Translate this page into:
Multimodality Imaging of Normal Hepatic Transplant Vasculature and Graft Vascular Complications
Address for correspondence: Dr. Marjorie W. Stein, Department of Radiology, Albert Einstein College of Medicine and Montefiore Medical Center, 111 E. 210th St., Bronx, NY 10467, USA mstein17@aol.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Orthotopic liver transplantation is an important treatment option for patients with end-stage liver disease. Advances in surgical technique, along with improvements in organ preservation and immunosuppression have improved patient outcomes. Post-operative complications, however, can limit this success. Ultrasound is the primary imaging modality for evaluation of hepatic transplants, providing real-time information about vascular flow in the graft. Graft vascular complications are not uncommon, and their prompt recognition is crucial to allow for timely graft salvage. A multimodality approach including CT angiography, MRI, or conventional angiography may be necessary in cases of complex transplant vascular anatomy or when sonography and Doppler are inconclusive to diagnose the etiologies of these complications. The purpose of this article is to familiarize radiologists with the normal post-transplant vascular anatomy and the imaging appearances of the major vascular complications that may occur within the hepatic artery, portal vein, and venous outflow tract, with an emphasis on ultrasound.
Keywords
Doppler
hepatic
spectral
ultrasound
vascular
INTRODUCTION

Orthotopic liver transplantation is an important treatment option for patients with end-stage liver disease. In the United States, 109,126 liver transplants were performed between January 1, 1988 and April 30, 2011 with 6291 transplants performed in 2010. Currently, approximately 17,000 people in the United States are awaiting liver transplantation. Indications for liver transplant include cirrhosis secondary to hepatitis C, hepatitis B, cryptogenic hepatitis, and autoimmune disease. Cholestatic liver diseases for which liver transplantation may be required include biliary cirrhosis, Caroli's disease, and primary sclerosing cholangitis. Other reasons for transplant include biliary atresia, acute hepatic necrosis, metabolic disorders, malignant neoplasms in certain cases, and Budd Chiari syndrome.[1]
Advances in surgical techniques over the past several decades, along with improvements in organ preservation and immunosuppressive therapy have improved patient outcomes after liver transplantation.[2] Post-operative complications, however, can limit this success, especially if not recognized early enough to allow for graft salvage. Among the most common and clinically significant complications to occur in the post-operative period are those of vascular origin. Imaging plays a crucial role in the early diagnosis of such complications. Ultrasound with Doppler is the primary imaging modality for the evaluation of hepatic transplants, providing detailed assessment of vascular flow in the graft. However, a multimodality approach including CT angiography, MRI, or conventional angiography may be necessary to diagnose the complications. This article describes the vascular anatomy of liver transplants and imaging appearances of the major vascular complications that may occur within the hepatic artery, portal vein, and venous outflow tract.
Protocol
All our images have been obtained from a single major urban medical center where all 52 transplants have been performed since our program began three years ago. All liver transplant patients undergo a liver sonogram with comprehensive Doppler evaluation on post-operative day one. The sonographic examination algorithm includes gray-scale evaluation of the graft and biliary tree, color, and spectral Doppler of the main hepatic artery, portal vein, hepatic veins and IVC, their anastomoses and intrahepatic branches. Further imaging is based on clinical follow-up including daily monitoring of laboratory values. If clinical parameters are abnormal, repeat sonography is performed and depending on the results, CT, MRI, or angiography may be requested.
Hepatic artery and hepatic artery complications
Typically, the donor hepatic artery is anastomosed end-to-end to the recipient hepatic or celiac artery. In recipients with a diseased or small hepatic artery, a donor iliac artery or PTFE (polytetrafluoroethylene) interposition graft can be anastomosed to the recipient aorta.[3] Hepatic arterial complications may lead to graft ischemia and are associated with high patient morbidity and mortality. Common complications of graft ischemia include biliary stenosis or necrosis as the biliary tree relies solely on the hepatic arteries for its blood supply [Figures 1 and 2].
- 55-year-old man S/P liver transplant. (a) Axial CT angiography image (CTA) performed 1-month post-transplant shows a patent interposition graft (white arrow) between the abdominal aorta (black arrow) and donor hepatic artery. The superior mesenteric artery (SMA) is indicated (black arrowhead). (b) 5 months later the graft has occluded (white arrow). Abdominal aorta (black arrow) and SMA (black arrowhead) are indicated. (c) Coronal reformat CTA demonstrates patent intrahepatic arterial collaterals (black arrows). (d) MRCP coronal thick slab demonstrates two biliary strictures in the common duct secondary to arterial ischemia (white arrows).
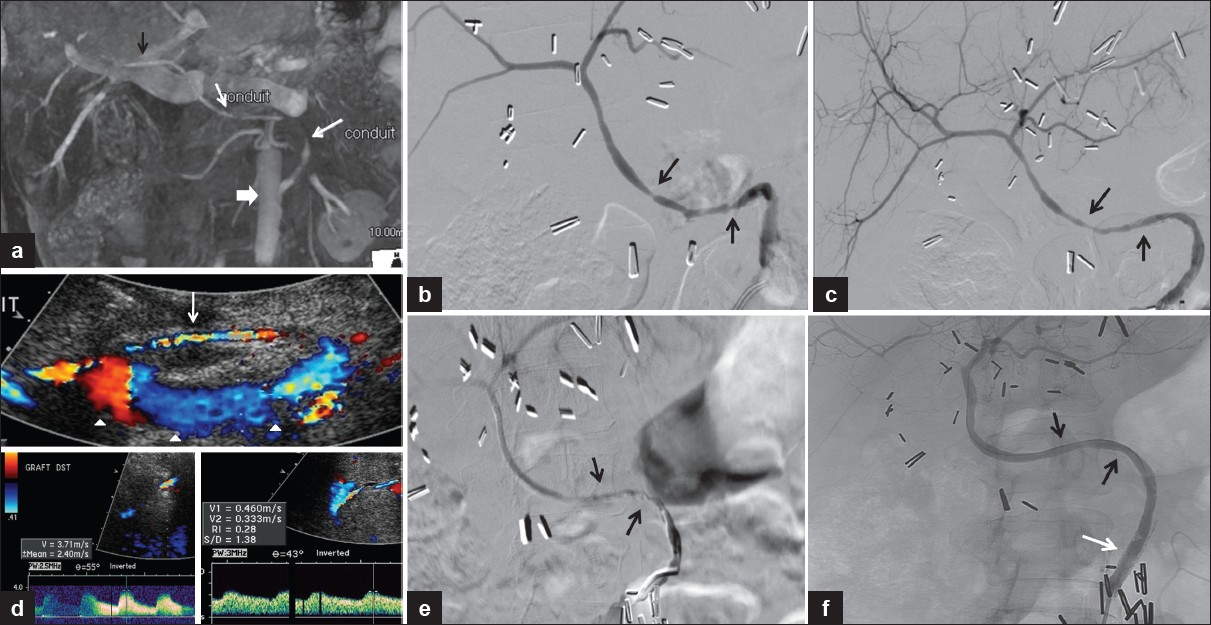
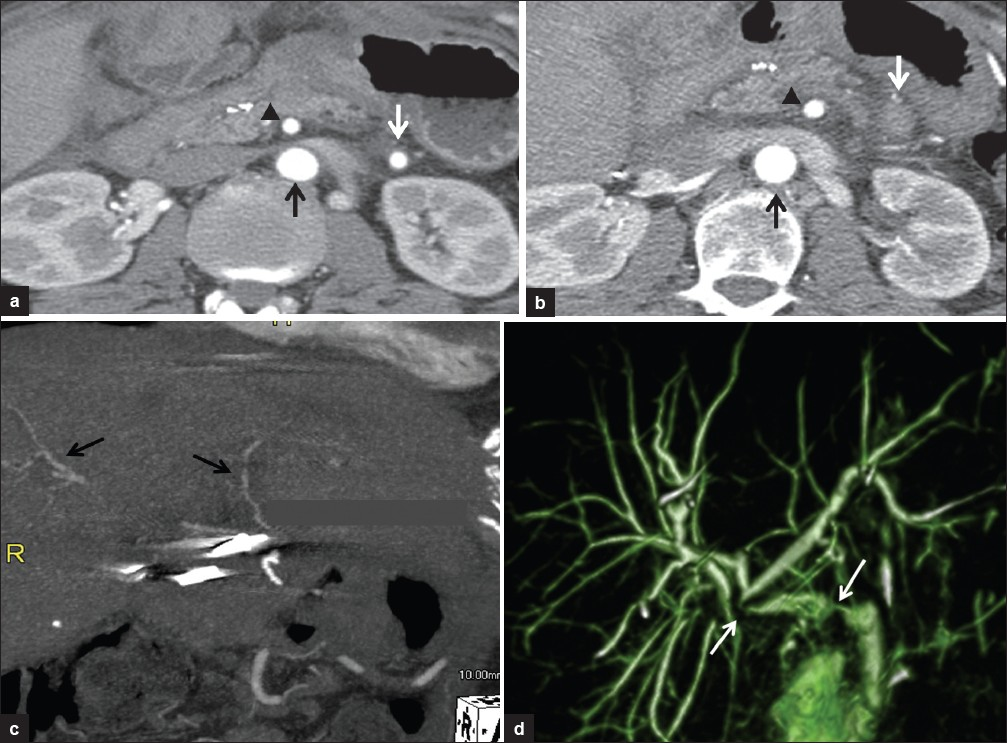
- 60-year-old woman status post-liver transplant with an interposition graft between the aorta and the donor hepatic artery with questionable findings for graft stenosis on sonography. (a) Coronal MIP reformatted image of contrast-enhanced MRI reveals areas of interruption of the contrast column in the graft (white arrows) between the aorta (block arrow) and donor hepatic artery (black arrow) consistent with significant stenoses. (b) Conventional angiography confirming the strictures (arrows). (c) Improvement after balloon angioplasty (arrows). (d) Subsequent US color Doppler. Top image: Long segment of turbulent flow in the graft with color aliasing (white arrow). The abdominal aorta is indicated (arrowheads). Bottom right: Spectral Doppler reveals persistent graft stenosis post-angioplasty with elevated peak systolic velocity in the distal graft. Bottom left: Intrahepatic tardus-parvus waveform. (e) Conventional angiography showing persistent sequential stenoses (arrows). (f) Stenoses were treated with balloon angioplasty with excellent results (black arrows) and a stent was placed in the proximal graft (white arrow).
The incidence of hepatic artery thrombosis is 4-12% in adults and 42% in children. Risk factors include donor and recipient arterial caliber discrepancy, use of an interposition conduit, acute rejection, and excessive cold ischemia time.[2] Hepatic artery thrombosis can occur in the early or late post-transplant periods. Early thrombosis is a dreaded complication associated with liver failure, biliary leak, sepsis, and a high mortality rate of 20-60%.[4] Prompt diagnosis is crucial, generally necessitating immediate graft revascularization. Even with early recognition of thrombosis, 60% of patients will still require retransplantation. Patients with hepatic artery thrombosis can occasionally present, many years post-transplant, with sepsis and chronic rejection.[4]
Hepatic artery stenosis occurs in 5-11% of liver transplant recipients usually in the first 100 days post-transplant. It is often at the anastomotic site, due to clamp injury and intimal trauma.[25]
The normal hepatic arterial spectral flow waveform demonstrates a low resistance pattern with a brisk systolic upstroke. The systolic upstroke acceleration time should be <0.08 s. The normal hepatic arterial resistivity index (RI) ranges from 0.50 to 0.80. In the first 72 hours post-transplant, the RI may be greater than 0.80 due to vascular spasm and post-surgical swelling, although it generally returns to normal in a few days.[236]
In main hepatic artery thrombosis, the artery may not be seen on gray-scale. Abnormal Doppler findings of hepatic artery thrombosis are absent color power Doppler and spectral flow in the main or intra-hepatic arteries.
Hepatic arterial collaterals may develop in chronic thrombosis and demonstrate low intrahepatic arterial RIs, mimicking stenosis.[7] Therefore, the sensitivity of ultrasound for the detection of hepatic artery thrombosis decreases as the interval following transplant increases.[8]
With hemodynamically significant hepatic artery stenosis, a segmental increase in flow velocity is found at the post-stenotic jet. A focal peak systolic velocity (PSV) in the main hepatic artery of greater than 2.0 m/s indicates significant stenosis.[9] Color Doppler imaging can be used to rapidly identify the line of maximal velocity associated with the stenotic site, by carefully reducing the color Doppler scale to produce the slightest degree of aliasing, which can be recognized as a change in Doppler color at the brightest portion of the color spectrum. Intrahepatic arteries may have a tardus-parvus waveform with a prolonged systolic upstroke acceleration time and low intrahepatic RI(s) of less than 0.50[2][Figure 2]. In a recent study by Park et al, the use of post-stenotic hepatic arterial flow velocity in conjunction with the finding of a tardus-parvus wave pattern can significantly increase the positive predictive value of Doppler for diagnosing hepatic artery stenosis.[10]
Pseudoaneurysms
Pseudoaneurysms are a rare but potentially catastrophic complication of hepatic transplantation, often occurring at the anastomotic site.[4] They can rupture or impair flow in the graft vasculature or biliary tree. They may develop secondary to infection, graft biopsy, or transhepatic catheter/drain placement. They are often clinically occult or may present with nonspecific graft dysfunction or abnormally elevated serum liver enzyme levels. On sonography, they appear as cystic masses that fill on color Doppler. Contrast-enhanced CT is superior to sonography in identifying small pseudoaneurysms, as bowel gas may obscure a tortuous extrahepatic hepatic artery. CT angiography or conventional angiography is performed if there is suspicion of graft-related bleeding or unexplained graft malfunction.[1112]
Portal vein and portal vein complications
The donor portal vein is usually anastomosed end-to-end to the recipient portal vein. However, if there is pre-existing thrombosis of the recipient portal vein, thrombectomy or placement of a jump graft between the donor portal vein and recipient superior mesenteric or splenic vein can be performed.
Normally, the portal vein is smooth walled with an anechoic lumen. Mild focal narrowing at the anastomosis is a common finding which does not necessarily imply stenosis. On Doppler examination, there should be continuous monophasic hepatopetal venous flow with gentle respiratory undulation.[2] Turbulent flow with elevated portal venous flow velocities may be present in the early post-operative period, decreasing over time in the absence of significant stenosis.
Portal vein stenosis and thrombosis are rare complications with an incidence of 1-2%.[4] They occur more frequently in the pediatric population and after living donor transplants. Patients present with liver failure, portal hypertension, peripheral edema, and ascites.[4] Predisposing factors include previous portal vein surgery or thrombosis, high downstream resistance due to an inferior vena cava (IVC) stricture, hypercoagulable state, and technical problems during surgery such as mismatched vessel size or vascular kinking. Treatments include angioplasty, stent placement, thrombolysis, surgical repair with thrombectomy, venous jump graft or portosystemic shunt creation.[13]
Gray-scale findings of portal vein thrombosis may include a hyperechogenic clot; however, because acute thrombus can be isoechoic or hypoechoic, color Doppler imaging should be performed. With acute thrombosis, the portal vein distends; in chronic thrombosis, the vessel caliber decreases. Very slow portal venous flow may simulate thrombosis, even with power Doppler, and so further evaluation with other imaging modalities such as CT, MRI or angiography may be appropriate.[2] If arterial flow is detected within the clot, tumor recurrence must be suspected.
Sonographic findings of portal vein stenosis include vascular narrowing with segmental color Doppler aliasing and post-stenotic dilatation. Duplex imaging reveals at least a 3- to 4-fold velocity gradient across the stenosis[2][Figure 3]. In a study by Mullan et al, a peak portal vein flow velocity of 80 cm/s diagnosed portal vein stenosis with a sensitivity of 100% and specificity of 84%.[14] In a study by Chong et al, a peak portal vein velocity of greater than 125 cm/s had only 73% sensitivity but a specificity of 95%.[15] With chronic portal thrombosis and cavernous transformation, an attempt should be made to differentiate large venous collaterals in the porta hepatis from the main portal vein and intrahepatic branches.
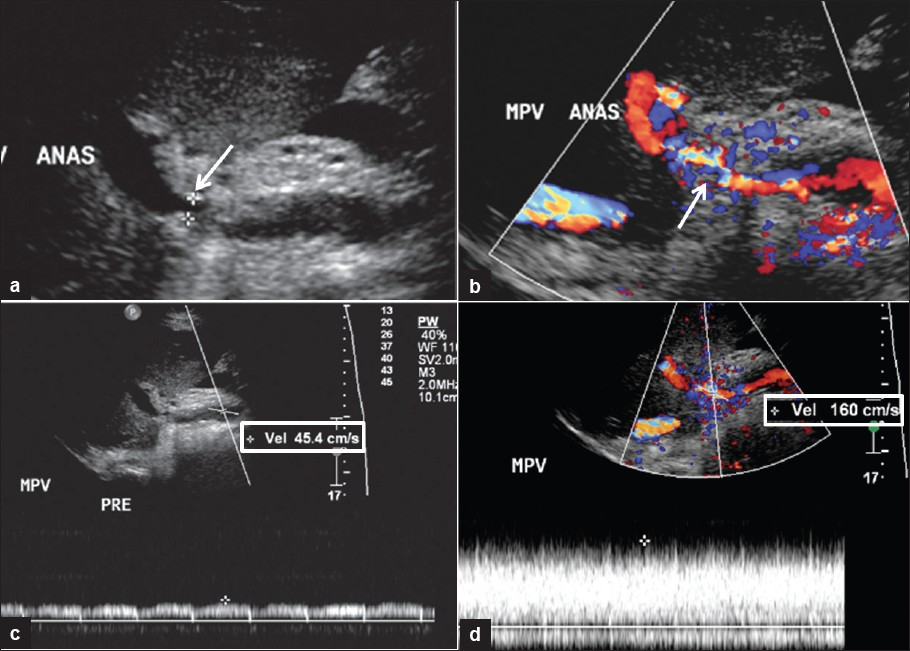
- 57-year-old man S/P liver transplant. Ultrasound images demonstrating portal vein stenosis. (a) Gray scale shows narrowing at the portal vein anastomosis (arrow). (b) Color Doppler shows aliasing at flow jet (arrow). (c) Spectral Doppler of pre-stenotic portal vein. (d) Spectral Doppler with high velocity at the post-stenotic jet, which is 3.5 times higher than peak velocity in the pre-stenotic portal vein.
Decreased portal venous flow velocity may be seen downstream from portal vein stenosis, but it can also be associated with increased hepatic resistance secondary to rejection or recurrent parenchymal disease. Vascular steal phenomenon directing portal flow away from the graft may occur when large pre-transplant portosystemic shunts, commonly splenorenal, are not ligated at the time of transplant.[16] In vascular steal, the portal venous flow may have decreased velocity or variable direction. A pulsatile portal venous waveform may be normal in the early post-operative period. If there is cardiac disease or arterioportal shunting, the pulsatile waveform will persist.[14]
Hepatic vein, inferior vena cava, and complications
In orthotopic liver transplantation, there are two main surgical techniques for reestablishing the venous outflow tract. In one, the intrahepatic segment of the recipient inferior vena cava is explanted with the diseased liver and replaced en bloc with the donor intrahepatic cava and graft. Anastomosis usually is performed end-to-end, at both the suprahepatic and infrahepatic segments of the recipient's residual IVC [Figure 4a].
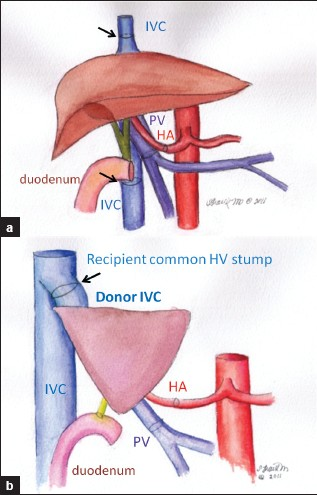
- Schematic drawings of surgical techniques. (a) Supra and infrahepatic end-to-end caval anastomoses. (b) Piggyback technique: The donor IVC or hepatic vein is anastomosed to the recipient common hepatic vein stump (arrow).
An alternative transplant procedure focuses on maintaining an intact recipient inferior vena cava avoiding venovenous bypass intraoperatively. In the Piggyback technique, a widely used form of caval-preserving surgery, a short segment of the donor IVC is anastomosed to the common trunk of the recipient's main hepatic veins [Figure 4b]. In partial hepatic transplants, one of several techniques can be used to anastomose the donor hepatic vein(s) to the recipient IVC.
Complications involving the venous outflow tract are uncommon, reportedly occurring in <1-10% depending on whether the procedure is orthotopic or partial.[21517] Patients can develop stenosis or thrombosis of the inferior vena cava or main hepatic veins, most commonly at an anastomotic site. Pediatric, re-transplant,[4] and partial graft recipients are especially at risk.
Patients with inferior vena caval or hepatic venous complications may present with pain, ascites, hepatomegaly, pleural effusion, peripheral edema,[18] or abnormal serum liver enzyme levels.[17] Hepatic venous congestion can cause graft malfunction and failure. Caval obstruction can result in renal failure. In the acute post-surgical period, caval stenosis is usually due to anastomotic stricture or caval compression from graft swelling, hematoma or other post-operative fluid collection.[3] With pediatric or living-related donor grafts, anastomotic vascular or graft-recipient size discrepancies predispose to stenosis. Vascular redundancy and graft rotation in the latter group may result in venous kinking[19] or frank torsion. Delayed stenosis can result from patient growth or graft enlargement, fibrosis, chronic thrombus, or neointimal hyperplasia.[4] Acute thrombosis can develop in patients with hypercoagulable states.[20]
On gray-scale sonography, findings of venous outflow compromise may include caval or anastomotic narrowing with pre-stenotic dilatation of the IVC and/or hepatic veins. Hyperechogenic intraluminal thrombus may be identified. Color Doppler imaging frequently reveals vascular narrowing and aliasing at the stenotic site where flow velocities are abnormally elevated. Isoechoic or hypoechoic thrombus, not visible on gray-scale imaging, may be identified as an intravascular filling defect with color Doppler [Figure 5].
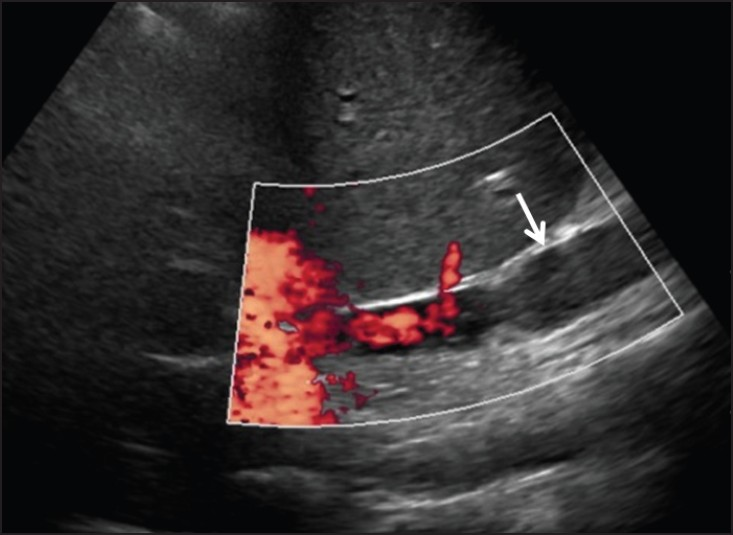
- 68-year-old man three months status post liver transplant with IVC clot demonstrated with color Doppler (arrow).
Duplex/spectral imaging typically demonstrates dampening with loss of pulsatility of the normally triphasic flow wave patterns in the pre-stenotic cava and/or hepatic veins. This is due to buffering of these latter regions from right atrial pressure changes. A monophasic wave pattern in the cava and hepatic veins is nonspecific, often seen in the acute post-surgical period [Figure 6].[15] Even a persistent monophasic flow wave pattern in the hepatic veins is non-specific for venous complications,[21] as it is also seen with graft rejection or recurrent parenchymal disease. Post-transplant caval or hepatic venous triphasic wave pattern has a high negative predictive value for downstream stenosis and graft rejection.[2122] With hemodynamically significant stenosis, there is an at least 3- to 4-fold flow velocity gradient across the stenosis [Figure 7].
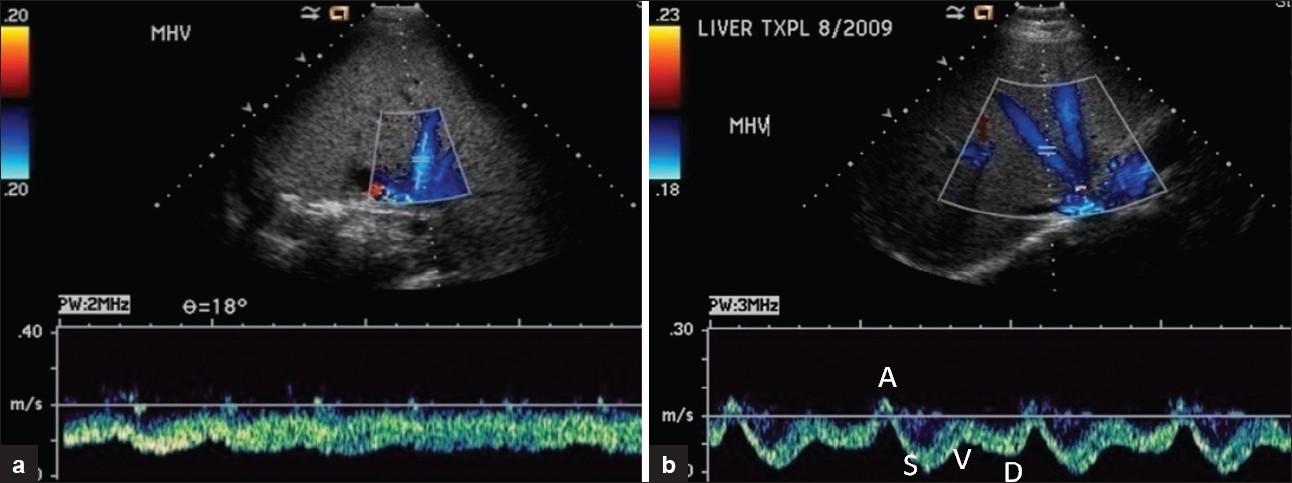
- (a) Orthotopic hepatic transplant on post-operative day 5. Spectral Doppler reveals dampened hepatic vein waveform. (b) Same patient one month after surgery with spectral Doppler showing normal triphasic hepatic venous flow.
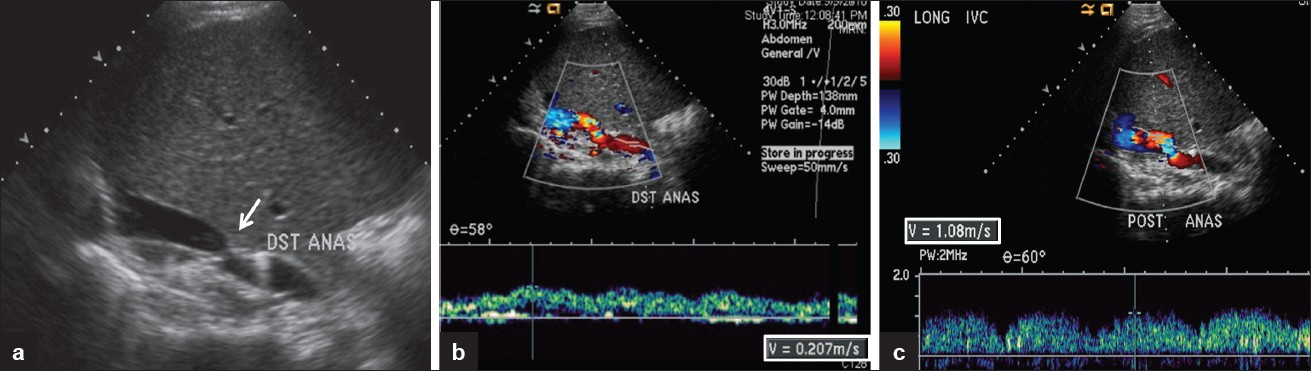
- 55-year-old female status post liver transplant. (a) Narrowing at the caval anastomosis on gray scale (arrow). (b) Pre-stenotic IVC. The spectral waveform is dampened with maximum flow velocity measured at 20 cm/s. (c) Spectral Doppler shows dampened waveform and increased flow velocity at the post-stenotic jet 4.8 times higher than the maximum flow velocity in the pre-stenotic IVC.
No intravascular spectral flow is demonstrated in regions of venous thrombosis. In severe cases of venous outflow stenosis or in thrombosis, there can be reversed flow in one or more main hepatic veins[18] or in the portal vein.
Role of computed tomography, magnetic resonance imaging, and conventional angiography
Post contrast computed tomography/angiography (CT/CTA) or magnetic resonance imaging/angiography/cholangiopancreatography (MRI/MRA/MRCP) are ancillary modalities used to evaluate vascular and nonvascular complications of hepatic transplants[ Figure 1]. These imaging techniques are especially valuable in patients with complex, atypical, or obscured post-surgical anatomy on sonography, or in patients with inconclusive or conflicting ultrasound findings. CT and MRI have additional imaging advantages not offered by sonography. These include demonstrating “nutmeg” enhancement pattern of the congested liver in the setting of venous outflow obstruction, hepatic infarction [Figure 8], and biliary abnormalities. These modalities also demonstrate the extent of post-operative fluid collections and other abnormalities, which may be partially obscured by gaseous interference on sonography [Figure 9].
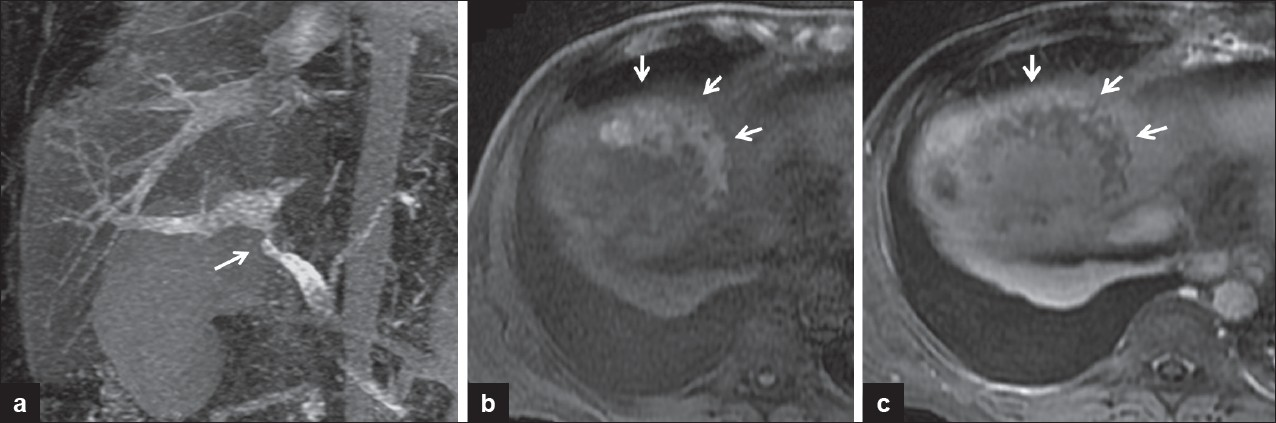
- (a) MIP coronal reformatted image of contrast-enhanced MRI. Portal vein stenosis (arrow). (b) Axial pre-contrast fat saturated T1-weighted MRI image demonstrates hemorrhagic infarction in the right hepatic dome evidenced by increased signal (arrows). (c) Axial post-contrast T1-weighted MRI image with subtraction showing no enhancement in the infarcted area (arrows).
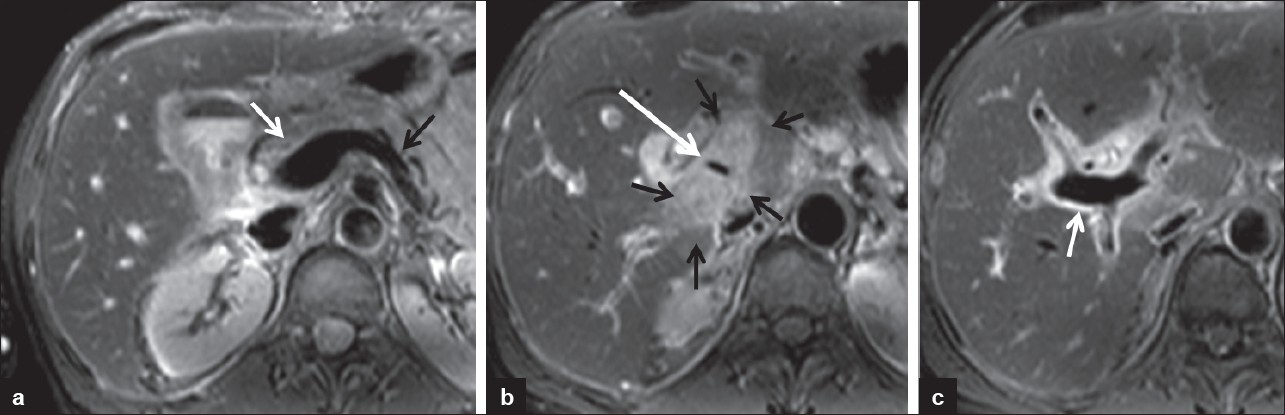
- 52-year-old man S/P liver transplant with extrinsic portal vein compression by an inflammatory mass. MRI axial T2-weighted images shows (a) signal void of the pre-stenotic main portal vein (white arrow) at the confluence of splenic vein (black arrow) and superior mesenteric vein. (b) An inflammatory mass which is hyperintense on T2-weighted images (small black arrows) causes extrinsic compression of the main portal vein evidenced by decreased caliber of signal void (long white arrow). (c) Signal void of the post-stenotic portal vein at the hilum (arrow).
Conventional angiography is generally used for problem solving and in preparation for intended vascular interventions. During catheterization, pressure gradients across stenotic vascular segments can be determined. Subsequent balloon angioplasty and stent placement[1723] are frequently successful in salvaging the graft, especially if early diagnosis is made [Figure 2].
SUMMARY
Sonography is the primary imaging tool for diagnosis of early and delayed vascular complications in liver transplant patients. After rejection, vascular complications are the most common cause of hepatic graft failure. This review of the normal sonographic post-transplant vascular anatomy and the imaging appearances of the major hepatic artery, portal vein, and venous outflow tract complications that may occur will assist the radiologist in identifying post-transplant vascular pathology. Recognition of the Doppler and cross-sectional imaging manifestations of normal and abnormal hepatic graft vasculature is essential for appropriate and timely management to ensure graft salvage. In cases of inconclusive ultrasound findings, a multimodality approach, including CT angiography, magnetic resonance imaging, or conventional angiography may be necessary.
Source of Support: Nil
Conflict of Interest: None declared.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2011/1/1/50/86665
REFERENCES
- US Government Web Site. US Department of Health and Human Services. Available from: http://optn.transplant.hrsa.gov
- [Google Scholar]
- Complications of liver transplantation: Multimodality imaging approach. Radiographics. 2007;27:1401-17.
- [Google Scholar]
- Postoperative imaging in liver transplantation: What radiologists should know. Radiographics. 2010;30:339-51.
- [Google Scholar]
- Hepatic artery stenosis after liver transplantation: Incidence, presentation, treatment and long-term outcome. Transplantation. 1997;63:250-5.
- [Google Scholar]
- Significance of and contributing factors for a high resistive index on Doppler sonography of the hepatic artery immediately after surgery: Prognostic implications for liver transplant recipients. AJR. 2003;181:831-8.
- [Google Scholar]
- Doppler ultrasound findings in the hepatic artery shortly after liver transplantation. AJR. 2009;193:128-35.
- [Google Scholar]
- Sonographic diagnosis and outcome of hepatic artery thrombosis after orthotopic liver transplantation in adults. AJR. 2007;189:346-51.
- [Google Scholar]
- Noninvasive imaging of liver transplant complications. Tech Vasc Interv Radiol. 2007;10:191-206.
- [Google Scholar]
- Hepatic arterial stenosis assessed with Doppler US after liver transplantation: Frequent false-positive diagnoses with tardus parvus waveform and value of adding optimal peak systolic velocity cutoff. Radiology. 2011;260:884-91.
- [Google Scholar]
- Pseudoaneurysms following orthotopic liver transplantation: Clinical and radiologic manifestations. Transplant Proc. 1989;21(1 Pt 2):2457-9.
- [Google Scholar]
- Hepatic artery pseudoaneurysms in adult living-donor liver transplantation: Efficacy of CT and Doppler Sonography. AJR Am J Roentgenol. 2005;184:1549-55.
- [Google Scholar]
- Clinical Doppler Ultrasound. (2nd ed). Philadelphia, USA: Churchill Livingstone Elsevier; 2006. p. :215-44.
- [Google Scholar]
- Can Doppler sonography discern between hemodynamically significant and insignificant portal vein stenosis after adult liver transplantation. AJR 2010. ;195:1438-43.
- [Google Scholar]
- Sonographic evaluation of venous obstruction in liver transplants. AJR Am J Roentgenol. 2007;188:W515-21.
- [Google Scholar]
- Vascular steal of the portal vein after orthotopic liver transplant: Intraoperative sonographic diagnosis. J Ultrasound Med. 2010;29:125-8.
- [Google Scholar]
- Endovascular treatment of hepatic venous outflow obstruction after living-donor liver transplantation. J Vasc Interv Radiol. 2002;13:591-9.
- [Google Scholar]
- Pediatric liver transplantation: A pictorial essay of early and late complications. Radiographics. 2006;26:1187-209.
- [Google Scholar]
- Complications of orthotopic liver transplantation: Spectrum of findings with helical CT. Radiographics. 2001;21:1085-102.
- [Google Scholar]
- Hepatic vein stenosis after living donor liver transplantation: Evaluation with Doppler ultrasound. Radiology. 2003;229:806-10.
- [Google Scholar]
- Orthotopic Liver Transplants in Children: Change in Hepatic Venous Doppler Wave Pattern as an Indicator of Acute Rejection. Radiology. 2003;226:105-12.
- [Google Scholar]
- Primary Gianturco stent placement for inferior vena cava abnormalities following liver transplantation. J Vasc Interv Radiol. 2000;11:177-87.
- [Google Scholar]






