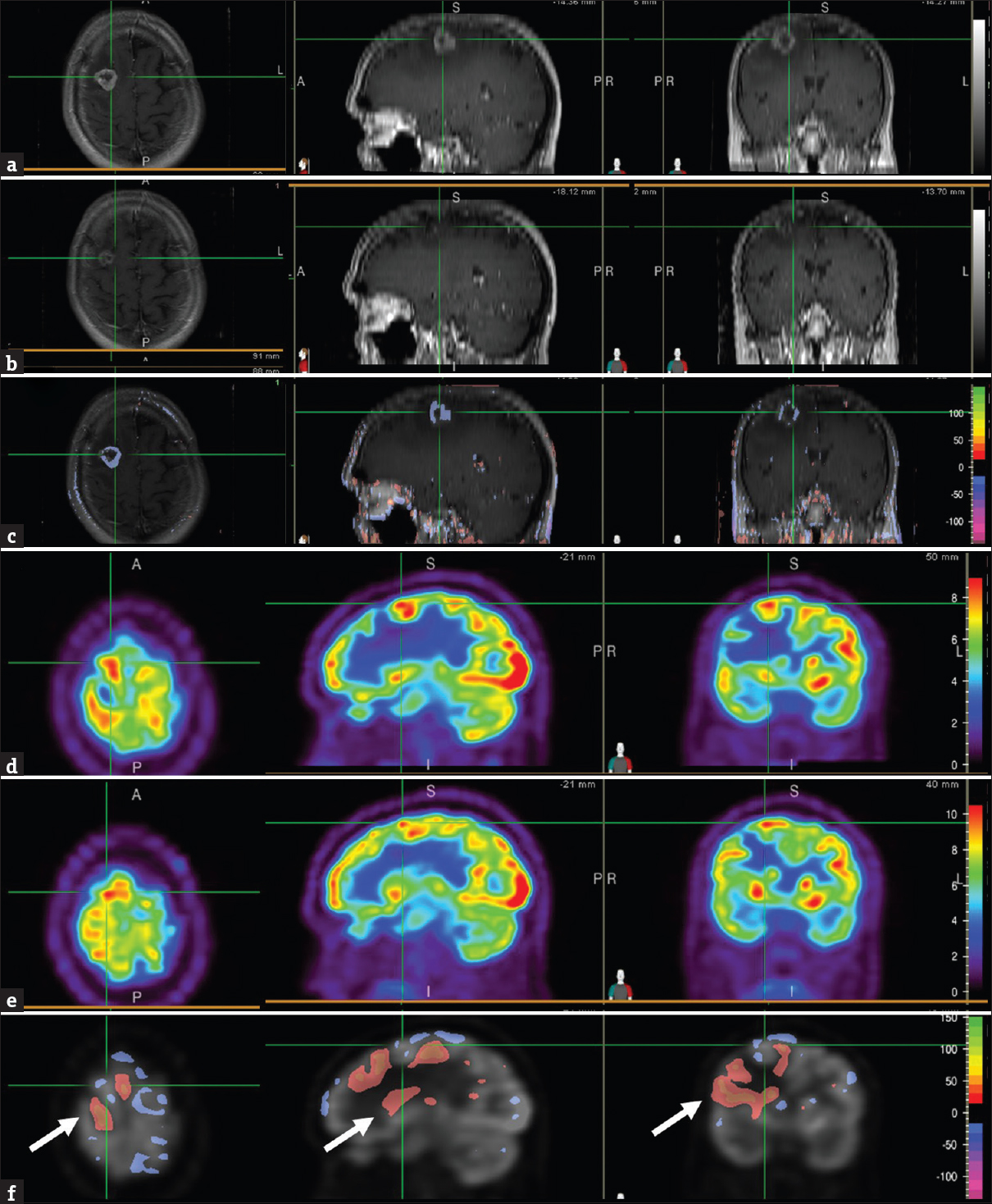
- Brain magnetic resonance images, T1-weighted contrast-enhanced, at 1 month (a) and 7 months (b) after stereotactic radiosurgery showed the area of the right frontal brain lesion (cross-hair). Normalized subtraction result of A and B, overlaid with the magnetic resonance image (c), showed marked decrease in the signal intensity and size of the ring enhancement. Corresponding positron emission tomography images at 1 month (d) and 7 months (e) after stereotactic radiosurgery showed the area of the right frontal lesion (cross-hair). Normalized subtraction result overlaid with positron emission tomography image (f) demonstrated small areas of mild interval decreased uptake (purple) within the brain lesion and other areas with no interval change. Given the residual fludeoxyglucose avidity compared to the surrounding and contralateral brain parenchyma, residual tumor was suspected. Interval increased (orange-red) uptake in brain tissue surrounding the tumor lesion reflected metabolic recovery and improving vasogenic edema (F, arrow).
Brain MRIs at 17 months showed an enlarging lesion with increased signal intensity compared with the scan at 7 months [Figure 3 a, b, and c], which raised the suspicion for recurrent tumor. Similarly, FDG PET scan of the same periods demonstrated interval increase in lesional uptake suggestive of recurrent disease [Figure 3 d, e, and f]. Recurrent disease was also suspected in subsequent proton MR spectroscopy. A recurrent tumor was, therefore, assumed and a tissue sampling was foregone. The patient was retreated again with SRS in a single dose of 21 Gy (2nd SRS). Although the patient remained free of neurologic symptoms, the lesion size progressed on follow-up MRI scans. A biopsy was positive for residual metastasis, and subsequent surgical resection of the lesion revealed metastatic tumor and an additional area of increased astrocytic component suggestive of radiation treatment effect. The patient then completed six cycles of carboplatin and pemetrexed, which achieved stable extracranial disease. He was started on the single agent gemcitabine before his medical care was transferred to another medical facility. Brain MRI scan at that time showed postoperative changes in the right frontal lobe without evidence of new brain metastasis. The authors certify that they have obtained the waiver of informed consent from their Institutional Review Board (IRB) for this patient data and publication.
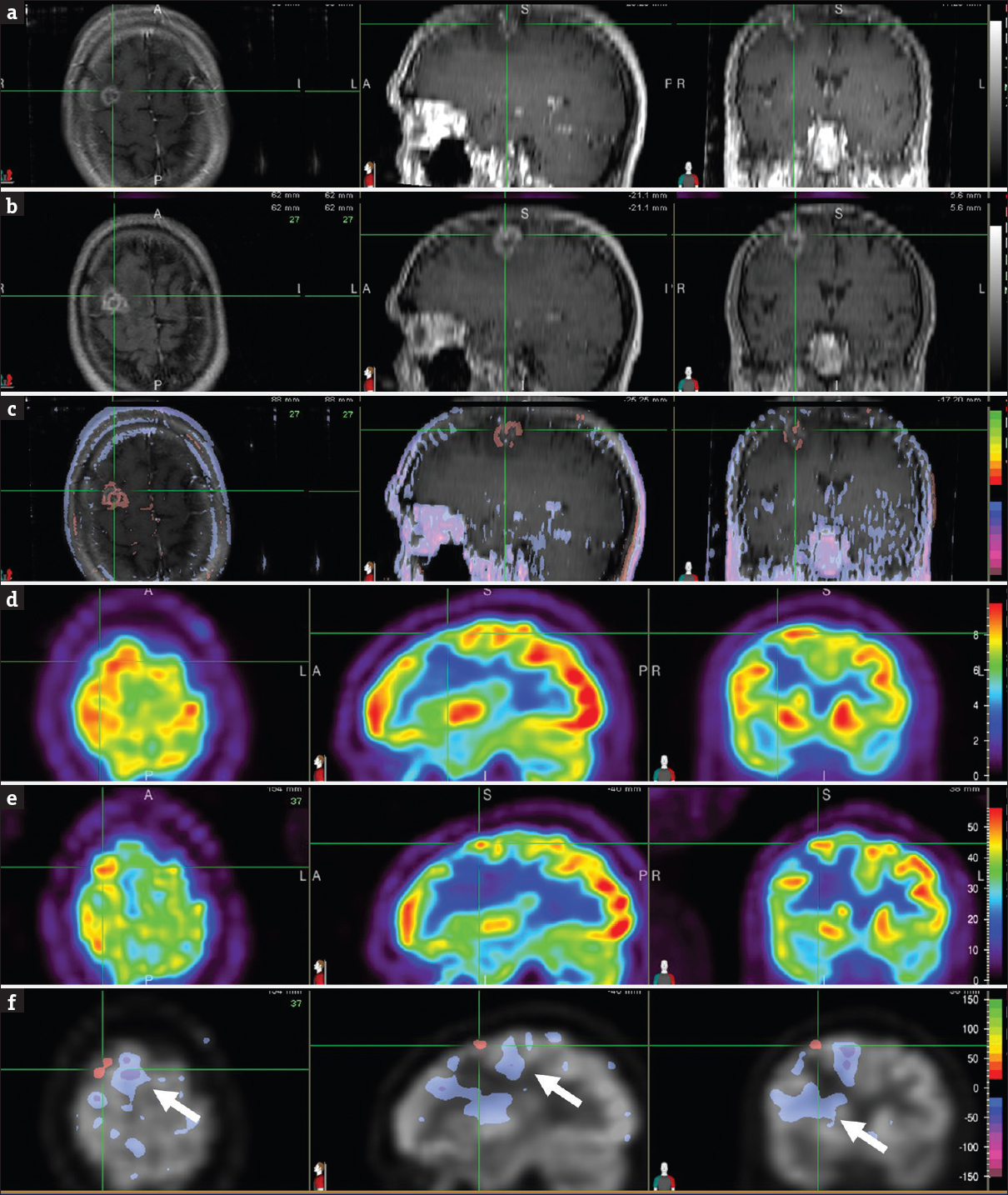
- Brain magnetic resonance images, T1-weighted contrast-enhanced, at 7 months (a) and 17 months (b) after stereotactic radiosurgery focused on the right frontal lesion (cross-hair). Normalized subtraction result, overlaid with the magnetic resonance image (c), demonstrated interval increase (orange-red) in signal intensity and size of the contrast enhancing lesion. Corresponding positron emission tomography images at 7 months (d) and 17 months (e) after stereotactic radiosurgery focused on the right frontal lesion (cross-hair). Normalized subtraction result, overlaid with the positron emission tomography image (f), showed interval increased (orange-red) fludeoxyglucose uptake within the tumor bed suspicious for tumor recurrence. Interval decreased (purple) uptake in brain areas surrounding the tumor (F, arrow) represented metabolic dysfunction due to vasogenic edema associated with tumor recurrence. Subsequent proton magnetic resonance spectroscopy was also suspicious for recurrent tumor, so a tissue sampling was foregone. The patient was retreated again with Still Birth Rate in a single dose of 21 Gy. Although the patient remained free of neurologic symptoms, the lesion size progressed on follow-up magnetic resonance imaging scans. A biopsy was positive for residual metastasis, and subsequent surgical resection of the lesion revealed metastatic tumor and an additional area of increased secondary to radiation treatment effect.
Image acquisition
MRI was performed on a 3.0-T scanner (Achieva 3T, Philips Medical Systems) with an 8-channel head coil. Imaging sequences included T1-weighted axial and sagittal (both TR/TE, 450/10), T2-weighted axial (TR/TE, 3000/80) and T2 FLAIR (TR/TE, 11000/120), and postcontrast T1-weighted axial and sagittal (both TR/TE, 450/10) and coronal (TR/TE, 493/10). The MR contrast agent gadobenate dimeglumine (Multi-Hance®; Bracco Diagnostics, Inc., Princeton, NJ) was administered IV in a dose of 0.1 mmol/kg. Image slice thickness was 4 mm with 1 mm gap between image slices.
The patient fasted at least 4 h before the PET/CT examination and received an IV injection of approximately 5.18 MBq/kg of body weight of FDG. Blood glucose concentration was < 150 mg/dL immediately before tracer injection. The patient sat in a quiet room during the subsequent 60-min uptake phase. The PET/CT scanner (Gemini TF, Philips Healthcare) consisted of a 64-MDCT unit, and low-dose CT was performed before PET for attenuation correction and image fusion (120–140 kV and 33–100 mAs). The 3D emission scans were acquired at 2.5 min per bed position, with a 128 × 128 matrix and a slice thickness of 4 mm. The brain PET data were extracted from the whole-body scans for the subsequent co-registration with MRI and NS analyses.
Normalized subtraction
The NS method was used to identify the differences in voxel intensities of the same modality, such as MRI and PET images. We used MIM 4.1 (MIM Software Inc., Cleveland, OH) to co-register MRI with PET data and to perform NS on the serial MRI and PET data. MIM 4.1 provides fully automated alignment of imaging data by maximization of mutual information.[3] Serial MR and PET scans often show minor intensity differences even in normal brain areas; thus, the image volumes require a normalization before NS is applied. In our manual approach, the interpreting physician uses normal cortical regions distant from the abnormal brain lesion to adjust for the intensity differences between scans, so the voxel differences in normal brain regions were near zero in the NS image. In this particular case, we used the temporal lobe contralateral to the brain tumor for both MRI and PET data. Subsequently, a difference of 15% or greater in voxel densities was used for both MRI and PET, above or below which a change in MR signal intensity and FDG avidity was considered significant.
DISCUSSION
The interpretation of serial MRI or PET scans usually occurs by visually comparing the images side by side. The angle of image acquisition of the head, however, is usually different between scans resulting in repositioning errors.[45] This may lead to low intra-and inter-observer agreement.[67] By accounting for this difference in angulation, formal co-registration of imaging data can provide a more accurate characterization of intracranial lesions.[589] Co-registered PET and MRIs have been shown to improve the diagnostic accuracy of PET; thus, it is essential to have co-registered images available while PET images are being interpreted.[21011]
We have demonstrated with this case that it is feasible to perform concurrent NS of serial MR and PET images using a single software application. With NS, the morphological and metabolic changes within the tumor bed could be easily followed and correlated. Although the temporal relations between MRI contrast enhancement and FDG metabolism in predicting response to therapy, recurrent disease, and posttreatment changes are not well studied and understood, our case showed that the PET changes may precede those on MRI at 1 month post-SRS, and the degree of metabolic change as determined by NS may complement MRI for early treatment assessment and prognostication.[121314]
We found that a cutoff of 15% in voxel intensity difference provides an acceptable trade-off between sensitivity and specificity for detecting both abnormal metabolic and morphologic changes in the brain. This cutoff would be equivalent to a subtraction cluster analysis at 2 SD level and cluster P < 0.05 (unpublished data). The total procedure from image co-registration to the completion of NS takes approximately 3 min.
Criteria to define lesion changes on subtraction images have been described for MRI.[89] A new lesion, enlarging lesion on unenhanced images, or lesion with increasing contrast enhancement appears hyperintense, whereas a resolving lesion or lesion with decreasing contrast enhancement appears hypodense. On the NS images, voxels with interval increased or decreased signal intensities can be displayed in color scales as presented in this case. Similar to MRI, a new FDG focus or focus with increasing metabolism appears more intense, whereas a focus with decreasing metabolism appears less intense on subtraction images. Lesions with stable metabolism cancel out in the NS images.
In the brain, NS of MRI data has been used to monitor brain tumors.[8915] Previous MRI studies on the topic of NS have reported good interobserver agreement for the detection of active lesions.[1617] In FDG PET, NS has been used to diagnose epilepsy and to follow-up postsurgical changes after epilepsy surgery.[1819] NS of FDG PET data to assess brain tumors, however, has not been reported. To date, the concurrent use of NS for both MRI and PET has not been reported in the literature.
As an alternative to visual evaluation, NS not only helps reduce the effect of repositioning errors but also allows for an assessment at the voxel level. This is particularly helpful in cases with mild metabolic changes, which can be missed at visual evaluation.[8] As a result, NS allows subjective and robust comparison of serial images of both MRI and PET image. NS offers detailed cross-correlation of multimodality images and thus helps better understand the correlation between tumor contrast enhancement and metabolic activity. Because of the complementary information gained from both morphological and metabolic evaluations, the concurrent use of NS holds the potential to improve tracking of brain tumors in a way that would have been difficult to achieve with nonconcurrent interpretations.
Another potential advantage of NS is the quantification of lesion volume in both increasing and decreasing disease activity, allowing comprehensive monitoring of treatment.[891519] The MRI and PET tumor volumes obtained from NS may enhance the definition of target volumes for radiation treatment, thus contributing to imaging diagnosis and treatment planning. Although a volume quantification would have been great in our case, this feature was not available in current MIM Software versions. In addition, we are not aware of any clinical software application capable of providing quantitative NS analysis of both voxel intensity and volume changes on both MRI and PET data. We hope that these features may be implemented in future software update, so clinicians and researchers can validate the potentials of concurrent NS.
Apart from the proposed NS technique using conventional contrast-enhanced MRIs, increasing evidence suggests that perfusion-weighted MRI such as dynamic susceptibility contrast MRI is capable of distinguishing active tumors from pseudoprogression and radiation necrosis in that active tumors usually show high relative cerebral blood volume (rCBV), particularly high rCBV ratio compared with normal brain when corrected for leakage with preloading dose and baseline subtraction.[20] Dynamic contrast-enhanced T1 perfusion MRI has also shown promise with rapid enhancement being typical of progressive tumor.[21] These techniques provide information about tissue perfusion and permeability and are increasingly utilized in tumor grading, pretreatment planning, and response to therapy assessment. However, these perfusion MR techniques are more technically challenging and time-consuming than the proposed NS of conventional contrast-enhanced MRIs and require significant training for image interpretation. In addition, the direct use of contrast-enhanced MRIs for the NS is simple and ease and is associated with less distortion artifacts and static susceptibility effects from air-filled sinuses or old hemorrhage, resulting in better image quality compared with perfusion-weighted MRI. However, it remains to be seen whether the proposed NS method for MRI data performs similarly to or has any added value to perfusion-weighted MRI for distinguishing pseudoprogression from true progressive disease. Further study is required to determine whether the proposed NS for both MRI and PET data can serve as clinically useful surrogates to guide treatment decisions. The NS method is not without limitations. Three types of artifacts may occur on the MR subtraction images: residual registration errors (partly due to patient movement during acquisition), lesion signal intensity differences between the baseline and follow-up images, and flow artifacts on the native images that may result in false-positive lesions.[8] These artifacts, however, can be minimized by inspecting the nonsubtracted, registered images.[17] Similarly, residual registration errors may also be encountered with PET data.[5] Some variations in blood glucose concentrations between PET scans remain although blood glucose concentrations were < 150 mg/dL in this patient. These blood glucose variations, however, might have little impact on the NS analysis because the images were first normalized to remove confounding effects. Nonetheless, intensity normalization may be adversely affected when large areas of FDG reduction are present, resulting in artifactual increases or decreases in normal tissue and misinterpretation of lesions in the NS result.[22] The brain images were extracted from the whole-body PET scanning and acquired at 2.5 min per bed position. This acquisition protocol was shown to be almost equivalent to an acquisition protocol dedicated to the brain.[23]
CONCLUSION
The concurrent NS to serial MR and PET images may allow for an enhanced correlation of morphological and functional information, which may have added value in early treatment monitoring and help distinguish recurrent tumor from posttreatment changes. Clinical studies are warranted to further validate the diagnostic potential of concurrent NS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2018/8/1/25/235509.
REFERENCES
- The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int J Cancer. 2001;96:191-7.
- [Google Scholar]
- Brain functional localization: A survey of image registration techniques. IEEE Trans Med Imaging. 2007;26:427-51.
- [Google Scholar]
- The effect of repositioning error on serial magnetic resonance imaging scans. Arch Neurol. 1993;50:569-71.
- [Google Scholar]
- Correlating MRI and clinical disease activity in multiple sclerosis: Relevance of hypointense lesions on short-TR/short-TE (T1-weighted) spin-echo images. Neurology. 1995;45:1684-90.
- [Google Scholar]
- Visual analysis of serial T2-weighted MRI in multiple sclerosis: Intra- and interobserver reproducibility. Neuroradiology. 1999;41:882-8.
- [Google Scholar]
- Use of subvoxel registration and subtraction to improve demonstration of contrast enhancement in MRI of the brain. Neuroradiology. 1996;38:717-23.
- [Google Scholar]
- Subvoxel image registration of multislice (2D) magnetic resonance images in patients with high-grade gliomas of the brain. Clin Radiol. 2002;57:1098-108.
- [Google Scholar]
- MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536-46.
- [Google Scholar]
- FDG PET/MR imaging coregistration helps predict survival in patients with glioblastoma and radiologic progression after standard of care treatment. Radiology. 2017;283:508-14.
- [Google Scholar]
- 3’-deoxy-3’-18F-fluorothymidine PET and MRI for early survival predictions in patients with recurrent malignant glioma treated with bevacizumab. J Nucl Med. 2012;53:29-36.
- [Google Scholar]
- Meninges: Benign postoperative enhancement on MR images. Radiology. 1990;174:99-102.
- [Google Scholar]
- Cranial postoperative site: Assessment with contrast-enhanced MR imaging. Radiology. 1990;174:93-8.
- [Google Scholar]
- Magnetic resonance image registration and subtraction in the assessment of minor changes in low grade glioma volume. Eur Radiol. 2004;14:2061-6.
- [Google Scholar]
- Image registration and subtraction to detect active T (2) lesions in MS: An interobserver study. J Neurol. 2002;249:767-73.
- [Google Scholar]
- Subtraction MR images in a multiple sclerosis multicenter clinical trial setting. Radiology. 2009;250:506-14.
- [Google Scholar]
- Opposite ictal perfusion patterns of subtracted SPECT. Hyperperfusion and hypoperfusion. Brain. 2000;123(Pt 10):2150-9.
- [Google Scholar]
- Postoperative alteration of cerebral glucose metabolism in mesial temporal lobe epilepsy. Brain. 2005;128:1802-10.
- [Google Scholar]
- Comparison of 3 tesla proton MR spectroscopy, MR perfusion and MR diffusion for distinguishing glioma recurrence from posttreatment effects. J Magn Reson Imaging. 2012;35:56-63.
- [Google Scholar]
- Differentiating treatment-induced necrosis from recurrent/progressive brain tumor using nonmodel-based semiquantitative indices derived from dynamic contrast-enhanced T1-weighted MR perfusion. Neuro Oncol. 2011;13:1037-46.
- [Google Scholar]
- Statistical parametric mapping: Assessment of application in children. Neuroimage. 2000;12:538-49.
- [Google Scholar]
- Statistical image analysis of cerebral glucose metabolism in patients with cognitive impairment following diffuse traumatic brain injury. J Neurotrauma. 2007;24:919-26.
- [Google Scholar]






