Translate this page into:
Mycotic and non-mycotic coronary artery aneurysms—A review of the rarity

*Corresponding author: Vishal Kukkar, Department of Radiology, UT Southwestern Medical Center, Dallas, Texas, United States. Vishal.Kukkar@utsouthwestern.edu
-
Received: ,
Accepted: ,
How to cite this article: Kukkar V, Kapoor H, Aggarwal A. Mycotic and non-mycotic coronary artery aneurysms—A review of the rarity. J Clin Imaging Sci 2022;12:13.
Abstract
Sir William Osler coined the term “mycotic” to identify aneurysms secondary to an infectious cause, which may not be necessarily fungal and are caused mainly by bacteria. The literature’s reported incidence of coronary artery aneurysms (CAA) is from 1.5-5%. The right coronary artery (RCA) is mainly involved, followed by the left side coronary circulation. Mycotic aneurysms are more commonly associated with infective endocarditis. More recently, coronary artery stents, particularly drug-eluting stents, are typically causing mycotic coronary aneurysms. CT angiography (CTA) has been the forefront diagnostic modality, showing both the lumen and wall of the coronary arteries. It also aids in preoperative planning. MRI is useful in diagnosing and following children with Kawasaki’s disease. Smaller mycotic coronary aneurysms may resolve with antibiotic therapy; however, aneurysms more significant than 1-2 cm diameter needs corrective surgery. Early diagnosis and appropriate management are the critical factors in the successful treatment of infective coronary artery aneurysms.
Keywords
Mycotic
Bacteria
Drug-eluting stents
Kawasaki’s disease
CT angiogram
INTRODUCTION
Sir William Osler coined the term “mycotic” to identify aneurysms secondary to an infectious cause, which may not be necessarily fungal. Bacteria are the most causative organisms implicated in the etiopathogenesis of the mycotic coronary aneurysm. Staphylococcus aureus and Streptococcus viridans are commonly reported causative bacteria.[1] Although rare, coronary aneurysms are now more widely seen in clinical practice, mainly due to increased imaging of the Heart and coronary vessels, especially CT Heart angiogram (CTA Heart) and to less MRI Heart, but also due to ever-increasing coronary stent placement procedures, with an exceptionally high incidence of mycotic coronary aneurysm after these procedures.
Incidence and anatomic distribution
The literature’s reported incidence of coronary artery aneurysms (CAA) is from 1.5-5%.[2]
Coronary artery aneurysms, in general, are most seen in the right coronary artery (RCA), followed by left anterior descending and circumflex arteries.[3]
However, mycotic (infective) coronary artery aneurysms are exceedingly rare, accounting for less than 3% of all coronary aneurysms [Table 1].[5]
| Coronary artery aneurysm distribution | % of cases |
|---|---|
| Right coronary artery (RCA) | 40-70 |
| Left anterior descending artery | 32.3 |
| Left circumflex artery | 23.4 |
| All three or left main | 3.5 |
ETIOLOGY
The most common etiology of coronary aneurysms is atherosclerosis in adults and Kawasaki disease in children and adolescents.[6] Daoud et al. [7] found that atherosclerosis caused 52% of cases, 17% were congenital, 11% were mycotic-embolic, 11% were dissecting, and 4% were luetic. More recently, coronary artery aneurysms are seen as a complication of surgical interventions, including coronary angioplasty (balloon, laser, atherectomy) and stent implantation, particularly the use of drug-eluting stents.[4]
Mycotic aneurysms are usually associated with infective endocarditis (IE). They can also be seen in patients with sepsis, particularly in immune-compromised patients.[9] The most common pathogens identified as causative factors are Staphylococcus aureus and Streptococcus viridans.[3,10] Salmonella and pseudomonas are also reported. Other less frequently implicated pathogens are coagulase-negative Staphylococcus, Enterococcus, Candida species, Enterobacter, Mycobacterium Bovis, Tuberculosis, Escherichia coli, Burkholderia, and clostridium.[11] However, coronary stents are increasingly implicated in forming a mycotic coronary aneurysm[5,9] and the organisms associated are similar to one seen in IE.
PATHOGENESIS
Risk factors implicated in mycotic aneurysms’ development include sepsis, endocarditis, arterial trauma, various immunocompromised states, and congenital cardiovascular defects.[12] Out of these, the most common is endocarditis.
Several pathophysiological mechanisms may explain the origin of mycotic coronary aneurysm—(a) Embolic occlusion of the arterial wall vasa vasorum of a normal-caliber artery or a pre-existing aneurysm resulting in vessel wall infarction. (b) Direct infiltration of the arterial wall from an adjacent source of sepsis. (c) Arterial injury from immune complex deposition. These all damage the different layers of the vessel wall and cause rapid dilation and aneurysm formation.[13,14]
Mycotic aneurysms associated with infective endocarditis are believed to be caused by microemboli to the vasa vasorum or by local spread from impacted macro emboli from the primary site of infection.[15]
Mycotic aneurysms secondary to intracoronary stent placement are related to multiple mechanisms, including contamination at the time of stent delivery, transient bacteremia from skin flora via access-site hematomas, pseudoaneurysms, delayed bleeding, prolonged arterial sheath insertion, and several procedures performed from the same access site over a short period.[16]
Mycotic aneurysms secondary to systemic infection/sepsis are mostly related to immunocompromised states such as leukemia, AIDS, SLE, or renal transplant patients.[11] Sometimes patients who underwent frequent vascular access, such as patients on dialysis or receiving parenteral nutrition can develop sepsis and subsequently mycotic aneurysms.
EPIDEMIOLOGY
The incidence of coronary aneurysms has been reported from 1.5-5% in the literature.[2] The CAA incidence is lower in Asia than in North America and Europe, highlighting the possible genetic and environmental susceptibility to CAAs [Table 2].[4]
| Causes of coronary artery aneurysm |
|---|
| Atherosclerosis-50% of cases |
|
Vasculitis • Kawasaki’s disease • Takayasu’s arteritis • Systemic lupus erythematous • Giant cell arteritis • Others (Bechet’s syndrome, sarcoidosis, rheumatoid arthritis, Fibromuscular dysplasia, CREST syndrome) |
|
Compensatory dilatation • Post stenotic dilatation • Fistula • Coronary anomalies (ALCAPA) |
|
Connective tissue disorder • Marfan’s syndrome • Ehlers-Danlos syndrome • Cystic medial necrosis • Neurofibromatosis (NF) |
| Mycotic |
|
Drug abuse: Cocaine, amphetamines, and protease inhibitors |
| Trauma |
|
Percutaneous coronary interventions • Balloon angioplasty • Stent implantation, particularly drug-eluting stents |
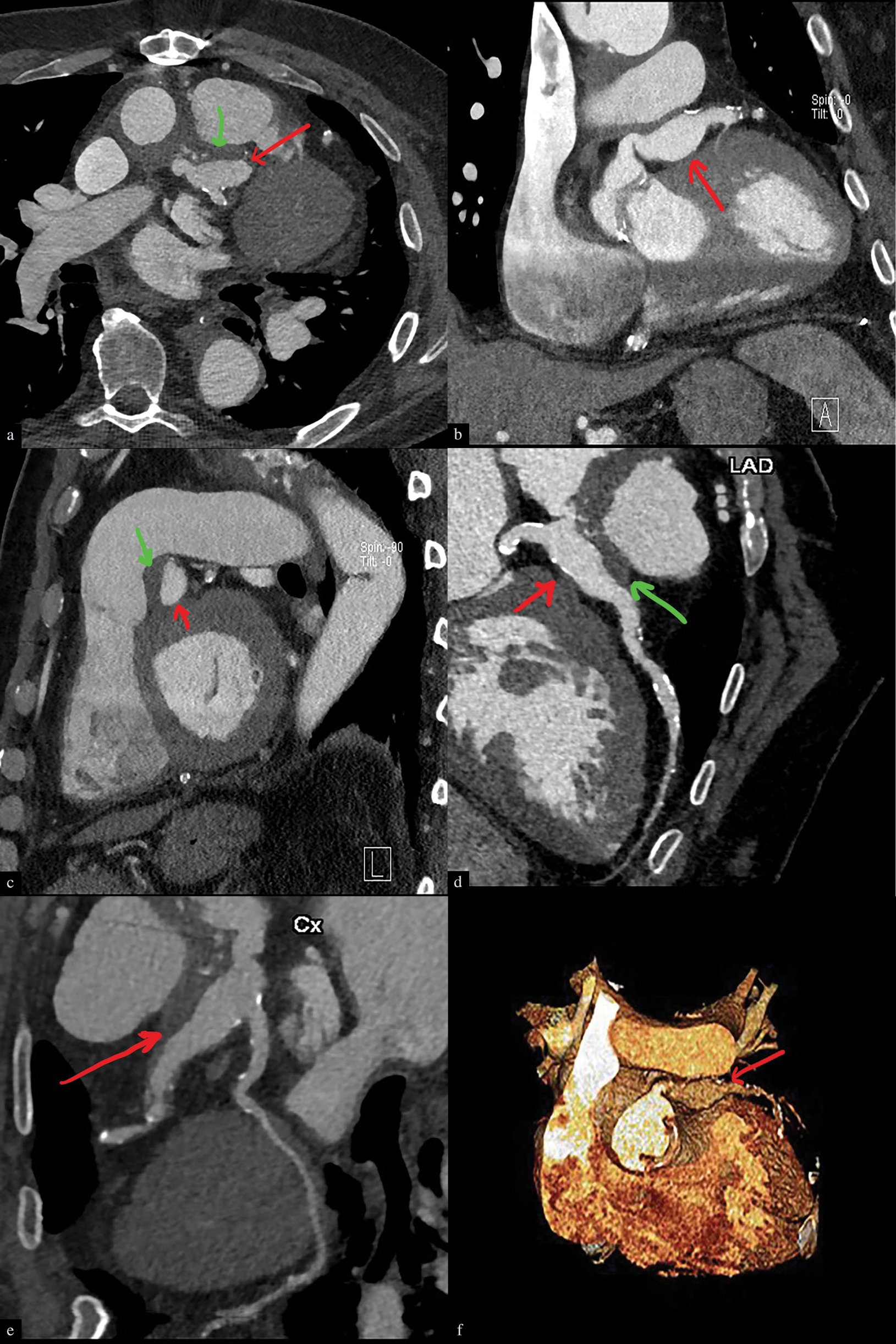
- Infected left coronary artery aneurysm in a 72-year-male, 2-3 weeks post aortic dissection repair and replacement of the aortic valve with fever. Cardiac CT angiography, axial (a), coronal (b), sagittal (c), planar oblique reconstruction (d, e), and 3D reconstruction (f). Images demonstrate a lobulated aneurysm (denoted by red arrows in each image) characterized by a thick wall and perianeurysmal soft tissue stranding. In addition, there is an eccentric thrombus (denoted by green arrows) in the aneurysm.
Hartnell et al. reported a 2.2% incidence of coronary artery aneurysm in male patients with coronary artery disease and 0.5% in women patients.[17]
Coronary artery aneurysms are associated with inflammatory disorders such as Kawasaki’s disease or are congenital in origin, usually present at an earlier age than those associated with atherosclerosis.
Interestingly, genetic predisposition to coronary artery aneurysms is seen mainly related to atherosclerosis and Kawasaki’s disease.[4]
Giant coronary artery aneurysms, vaguely defined as having a diameter of 5-15 cm, are primarily congenital, and the reported incidence is 0.02%.[18]
CLINICAL FEATURES
Most patients are asymptomatic, and coronary artery aneurysms are only detected when the patient is imaged for some other disease.
Whenever CAA are symptomatic, they present with angina or myocardial infarction symptoms. Some authors have also described a precordial systolic murmur associated with CAA. The possibility of coronary artery aneurysms should be considered in young patients with chest pain.[2]
Mycotic (infective) coronary artery aneurysms present mainly with systemic symptoms and can cause acute myocardial infarction.[9] Sometimes pericardial effusions as the presenting complaint of mycotic aneurysms, hence a high index of suspicion should be maintained when evaluating patients with purulent pericarditis.
COMPLICATIONS
The potential complications secondary to mycotic coronary artery aneurysms include rupture, cardiac tamponade, fistulation, myocardial ischemia, or infarction secondary to septic embolization and sudden cardiac death.[19] The literature’s reported mortality rate associated with mycotic coronary aneurysms is 43-53%.[11,20,21]
DIAGNOSIS
Most of the time, coronary artery aneurysms are asymptomatic and are only incidentally discovered when the patient is imaged for other concerns.
The optimal investigation should evaluate the CAA characteristics such as shape (fusiform versus saccular), diameter, wall calcification, and whether other aneurysms are seen elsewhere in the coronary tree. It should also be capable of detecting aneurysm luminal thrombosis and significant stenosis. Possible complications such as myocardial infarction, fistula formation, and rupture should also be ruled in or out at the same time. Preoperative assessment is essential to define the relationship of the aneurysm to the surrounding structures.[5]
Catheter coronary angiogram was previously the “Gold Standard” for evaluating coronary aneurysms; however, it can only evaluate the lumen with no information about the vessel wall or a potential luminal thrombus occluding the lumen and hence can lead to underestimation of the aneurysm size.[22] Coronary angiography is also limited in differentiating between a true aneurysm and a pseudoaneurysm.[4]
Intravascular ultrasound (IVUS) is another helpful modality that evaluates lumen and provides information about the arterial wall. Hence, it can determine complex plaques and detect pseudoaneurysms.[23]
Multidetector CT angiography is the current imaging modality of choice for evaluating suspected mycotic aneurysms, with the advantage of rapid image acquisition, high sensitivity, accessible and widespread availability, high spatial resolution, and multi-planar capabilities.[13,24] Its only limitations are exposure to radiation and iodinated contrast media. That is why it was not considered the gold standard in the evaluation and follow-up of young patients with aneurysms and patients with concurrent renal failure.
Echocardiography, including both transthoracic (2-DE) and transesophageal (TEE), has been used in the diagnosis and follow-up of coronary artery aneurysm, particularly in patients with Kawasaki’s disease. However, limitations in evaluating distal coronary segments and limited information regarding perianeurysmal/perivascular inflammation make this modality out of favor in evaluating coronary aneurysms, particularly mycotic aneurysms.[2,4]
MR Angiography demonstrates similar findings as CTA but is limited by long acquisition times, less common availability, motion susceptibility, and relatively lower spatial resolution.[24] It is mainly used to evaluate children with Kawasaki’s disease and is also helpful in patients in whom CT is contraindicated, related primarily to iodinated contrast media-related adverse effects.
TREATMENT AND PROGNOSIS
Because of the rarity of the CAA, it is not easy to standardize treatment. Also, the natural history of mycotic coronary aneurysms is unclear.[24] Aneurysms smaller than 1-2 cm may resolve with antibiotics; however, larger ones may enlarge and ultimately rupture with resultant cardiac tamponade and sudden death. Therefore, more giant aneurysms should be excised or excluded from circulation, and the distal coronary artery should be revascularized.[25] Most commonly, surgical mycotic coronary aneurysm and distal bypass of the artery are performed. Sometimes deroofing and debridement of the aneurysm combined with arterial ligation and distal bypass are performed. Some authors have advocated the percutaneous placement of covered stents to treat mycotic coronary artery aneurysms; however, surgical treatment is the gold standard of care unless the patient is deemed at higher risk for surgery.[26]
CONCLUSION
Coronary artery aneurysms are rare diseases with mycotic coronary artery aneurysms being even more infrequent, however potentially fatal illness and hence high index of clinical suspicion and increased awareness among radiologists of the pertinent diagnostic/imaging features goes a long way in the management. In summary, early diagnosis, appropriate antibiotic therapy, and immediate surgery remain the mainstay of current treatment for mycotic coronary artery aneurysms.
Declaration of patients consent
Patient consent is not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Coronary artery mycotic aneurysm following endocarditis of a composite aortic graft-a case report and literature review. Angiology. 1998;49:145-50.
- [CrossRef] [PubMed] [Google Scholar]
- Mycotic coronary artery aneurysm from fungal prosthetic valve endocarditis. Ann Thorac Surg. 2007;84:280-2.
- [Google Scholar]
- Coronary artery aneurysms: A review of the epidemiology, pathophysiology, diagnosis, and treatment. Front Cardiovasc Med. 2017;4:24.
- [CrossRef] [PubMed] [Google Scholar]
- Giant mycotic pseudoaneurysm of the left main coronary artery after pneumococcal pneumonia. J Thorac Cardiovasc Surg. 2010;140:e50-2.
- [CrossRef] [PubMed] [Google Scholar]
- Giant saccular aneurysm of the left main coronary artery. J Geriatr Cardiol. 2013;10:110-2.
- [CrossRef] [PubMed] [Google Scholar]
- Aneurysms of the coronary artery. Report of ten cases and review of literature. Am J Cardiol. 1963;11:228-37.
- [CrossRef] [PubMed] [Google Scholar]
- Thoracic Imaging: Pulmonary and cardiovascular radiology 2016.
- Mycotic aneurysm of the right coronary artery presenting as infected pericardial effusion. Circulation. 2014;130:e7-8.
- [CrossRef] [PubMed] [Google Scholar]
- Infected (mycotic) aneurysms: Spectrum of imaging appearances and management. Radiographics. 2008;28:1853-68.
- [CrossRef] [PubMed] [Google Scholar]
- Left main mycotic aneurysm causing myocardial infarction. Can J Cardiol. 2010;26:e276-7.
- [CrossRef] [PubMed] [Google Scholar]
- Mycotic aneurysm of the left anterior descending coronary artery after aortic endocarditis. A case report and brief review of the literature. Tex Heart Inst J. 1994;21:231-5.
- [PubMed] [Google Scholar]
- Successful surgical treatment of an infected right coronary artery aneurysm-to-right ventricle fistula after sirolimus-eluting stent implantation. Intern Med. 2007;46:865-71.
- [CrossRef] [PubMed] [Google Scholar]
- Coronary artery ectasia. Its prevalence and clinical significance in 4993 patients. Br Heart J. 1985;54:392-5.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical treatment of giant coronary artery aneurysm. J Thorac Cardiovasc Surg. 2005;130:817-821.
- [CrossRef] [PubMed] [Google Scholar]
- Successful management of a giant unruptured mycotic coronary artery aneurysm after coronary angioplasty. Indian Heart J. 2016;68:S44-S46.
- [CrossRef] [PubMed] [Google Scholar]
- Infected coronary artery pseudoaneurysm after repeated percutaneous coronary intervention. Ann Thorac Surg. 2011;91:e17-9.
- [CrossRef] [PubMed] [Google Scholar]
- Left main stem rupture caused by methicillin resistant staphylococcus aureus infection of left main stent treated by covered stenting. Int J Cardiol. 2010;144:e39-41.
- [CrossRef] [PubMed] [Google Scholar]
- Spectrum of coronary artery aneurysms: From the radiologic pathology archives. Radiographics. 2018;38:11-36.
- [CrossRef] [PubMed] [Google Scholar]
- Intravascular ultrasound as a significant tool for diagnosis and management of coronary neurysms. CardioVasc Intervent Radiol. 2004;27:666-8.
- [CrossRef] [PubMed] [Google Scholar]
- Left anterior descending coronary artery and multiple peripheral mycotic aneurysms due to mycobacterium bovis following intravesical bacillus calmette-guerin therapy: A case report. J Radiol Case Rep. 2016;10:12-27.
- [CrossRef] [PubMed] [Google Scholar]
- Mycotic left main coronary artery aneurysm following double-valve replacement for active infective endocarditis. Ann. Thorac Cardiovasc Surg. 2013;19:70-2.
- [CrossRef] [PubMed] [Google Scholar]
- Giant mycotic coronary aneurysm associated with late stent infection. Eur Heart J Cardiovasc Imaging. 2014;15:630.
- [CrossRef] [PubMed] [Google Scholar]






