Translate this page into:
MRI Virtual Biopsy of T2 Hyperintense Breast Lesions

*Corresponding author: Swati Sharma, Department of Radiology, University of Florida, 655 W 8th Street, 2nd Floor, Clinical Center, Jacksonville - 32209, Florida, United States. swati.sharma@jax.ufl.edu
-
Received: ,
Accepted: ,
How to cite this article: Sharma S, Nwachukwu C, Wieseler C, Elsherif S, Letter H, Sharma S. MRI Virtual biopsy of T2 hyperintense breast lesions. J Clin Imaging Sci 2021;11:18.
Abstract
A wide variety of benign and malignant breast processes may generate hyperintense signal at T2-weighted magnetic resonance imaging (MRI). MRI has been traditionally used in the pre-treatment planning of breast cancer, in assessing treatment response and detecting recurrence. In this comprehensive review, we describe and illustrate the MRI features of a few common and uncommon T2 hyperintense breast lesions, with an emphasis on MRI features that help to characterize lesions based on morphological features, specific appearances on T1-and T2-weighted imaging, and enhancement characteristics on the dynamic post-contrast phase that are either diagnostic or aid in narrowing the differential diagnosis.
Keywords
Breast
Mass
Lesion
Magnetic resonance imaging
T2 hyperintense
INTRODUCTION
T2 hyperintense breast lesions can have inflammatory, infectious, or neoplastic etiologies. The histopathologic background for T2 hyperintensity of these breast lesions can be cystic or microcystic components, adipose or sebaceous components, mucinous or loose myxoid stroma, edema, necrosis or hemorrhagic changes.[1] The breast is made of three components: Glandular, adipose, and fibrous tissues. Cooper’s ligaments are the suspensory ligaments of the breast gland and divide the parenchyma into lobes. The glandular structure is composed by 15–20 lobes arranged in clusters with an irregular radial pattern around and behind the nipple. Each lobe is an independent glandular entity made of numerous lobules, constituted by alveoli, which are the secreting units. The alveolar ducts converge into the lobular ducts which, in turn, converge into the milk ducts. The milk ducts, then, converge to the nipple with an ampullary dilatation, the lactiferous sinus. The stroma is composed of dense fibrous and adipose tissues that surround the entire gland and penetrate between the lobes. The breast parenchyma is contained by a two-layer fold of the subcutaneous fascia that may be divided in two parts: The superficial layer that covers the gland and the deep layer, which covers the posterior portion of the gland and separates it from the pectoralis major muscle.[2]
Magnetic resonance imaging (MRI) is the most sensitive technique for characterization of the breast anatomy and disease processes. It allows the study of areas that are not clearly visible on conventional techniques such as the posterior part of the breast and the chest muscles. MRI has the advantage of superior soft-tissue contrast resolution between lesion and adjacent breast tissue, which is enhanced by the use of fat saturation techniques.
T2-weighted MRI sequences are useful for identifying diseased tissue in almost any part of the body; such tissue often is associated with free water, which produces hyperintense signal on T2-weighted images. Following gadolinium administration, three possible enhancement kinetics have been described for lesions on breast MRI; continuous increase in enhancement with time (progressive or persistent pattern), initial uptake followed by plateau phase later (plateau pattern) and rapid initial uptake, and reduction in enhancement later (washout pattern). The wash-in of contrast media inside a lesion and the subsequent wash-out, can be used to differentiate lesions that have a slow continuous wash-in (typically benign lesions) from lesions that have a fast wash-in and a fast wash-out (typically malignant lesions).[2] Broadly, the potential for malignancy increases from persistent to washout kinetics.
The breast can be involved in various disease processes, including benign and malignant lesions. In this article, we review the MRI features of T2 hyperintense breast lesions and discuss how MRI can help narrow the differential diagnosis and be used by the radiologist as a virtual biopsy tool.
BENIGN LESIONS
Cysts
Breast cysts [Figure 1] are the most common benign breast lesion which most commonly occur between the ages of 30 and 50 years old.[3,4] Similar to most benign breast lesions, breast cysts present as palpable round, mobile breast nodules on physical examination.[3,4] They originate from dilated terminal duct lobular units containing serous, serosanguinous, and proteinaceous fluid.[4,5] Based on pathogenesis, internal contents, and imaging features, breast cysts can be categorized into simple versus complicated.
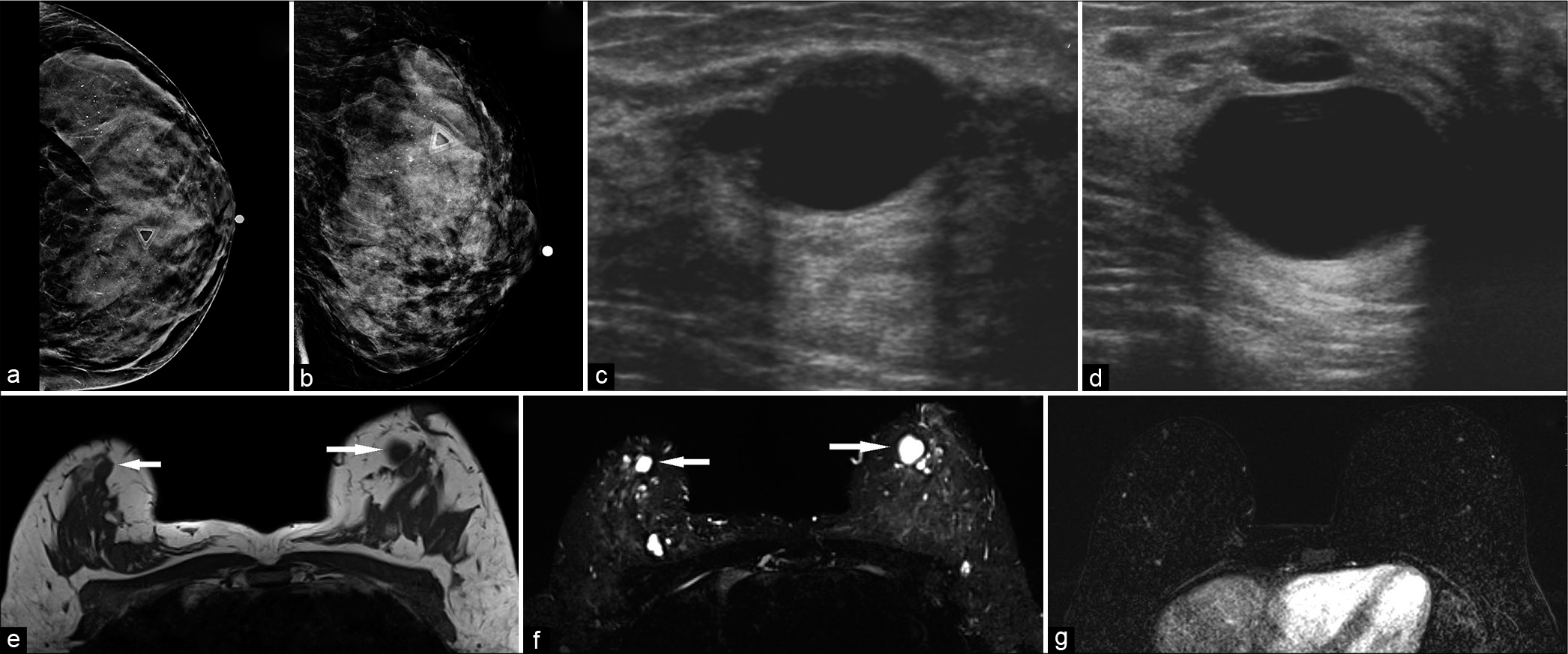
- A 42-year-old female at high risk for breast cancer (multiple family members with breast cancer), presented with palpable lump in the left breast. Diagnostic mammogram CC (a) and MLO (b) views demonstrated extremely dense breasts with indeterminate calcifications. No definite abnormality could be identified underneath the palpable lump marker. The left breast ultrasound transverse (c) and sagittal (d) views demonstrated a 2.6 × 1.6 × 2.8 cm (AP × TR × CC) anechoic mass, with thin imperceptible walls and posterior acoustic enhancement, consistent with cyst, corresponding to the area of palpable lump in the left breast, at 11:30 position, 2 cm from the nipple. Magnetic resonance imaging both breasts demonstrated T1 hypointense (e) and T2 hyperintense (f) well-circumscribed round or oval masses in both breasts (white arrows) which did not show any post-contrast enhancement (g). The palpable lump in the left breast, 2 cm from the nipple, also corresponded to a cyst.
On mammography, both simple and complicated cysts exhibit round or oval morphology with circumscribed margins, typically of equal density to the adjacent fibroglandular tissue. Ultrasound is the diagnostic modality for detecting cysts and helps delineate simple versus complicated cysts. For example, simple cysts will appear anechoic with a thin, imperceptible wall and posterior acoustic enhancement while complicated cysts contain septations and internal echogenic debris with possible fluid-fluid levels.[3,4] MRI features depend on the internal cystic components. Simple cysts typically contain serous fluid and therefore hyperintense on T2-weighted imaging (WI), hypointense on T1WI, and lack contrast enhancement.[3-5] Complicated cysts arise from infectious or inflammatory etiologies and contain internal blood products or proteinaceous debris. As a result, complicated cysts demonstrate a heterogeneously increased or decreased T2 signal intensity, increased T1 signal intensity, and positive rim enhancement on post-contrast imaging.[3-5]
Seroma and hematoma
Breast seromas and hematomas [Figure 2] are loculated fluid collections which accumulate in the post-surgical bed with a reported frequency of 20–50% following radical mastectomy and 9–20% following lumpectomy.[6] On ultrasound, seromas may present as simple or complex cysts with internal echogenic debris.[6] On MRI, seromas display decreased T1 signal intensity, increased T2 signal intensity, and a thin layer (<4 mm) of peripheral enhancement.[3]
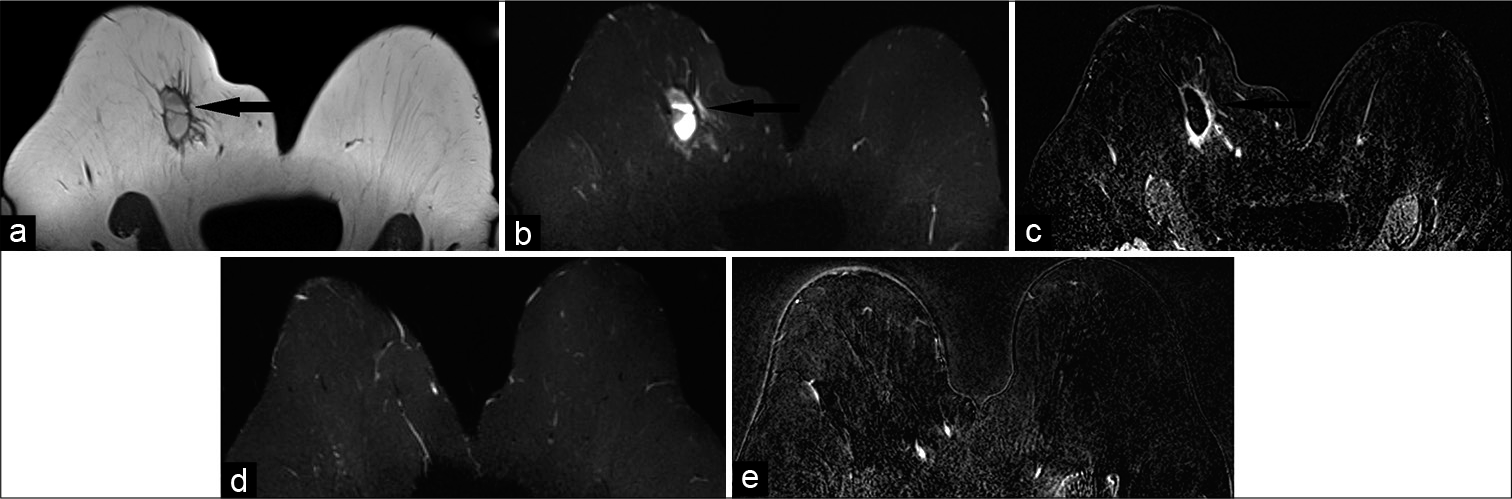
- A 65-year-old female who was status post right breast lumpectomy for invasive ductal carcinoma at 12’o position, 9 cm from the nipple, presented for follow-up imaging. Magnetic resonance imaging (MRI) breasts demonstrated T1 hypointense (a), T2 hyperintense (b) mass measuring 4.2 × 1.9 × 2.5 cm (AP × TR × CC) in the upper slightly inner right breast (black arrow), 10.5 cm from the nipple. Dynamic contrast-enhanced MRI (c) demonstrated rim enhancement of the mass (black arrow), consistent with post-surgical hematoma/ seroma. In addition, mild non-mass-like enhancement was noted adjacent to the collection. After 6 months, follow-up MRI-T2 (d) and contrast-enhanced (e) images showed resolution of the mass, confirming prior diagnosis of post-surgical hematoma/seroma.
Breast hematomas may clinically present with skin ecchymosis and result following trauma, anticoagulation therapy, and surgery with a reported incidence of 2–10%.[6] MRI features are variable due to age of blood products, but will classically exhibit T1 hyperintensity, T2 hypointensity or hyperintensity, and lack of contrast uptake.[3,4]
Intramammary lymph nodes
Intramammary lymph nodes [Figure 3] are a relatively common incidental finding on breast imaging. They typically reside in the upper-outer quadrant of the breast and/or axilla. Normal lymph nodes are composed of lymphoid clusters surrounded by a thin fibrous capsule with internal vessels and a central fatty hilum.
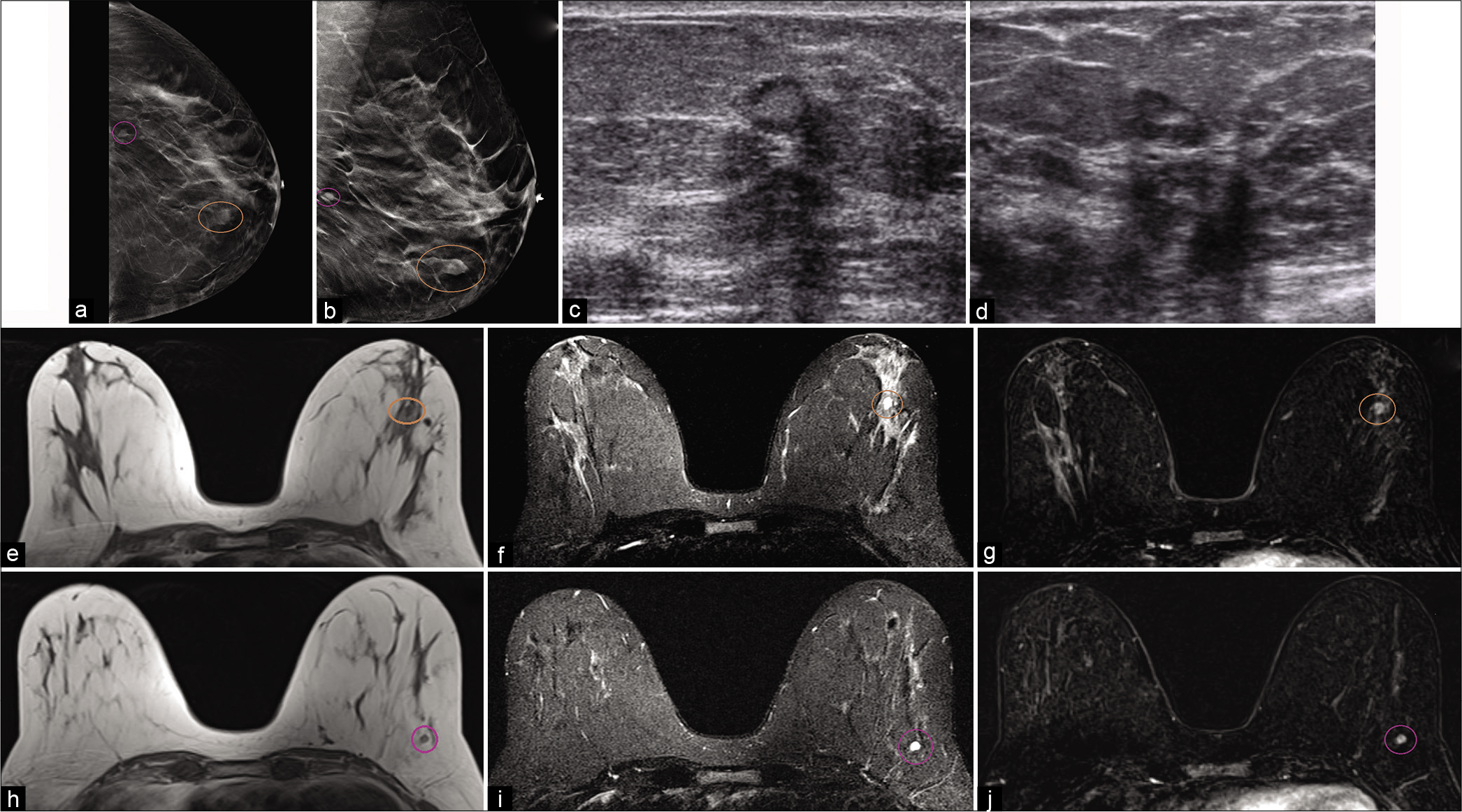
- A 51-year-old female had an abnormal screening mammogram. CC (a) and MLO (b) tomosynthesis views demonstrated an oval mass with indistinct margins in the left lower breast, 4.5 cm from the nipple (brown circle) and another mass with circumscribed margins in the left lower outer breast, 9 cm from the nipple (purple circle). Targeted ultrasound of the left breast – transverse (c) and sagittal (d) images demonstrated sub-centimeter masses, one was an oval parallel hypoechoic mass with central fat, well-circumscribed margins, at 3’o position, 4 cm from the nipple and another was a well circumscribed mass in the left lower outer quadrant, 9 cm from the nipple. Magnetic resonance imaging (MRI) breasts demonstrated similar circumscribed T1 hypointense and T2 hyperintense masses one at 3’o position (e-g) T1, T2, and contrast enhanced T1 images, respectively, 4 cm from the nipple (brown circle) and another in the left lower outer quadrant (h-j) T1, T2, and contrast-enhanced T1 images, respectively, 9 cm from the nipple (purple circle). Dynamic contrast-enhanced MRI demonstrated homogeneous enhancement within the masses. Findings consistent with intramammary lymph nodes.
Normal intramammary lymph nodes with preserved architecture are small hypoechoic masses surrounded by a thin capsule and hyperechoic fatty hilum on ultrasound. On mammogram, they are reniform or oval in shape with circumscribed borders and may contain a small fat density hilum.
On MRI, intramammary lymph nodes are T2 hyperintense and predominantly T1 hypointense with a central hyperintense fatty hilum.[3,5] Secondary to their prominent vascular supply, they can demonstrate washout enhancement pattern.
Cavernous hemangioma
Cavernous hemangiomas [Figure 4] of the breast are rare, benign vascular malformations with a reported incidence of 1.2–11%.[6,7] Microscopically, they are dilated, thin-walled vessels congested with erythrocytes and potential calcifications.[8] Furthermore, these vascular lesions are subdivided into capillary, cavernous, and venous subtypes depending on vascular channel size.[6,8] Typically, they are located within the superficial subcutaneous tissue and therefore potentially palpable on physical examination.[6]

- A 15-year-old male presented with enlarging left breast mass. Targeted ultrasound – transverse (a) and sagittal (b) images demonstrated a 4.2 × 3.9 × 2.5 cm (CC × AP × TR) heterogeneous, hypoechoic mass, with indistinct margins and posterior acoustic enhancement. The mass showed internal vascularity with both arterial and venous waveforms detected on Doppler (c and d). Magnetic resonance imaging breasts demonstrated a circumscribed mass (red arrow), measuring 4.2 × 4 × 2.9 cm (CC × AP × TR), hypointense on T1 (e), hyperintense on T2 (f) and with heterogeneous enhancement on post-contrast imaging (g and h). It had mass effect on pectoralis muscle with a preserved fat plane. Ultrasound-guided core biopsy showed cavernous hemangioma.
Cavernous hemangiomas may exhibit ovoid morphology with circumscribed or microlobulated borders on mammography.[8] The typical appearance on sonography is a circumscribed, oval mass with parallel orientation, and variable echogenicity. On MRI, these benign tumors are isointense to adjacent fibroglandular tissue on T1WI and frequently T2 hyperintense due to slow blood flow.[8] The MRI hallmark of hemangiomas is rapid peripheral contrast enhancement followed by delayed fill-in enhancement.[8] Smaller lesions are difficult to characterize and potentially mimic malignancy as a result of brisk contrast uptake.[6]
Apocrine cystic metaplasia
Apocrine cystic metaplasia of the breast [Figure 5] is a subclass of the umbrella term “fibrocystic changes” which is extremely common in females older than 25 years.[8-10] Histologically, these lesions are composed of dilated ducts and fluid-filled cystic spaces lined by apocrine epithelium.[5,9]
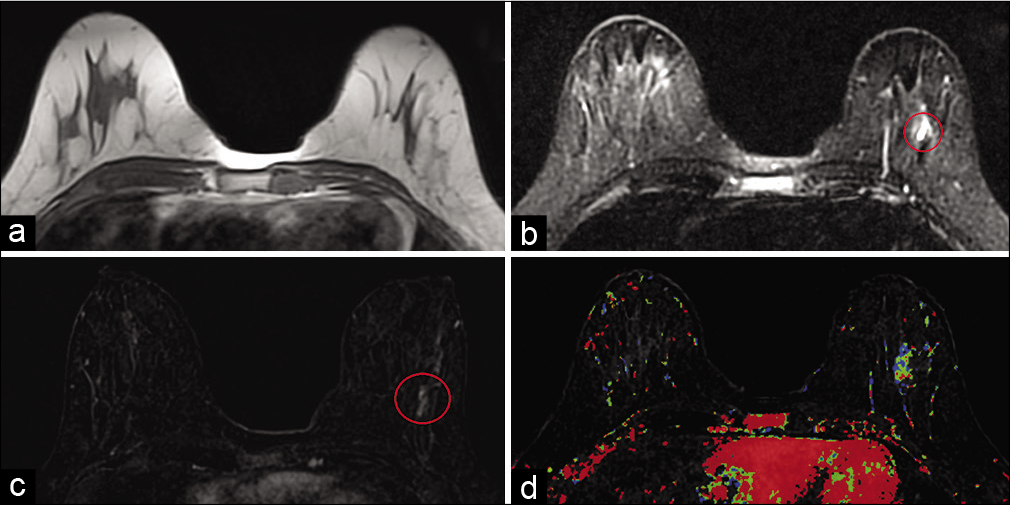
- A 31-year-old female presented for high-risk screening magnetic resonance imaging (MRI) bilateral breasts (maternal history of breast cancer diagnosis in her 30’s). T1-weighted MR image (a) did not demonstrate any abnormality. T2-weighted MR image (b) demonstrated hyperintensity in a segmental distribution in the lower outer left breast (red circle), measuring 3.8 × 1.8 × 3.7 cm (AP × TR × CC), approximately 6 cm from the nipple. Dynamic contrast-enhanced MRI (c) demonstrated segmental heterogeneous non-mass enhancement in the area of T2 signal abnormality in the lower outer left breast (red circle) and mixed kinetics (d). MRI guided core biopsy showed cystic apocrine metaplasia.
The mammographic appearance is equal or low-density mass with microlobulated margins. Apocrine cystic metaplasia may manifest on ultrasound as clustered microcysts with intervening septae.[5] On MRI, they have variable appearance ranging from subcentimeter masses to hybrid lesions with mass and non-mass features. Because of their cystic nature, these lesions are T2 hyperintense on MRI. Apocrine cystic metaplasia can demonstrate either heterogeneous contrast enhancement with plateau or washout kinetics or even lack of contrast uptake on post-contrast T1WI.[5]
Myxoid fibroadenoma
Breast fibroadenomas [Figure 6] are the second most common benign breast mass accounting for 50% of the benign etiologies.[3,11] Again, like most benign breast lesions, fibroadenomas present as palpable, freely movable breast lumps on physical examination.[5] Histopathologic analysis reveals epithelial lined glandular tissue and extracellular fibrous stroma with underlying myxoid and mucinous changes.[5]
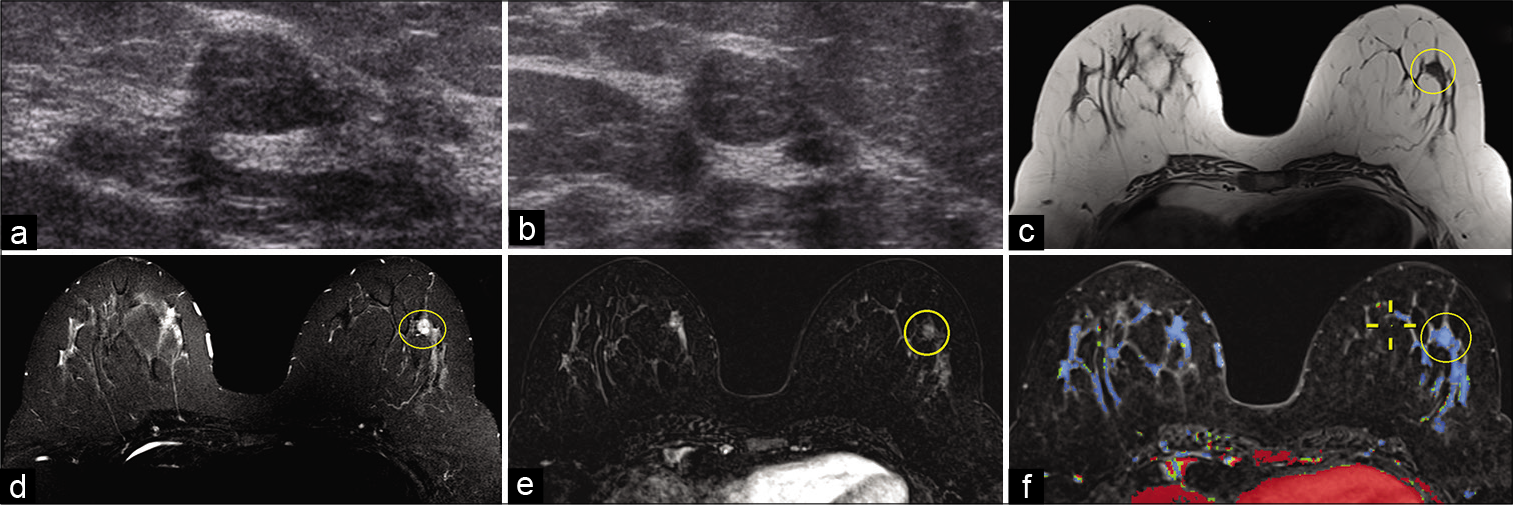
- A 37-year-old female presented for a palpable right breast mass. Targeted ultrasound – transverse (a) and sagittal (b) images of the left breast demonstrated an oval hypoechoic circumscribed mass, approximately 1 cm in greatest dimension, at 5’o position, 4–5 cm from the nipple. No mammographic correlate was identified. Magnetic resonance imaging (MRI) breasts demonstrated a 0.8 cm well-circumscribed T1 hypointense (c), T2 hyperintense (d) mass in the lower outer quadrant of the left breast (yellow circle), approximately 4.5 cm from the nipple. The mass showed thin internal septations on T2W imaging. Dynamic contrast-enhanced MRI (e) demonstrated homogeneous enhancement within the mass (yellow circle) with persistent kinetics (f). Ultrasound-guided biopsy revealed fibroadenoma.
Myxomatous fibroadenomas exhibit benign morphology on imaging, including smooth margins and round or ovoid shape.[5] The classic mammographic appearance is a mass containing “popcorn” or coarse calcifications. On ultrasound, they are typically oval shaped, hypoechoic, or heterogeneous with circumscribed or microlobulated borders. Fibroadenomas are classically T2 hyperintense as a result of their mucinous contents.[3,5] Progressive, homogenous contrast enhancement on post-gadolinium T1WI (Type I kinetics) with dark internal septations is the most specific MRI feature.[3,5]
Benign phyllodes tumor
Phyllodes tumor [Figure 7] is a relatively rare fibroepithelial tumor which resembles the histologic appearance of fibroadenomas. Similar to fibroadenomas, phyllodes tumors contain an admixture of epithelial cells and stromal connective tissue, but with hypercellular stroma arranged in leaf-like pattern.[1,12,13] Phyllodes tumors may also contain patchy areas of myxoid stroma.[1] The World Health Organization (WHO) classifies phyllodes tumors into benign, borderline, and malignant based on underlying histopathologic appearance.[12] Benign phyllodes tumors demonstrate a hypercellular stroma, lack of stromal hypertrophy, minimal nuclear atypia, non-infiltrative margins, and <4 mitoses/10 high-power fields.[14] Regardless of histopathologic subgroup, it is generally recommended to excise these masses considering approximately 20–30% are upgraded to malignant post-resection.[11] Wide surgical resection is preferred as there is a local recurrence rate up to 30% for benign phyllodes tumors.[12,15]
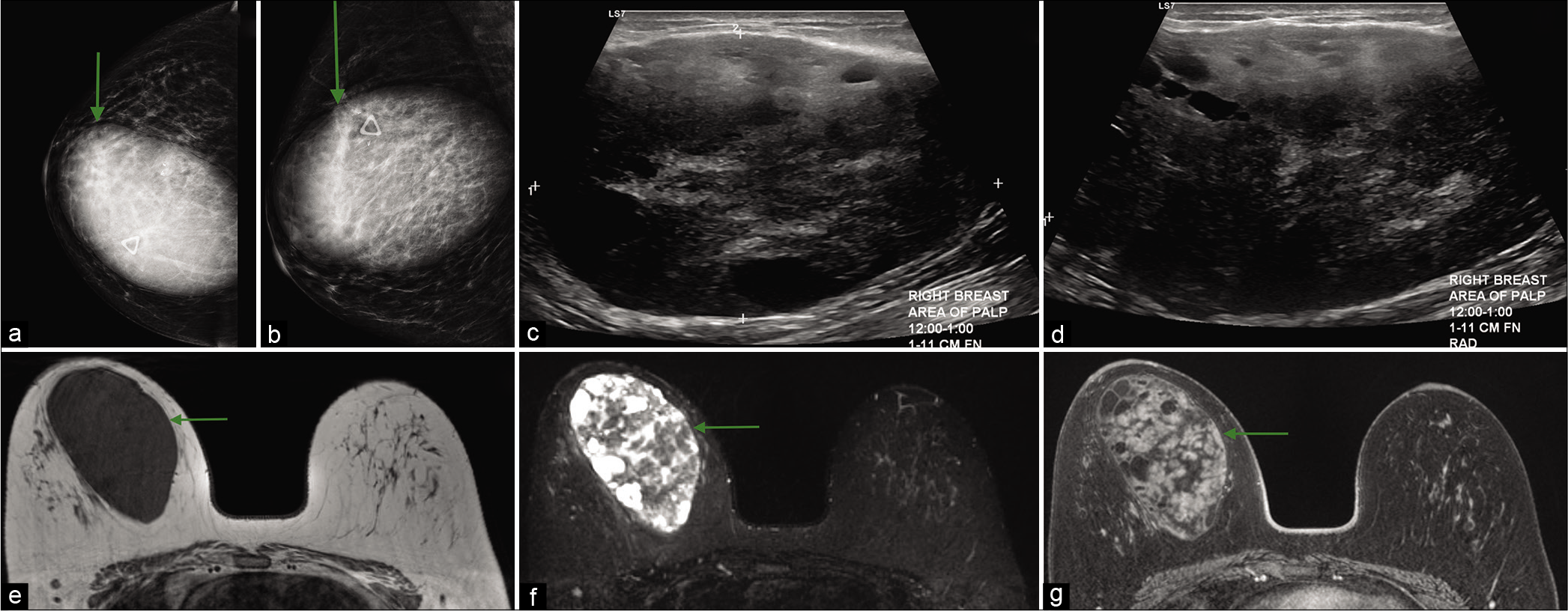
- A 45-year-old female presented with soft mobile lump in the right breast, which had been gradually increasing in size and a history of two prior benign biopsies of the same lump. CC (a) and MLO (b) views of mammogram demonstrated a large circumscribed oval mass (green arrow) in the upper inner right breast underneath the palpable lump marker, measuring approximately 8.6 × 12.5 × 9.2 cm and two biopsy clips at the superolateral margin of mass. Ultrasound transverse (c) and sagittal (d) images showed a 10 cm sized, heterogeneous, predominantly hypoechoic mass, with internal cystic areas, at 12–1’o position. Magnetic resonance imaging breasts demonstrated a circumscribed oval T1 hypointense (e) mass with multiple T2 hyperintense (f) internal areas and heterogeneous enhancement (g). These imaging features and interval growth in the known breast mass were consistent with phyllodes tumor.
Phyllodes tumors are most commonly round or oval in morphology with well-circumscribed or microlobulated margins on mammography.[11] Typical ultrasound features include a round, heterogeneous, or hypoechoic mass with circumscribed or microlobulated margins. Unfortunately, MRI is unable to accurately differentiate benign from malignant phyllodes tumors. As with mammography and ultrasound on MRI, these fibroepithelial tumors are round and oval in morphology and display variable T2 signal intensities and may demonstrate T2 hyperintensity if they possess myxomatous stroma.[1] In addition, heterogeneous enhancement on post-contrast T1WI has been described.[1]
MALIGNANT LESIONS
Mucinous/colloid carcinoma
Mucinous breast carcinoma [Figure 8], also known as colloid carcinoma, is a well-differentiated type of adenocarcinoma with abundant extracellular mucin secreted by tumor cells. It accounts for 1–7% of all breast cancers.[16,17] It usually occurs in women older than 55 years and is generally considered to have a favorable prognosis. Typically, mucinous carcinoma expresses estrogen and progesterone receptors and lack of HER2 amplification.[18]
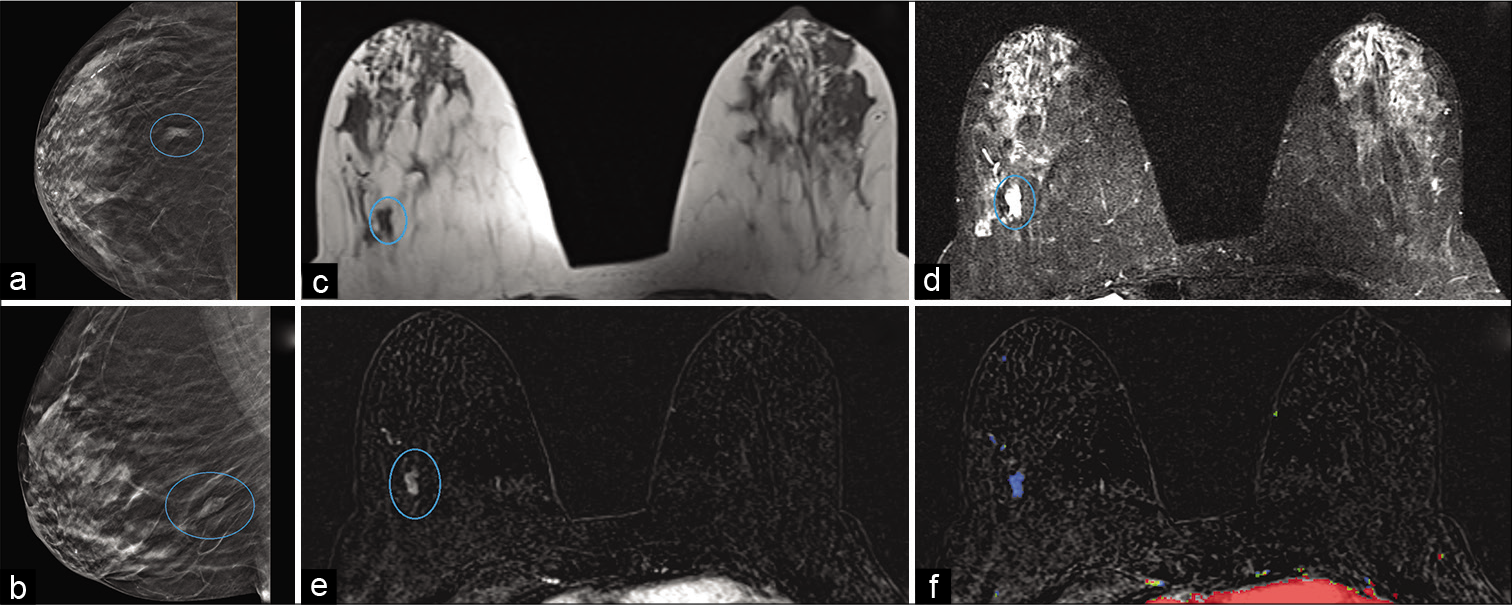
- An 85-year-old female had a history of benign left breast biopsy. Screening mammogram – CC (a) and MLO (b) views demonstrated a focal asymmetry in the posterior right breast (blue circle), 8 cm from the nipple. Magnetic resonance imaging (MRI) breasts demonstrated T1 hypointense (c) and T2 hyperintense (d) mass measuring 1 × 0.4 × 0.8 cm (AP × TR × CC) in the lower outer quadrant of the right breast (blue circle), approximately 8.5 cm from the nipple. Dynamic contrast-enhanced MRI (e) demonstrated heterogeneous enhancement (blue circle) and persistent kinetics (f) in the mass. MRI-guided stereotactic core biopsy of the mass showed mucinous/colloid carcinoma.
There are two types of mucinous breast carcinoma. In pure mucinous carcinomas, <10% of the tumor is composed of other histologic types of breast cancer, whereas in mixed mucinous carcinomas, more than 10% of the tumor is composed of other histologic subtypes, mainly invasive ductal carcinoma not otherwise specified. This distinction has important implications because pure mucinous cancers tend to be less aggressive and have a lower frequency of axillary metastases and a better overall survival rate than do mixed tumors.[19,20]
On mammography, the appearance of pure and mixed mucinous carcinoma is different. Pure mucinous carcinoma is usually round or oval in shape with circumscribed margins. The mixed form typically presents as an irregular mass with spiculated or indistinct margins. Microcalcifications are rare. On ultrasound, the pure type of mucinous carcinoma is isoechoic to subcutaneous fat with homogeneity. The mixed type of mucinous carcinoma may appear as a complex solid and cystic mass. Posterior enhancement is found in mucinous carcinoma in more than 50% of cases. On MRI, mucinous carcinoma has a round, oval, or lobulated shape. On T1-weighted MRI sequences, it has low signal intensity. On T2-weighted sequences, it is of homogeneous high signal intensity due to a large mucin content. Mucinous carcinoma is hyperintense on diffusion-weighted imaging, with high apparent diffusion coefficient values. On post-contrast T1-weighted fat saturation images, there is avid peripheral rim enhancement or heterogeneous internal enhancement. It can demonstrate persistent or plateau kinetics. The peripheral enhancement can be explained by presence of mucin in the center and epithelial cells with invasive component at the periphery.[1,18,19,21,22]
Metaplastic carcinoma
Metaplastic breast carcinoma (MPC) accounts for 0.2–5% of all invasive breast cancers.[23] It consists of a heterogeneous group of malignant neoplasms containing both glandular and non-glandular components with mixed epithelial and mesenchymal differentiation.[24] The WHO divides metaplastic tumors into two groups: Pure epithelial (squamous cell, adenocarcinoma with spindle cell, or adenosquamous cell) and mixed epithelial-mesenchymal (carcinosarcoma with possible chondroid or osseous differentiation).
MPC [Figure 9] often presents as a palpable breast mass in women more than 50 years of age. The lesions are characterized by large tumor size and rapid growth. MPC is often not associated with estrogen and progesterone receptors, or HER2 expression. There is a high hematogenous metastatic potential to lung and bone rather than lymphatic spread. There is increased risk of tumor recurrence and worse prognosis with MPC compared with invasive ductal or invasive lobular carcinoma.[25-27]
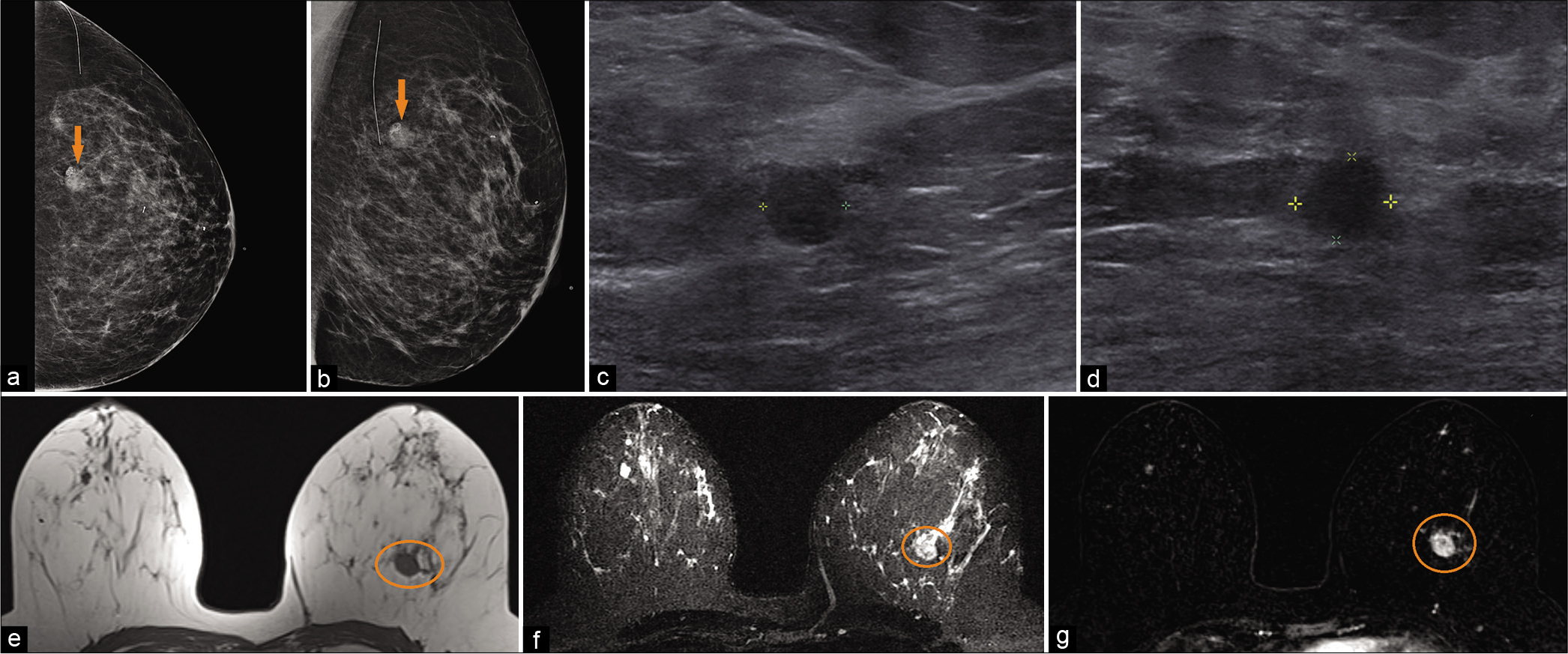
- A 49-year-old female with a history of benign left breast biopsy presented for screening mammogram. Mammogram CC (a) and MLO (b) views demonstrated new focal asymmetry with coarse heterogeneous and fine pleomorphic calcifications in the left upper outer breast (orange arrow), 7 cm from the nipple. Biopsy clips noted in the 12’o position. Targeted ultrasound – transverse (c) and sagittal (d) images of the left breast demonstrated an irregular, non-parallel hypoechoic mass, with indistinct and angular margins and internal calcifications, at 1’o position, 7 cm from the nipple. Magnetic resonance imaging (MRI) breasts demonstrated T1 hypointense (e), T2 hyperintense (f) irregular mass (orange circle) measuring 1.8 × 1.6 × 1.5 cm (AP × TR × CC) at 12’o position, 8 cm from the nipple. Dynamic contrast-enhanced MRI (g) demonstrated heterogeneous enhancement within the mass (orange circle). Ultrasound-guided biopsy of the mass showed metaplastic type poorly differentiated infiltrating carcinoma.
On mammogram, MPC presents as a high-density, round or oval, often non-calcified mass with variable non-specific margins.[28] The sonographic appearance of MPC can be a hypoechoic mass or a complex cystic and solid mass, typically with posterior acoustic enhancement.[25,29] MPCs may be oval, round, or irregular in shape with circumscribed or indistinct margins. Similar to mammogram and ultrasound, MPC presents as a mass on MRI with variable shape and margins, including round, lobular, or irregular shape, and often with smooth margins or sometimes with spiculated margins. MPCs frequently demonstrate high signal intensity on T2-WI; T2 hyperintensity being due to necrotic component and cystic degeneration. The reported enhancement characteristics of these lesions include rim-like, homogenous or heterogeneous or containing non-enhancing internal components. MPC often depicts washout kinetics, with early enhancement and delayed washout, corresponding to the enhancing peripheral portion of the mass.[25,30,31]
Papillary carcinoma
Papillary carcinoma [Figure 10], a rare malignant lesion, accounts for <2% of breast carcinomas, commonly initially presenting as bloody nipple discharge, though additional presentations may encompass palpable mass or nipple retraction.[1,32] The papillary component itself refers to a fibrovascular stalk lined by epithelial cells.[33] This malignant tumor is subcategorized based on pattern of growth as intracystic, intraductal, or invasive. The intracystic variant may also be referred to as encapsulated papillary carcinoma. Predominantly solid papillary carcinoma variants have also been described, though invasive components may be the most pertinent feature of encapsulated and solid variants, displaying features of invasive ductal carcinoma, not otherwise specified.[32,33] At times, the tumor may be associated with ductal carcinoma in situ also arising along the fibrovascular stalk.[32,33] The malignant papillary variants distinguish themselves from benign papilloma based on the absence of a myoepithelial cell layer.[32,33]
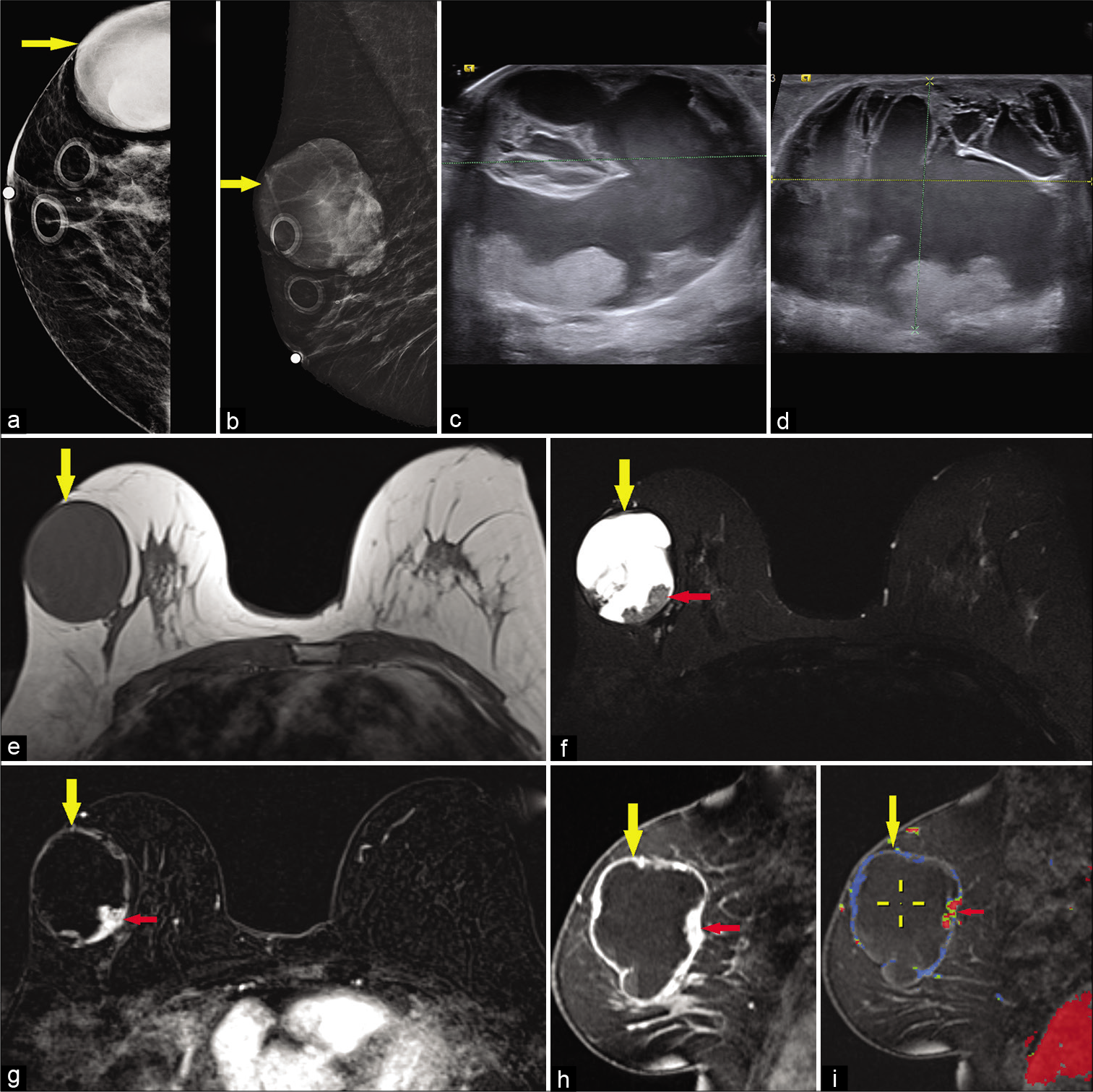
- A 65-year-old female presented for a palpable left breast lump. Diagnostic mammogram CC (a) and MLO (b) views demonstrated a large high-density mass in the upper outer right breast (yellow arrow). Targeted ultrasound – transverse (c) and sagittal (d) images of the left breast demonstrated a mixed cystic and solid mass measuring approximately 4.4 × 5.7 × 5.2 cm (AP × TR × CC) at 10’o position, 7 cm from the nipple. Magnetic resonance imaging (MRI) breasts demonstrated T1 hypointense (e), T2 hyperintense (f) large cystic and solid round mass in the upper outer right breast (yellow arrow) with mural nodularity (red arrow) and fluid-fluid levels. Dynamic contrast-enhanced MRI axial (g) and sagittal images (h) demonstrated peripheral and nodular mural enhancement (red arrow) in the mass (yellow arrow), with washout kinetics in the mural nodule (i), ultrasound-guided core biopsy of the mass showed papillary carcinoma.
The intracystic variant in particular displays a common appearance of a mixed solid and cystic mass with mural soft-tissue nodularity projected into a high T2 signal component, corresponding to a fibrovascular papillary stalk.[33,34] High T2 signal regions may also reflect necrosis with high T1 signal suggesting hemorrhagic necrosis and/or intracystic hemorrhage.[1] There may be characteristic enhancement of the soft-tissue components and the cyst walls and septae.[33] Mammography is typically nonspecific, and ultrasound may better display the expected soft-tissue focus projecting into cystic regions.[32,34]
The solid variant typically displays a nodular morphology with an underlying fibrovascular core that is in itself papillomatous, qualifying it as a papillary lesion.[33] Commonly these tumors may contain mucinous components, resulting in regions of T2 prolongation.[33] In general, invasive margins are suggested in the presence of an edematous border around the lesional margins.[35]
Papillary structures growing intraductally may commonly display malignant foci of partial or complete absence of the myoepithelial layer in addition to containing variants of ductal carcinoma.[33,34] Intraductal variants typically produce ductal dilation secondary to a centrally vascular lesion, though imaging findings may at times be confused with the intracystic variant. These lesions may display variable MRI findings varying from occult to uniform or heterogeneous enhancement with or without ductal findings.[34] Atypical or carcinomatous components within the papillary structure may also vary in appearance and may be MRI occult, though calcifications demonstrated on mammography may reflect components of ductal carcinoma in situ.[34]
Necrotic/hemorrhagic invasive ductal carcinoma
Invasive ductal carcinoma [Figure 11] is the most common malignant breast tumor, and cystic changes on imaging are usually attributable to necrosis.[1] Necrosis is an expected finding for high mitotic index malignant tumors unable to maintain an adequate vascular supply, usually centrally, resulting in apoptosis. Typically, these issues arise in larger lesions with more disorganized histopathologic architecture.
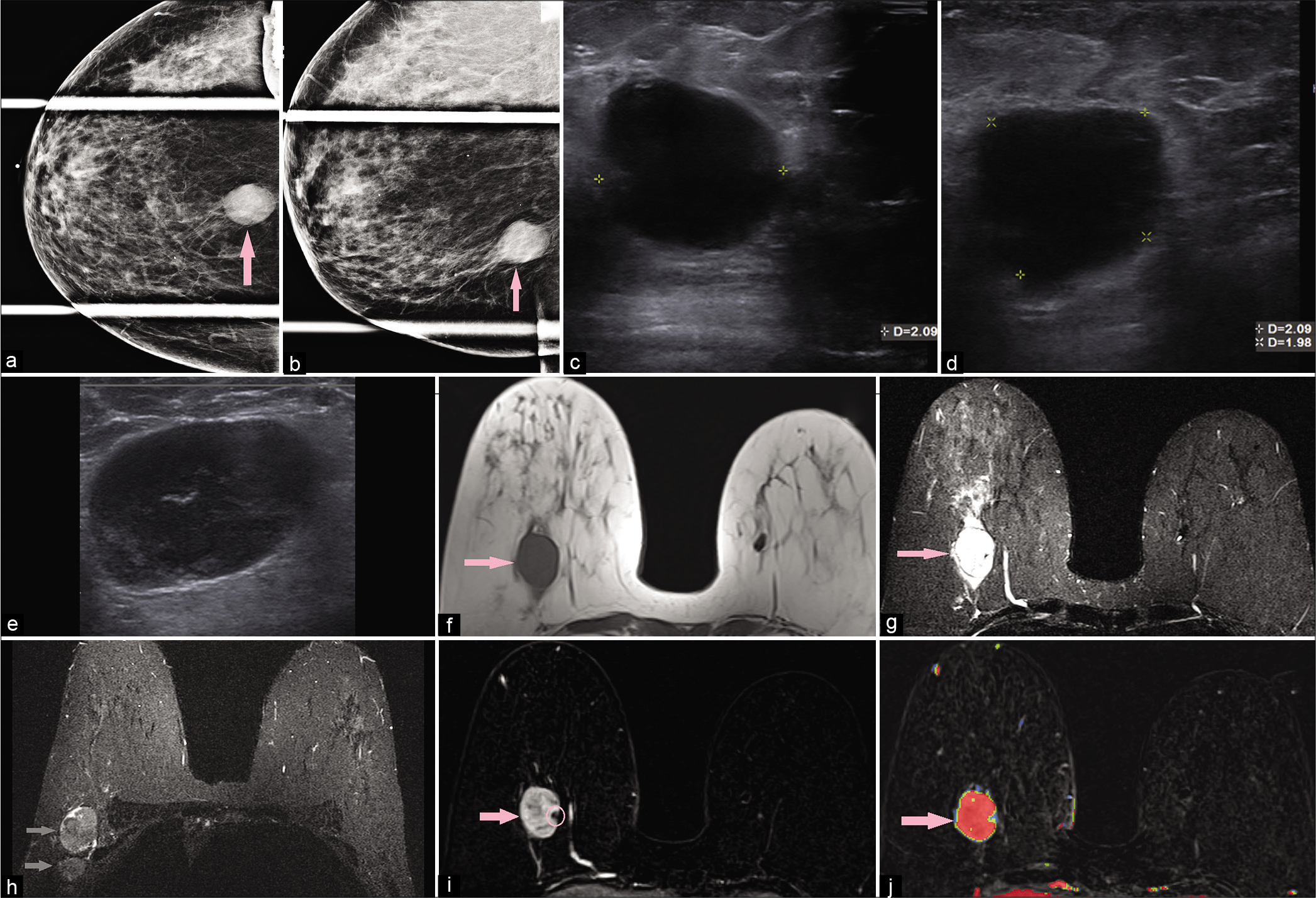
- A 58-year-old female had a screening mammogram which demonstrated a mass in the right breast. Diagnostic mammogram – compression CC (a) and MLO (b) views demonstrated an oval high-density mass (pink arrow) with indistinct margins at 6’o position. Targeted ultrasound – transverse (c) and sagittal (d) images demonstrated a round hypoechoic mass, measuring 2.1 × 2.1 × 2 cm (AP × TR × CC), with indistinct margins, at 6’o position, 7 cm from the nipple. In the right axilla (e), ultrasound showed an enlarged round lymph node with cortical thickening of 1 cm and near complete replacement of the fatty hilum. Magnetic resonance imaging (MRI) breasts demonstrated T1 hypointense (f), T2 hyperintense (g), oval mass (pink arrow) measuring 2.7 × 3 × 2.7 cm (AP × TR × CC) at 6’o position, with a peripheral biopsy clip and T2 hyperintensity/edema around the mass. Multiple enlarged right axillary lymph nodes (gray arrows) were noted (h). Dynamic contrast-enhanced MRI (i) demonstrated heterogeneous enhancement within the mass (pink arrow) and washout kinetics (j). Ultrasound-guided core biopsy of the mass showed triple-negative poorly differentiated invasive ductal carcinoma.
Malignant tumors are generally irregular or spiculated and hypointense to fibroglandular tissue on T2-WI with heterogeneous or rim enhancement.[5,36] Necrosis results in the predominantly central accumulation of fluid and debris. Fluid collections will typically result in T2 prolongation, though T1 hyperintensity may be present representing proteinaceous debris and/or hemorrhage.[1,5]
Triple-negative and basal-like breast cancers are more likely to present as round or oval high- density masses on mammogram and with fibroadenoma-like appearance on ultrasound. On MRI, they may be uniformly T2 hyperintense, with rim enhancement on post-contrast imaging.
Malignant phyllodes
The phyllodes tumors of the breast account for <1% of breast tumors and are generally a category of fibroepithelial lesions originating from hypercellular periductal stroma and most commonly affecting women aged 35–55 years.[1,37] Characteristically, they are subtyped into benign, borderline malignant, and malignant categories based on their histologically consistent architecture.[15,38] Malignant phyllodes tumors display sarcomatous features with a propensity for hematogenous metastasis and local recurrence, though metastases and recurrence generally occur within the first 2 years.[15,38] Local recurrence rates of >50% have been reported for malignant variants.[38]
Favorable clinical and surgical management of concerning phyllodes lesions must consider the risk of malignant potential, and imaging features can contribute to appropriate suspicion. The contribution of imaging to further suspicion is particularly important in the absence of malignant features demonstrated on histologic analysis after core biopsy or incomplete surgical resection, as samples may show predominantly benign features.[37,38] Phyllodes tumors may generally be indistinguishable from fibroadenoma based on imaging characteristics; however, phyllodes tumors do display more heterogeneous, patchy myxoid degeneration when compared to the homogenous degeneration of fibroadenoma.[1]
Phyllodes tumors may typically present as a palpable abnormality corresponding to a hypoechoic, oval mass with possible lobulation on ultrasound and a similar equal or lesser density mass on mammography.[11,12,37] Benign phyllodes tumors consistently display a complete or partial pseudocapsule without peripheral infiltration.[37] A hyperechoic border along the margin of the lesion of concern has a demonstrated association with infiltrating margins consistent with malignancy.[35] More heterogeneous and complex internal architecture is more consistent with phyllodes tumor as opposed to a benign fibroepithelial lesion, such as fibroadenoma, and additional complexity with hypervascular features suggests a higher grade phyllodes tumor.[11,37] Heterogeneity associated with higher grade phyllodes lesions is generally attributed to variable regions of necrosis with myxoid and cystic degeneration, and in the case of highly invasive malignant subtypes, peripheral edema and structural parenchymal distortion are characteristic.[1,11,37] Cystic spaces with smooth margins and exhibiting homogeneous hyperintense signal on T2WI are consistent with benign tumors while irregular margins and heterogeneous signal intensities would favor a borderline or malignant lesion. Heterogeneous internal architecture translates to associated heterogeneous tumor enhancement.[11]
Metastases
Extramammary metastasis [Figure 12] to the breast is a rare phenomenon with estimated prevalence well under 10% and ranging to as low as 0.5%.[19,39-41] This diagnosis carries a high mortality with <20% survival rate within 1 year.[40,41] The most common extramammary tumors to metastasize to the breast are melanoma, lymphoma, lung, sarcomas, ovarian neoplasms, and neuroendocrine tumors.[19,40,42] Tumors of the reproductive system, primarily of the uterus and ovaries, display an affinity for the breast.[40] In the female adolescent patient, rhabdomyosarcoma is the most common primary.[19,40] Metastasis to the breast should remain a prime differential consideration in patients with known extramammary malignancy. An unrecognized primary lesion may remain occult in the absence of conclusive pathology to indicate extramammary primary.

- A 73-year-old woman with a palpable mass and pain in the left breast and a history of malignant melanoma. On mammography showing the left craniocaudal (CC) view (a), two circumscribed round to oval-shaped hyperdense (solid arrow) and isodense (arrowhead) masses are seen in the left mid to inner deep portion, where the patient complained of a palpable mass. On sonography taken at a different hospital (not shown), the masses were circumscribed and had low echogenicity. The masses all showed mostly high signal intensity on axial fat-saturated T1-weighted imaging (b) and showed enhancement on contrast-enhanced T1-weighted image (c). They also showed high signal intensity on fat-saturated T2-weighted image (d). On chest CT scan (e), the larger mass showed a central hypodense area of possible necrosis. A loss of the fat plane in between the larger mass and the pectoralis muscle is also seen, indicating the possibility of muscle involvement. In addition, an enlarged internal mammary lymph node on the contralateral side is noted (dashed arrow). On 18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET-CT) (f), the masses and enlarged internal mammary lymph node all had increased FDG uptake, and the maximum SUV of the large mass was 7.2. (Acknowledgement for Figure 12 – We extend our sincere appreciation to Dr. Kyu Ran Cho, corresponding author of the article Radiologic Findings of Metastatic Malignant Melanoma of the Breast: Mammographic, Sonographic, Dynamic Contrast-Enhanced Breast Magnetic Resonance Imaging, and 18F-FDG PET-CT Features, Iran J Radiol. 2017; 14(3): e38392 for allowing us to use the images and annotations of Figure 1 from their article).
Unfortunately, imaging findings may fail to demonstrate the underlying malignant potential of the lesion of concern. Previous analyses have demonstrated that as many as 24% of lesions may be mistakenly characterized as primary breast cancer or a benign lesion after imaging analysis was performed.[40] Palpable masses or other alarming physical findings, such as edema and erythema, may prompt earlier presentation and assessment. Metastasis secondary to widespread lymphatic infiltration generally presents with diffuse erythema and skin thickening with adenopathy resembling mastitis or inflammatory breast carcinoma.[19,40] Commonly mammographic assessment reveals high-density, round, circumscribed masses without other alarming findings more common to primary breast malignancies, including spiculation and nipple retraction.[19,39,40,41] This deceptively benign appearance may occur secondary to the development of a fibrous pseudocapsule of varying thickness.[40]
Lesions may appear heterogeneous but predominantly hyperechoic or hypoechoic on ultrasound.[19,41] Neither calcifications nor axillary lymph node involvement are common in these lesions; however, calcified matrix has been described for metastatic disease in the setting of an osteosarcoma primary.[19,40] Lesions have been described as typically T2 intermediate and T1 hypointense, except for melanoma which displays T1 hyperintensity.[19] Necrosis and cystic degeneration of the lesion may result in heterogeneous regions of T2 prolongation. Variable, avid enhancement patterns have also been described for post-contrast sequences.[19] Possible imaging findings may manifest as numerous bilateral masses.[41] A tendency toward rapid tumor growth has been described, but the imaging characteristics of the lesions may be extremely variable because of the range of possible primaries, which makes a focused and detailed clinical assessment crucial.[42]
CONCLUSION
T2 hyperintense breast lesions can have inflammatory, infectious, or neoplastic etiologies. MRI is a far superior noninvasive imaging technique than ultrasound and mammogram in characterization of these breast processes. The differentiation of these lesions is based on a combined assessment of morphology, internal signal variability, and enhancement pattern; all of which are best delineated on MRI [Table 1].
| Lesion | Morphology | T1- and T2- WI features | Enhancement pattern | Diagnostic clues |
|---|---|---|---|---|
| Cyst | Round or oval shape, circumscribed margins | T1: Hypointense T2: Hyperintense |
No enhancement or thin rim enhancement | May be multiple, cyclical, may be tender on exam, history of cysts |
| Seroma/hematoma | Round shape, circumscribed margins | Seroma T1: Hypointense T2: Hyperintense Hematoma– Variable signal depending on age of blood products |
No enhancement or thin rim enhancement | History of trauma or surgery, hematoma may demonstrate fluid-fluid levels |
| Intramammary lymph node | Oval shape, circumscribed margins | T1: Hypointense with central hyperintense fatty hilum T2: Homogeneously hyperintense |
Homogeneous enhancement Wash-out kinetics |
Reniform shape, central fat, common location is upper outer quadrant of breast |
| Hemangioma | Oval shape, circumscribed or microlobulated margins | T1: Isointense T2: Hyperintense |
Rapid peripheral enhancement followed by delayed fill in | Features same as hemangiomas anywhere in the body |
| Apocrine cystic metaplasia | Round, oval or irregular shape, indistinct or microlobulated margins | T1: Hypointense T2: Hyperintense |
No enhancement or mild heterogeneous enhancement Variable kinetics |
Clustered microcysts on ultrasound, hybrid lesions with mass and non-mass features on MRI |
| Myxoid fibroadenoma | Round or oval shape, circumscribed margins | T1: Hypointense T2: Homogeneously hyperintense |
Dark internal non-enhancing septations Persistent kinetics |
May be multiple, mobile on examination, younger females |
| Phyllodes tumor | Benign-round or oval shape, circumscribed margins Malignant variable shape and margins |
T1: Hypointense T2: Patchy hyperintensity |
Heterogeneous enhancement | Middle aged, fibroadenoma like imaging appearance; however, large size and rapid growth are suggestive |
| Mucinous/colloid carcinoma | Round or oval shape, circumscribed margins | T1: Hypointense T2: Hyperintense with interspersed areas of moderate signal intensity |
Rim enhancement or heterogeneous enhancement Variable kinetics |
Older women, lobular margins, posterior enhancement on ultrasound |
| Metaplastic carcinoma | Variable shape and margins | T1: Hypointense T2: Central hyperintensity |
Variable enhancement | Older women, fast growing, propensity for hematogenous spread but not lymphatic spread |
| Papillary carcinoma | Round or irregular shape, variable margins | T1: Hypointense T2: Heterogeneous with areas of hyperintensity related to microcystic components |
Mass with thick enhancing rim and septate or clumped non-mass enhancement with focal, segmental, or regional distribution | Bloody nipple discharge, mixed cystic solid mass on ultrasound |
| Invasive ductal carcinoma | Irregular shape, non-circumscribed margins | T1: Hypointense T2: Variable or heterogeneous hyperintense signal |
Heterogeneous or rim enhancement Wash-out kinetics |
High-grade invasive ductal carcinomas can be T2 hyperintense due to central necrosis |
| Metastases | Variable shape, circumscribed or non-circumscribed margins | T1: Hypointense T2: Intermediate signal or heterogeneously hyperintense |
Heterogeneous enhancement Mixed kinetics |
Multiple or bilateral masses, known primary |
MRI can help narrow the differential diagnosis, acting as a “Virtual Biopsy” tool based on the imaging characteristics of the lesions and hence can be useful beyond its currently recognized usage as a breast cancer pre-treatment and surveillance tool. Through this article, we have highlighted how the specific MRI features of T2 hyperintense breast lesions can guide the interpreting radiologist toward the histology of these lesions.
Acknowledgement
*Acknowledgement for Figure 12- We extend our sincere appreciation to Dr. Kyu Ran Cho, corresponding author of the article- Radiologic Findings of Metastatic Malignant Melanoma of the Breast: Mammographic, Sonographic, Dynamic Contrast-Enhanced Breast MRI, and 18F-FDG PET-CT Features, Iran J Radiol. 2017; 14(3): e38392, for allowing us to use the images and annotations of Figure 1 from their article.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Radiologic and pathologic findings in breast tumors with high signal intensity on T2-weighted MR images. Radiographics. 2010;30:533-48.
- [CrossRef] [PubMed] [Google Scholar]
- Breast MRI imaging: What the radiologist needs to know. J Clin Imaging Sci. 2011;1:48.
- [CrossRef] [PubMed] [Google Scholar]
- Cystic masses of the breast. AJR Am J Roentgenol. 2010;194:122-33.
- [CrossRef] [PubMed] [Google Scholar]
- Using T2-weighted sequences to more accurately characterize breast masses seen on MRI. AJR Am J Roentgenol. 2014;202:183-90.
- [CrossRef] [PubMed] [Google Scholar]
- Breast hemangioma: MR appearance with histopathological correlation. J Clin Imaging Sci. 2012;2:53.
- [CrossRef] [PubMed] [Google Scholar]
- Cavernous hemangioma in the breast. J Breast Health. 2015;11:199-201.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular abnormalities of the breast: Arterial and venous disorders, vascular masses, and mimic lesions with radiologicpathologic correlation. Radiographics. 2011;31:E117-36.
- [CrossRef] [PubMed] [Google Scholar]
- Breast MRI-detected cystic apocrine metaplasia: Imaging features with microvessel analysis and histologic correlation. AJR Am J Roentgenol. 2015;204:211-8.
- [CrossRef] [PubMed] [Google Scholar]
- Non-operative breast pathology: Apocrine lesions. J Clin Pathol. 2007;60:1313-20.
- [CrossRef] [PubMed] [Google Scholar]
- Differentiation between phyllodes tumors and fibroadenomas based on mammographic sonographic and MRI features. Breast Care (Basel). 2016;11:123-7.
- [CrossRef] [PubMed] [Google Scholar]
- Phyllodes tumor of the breast: Ultrasound-pathology correlation. AJR Am J Roentgenol. 2018;210:W173-9.
- [CrossRef] [PubMed] [Google Scholar]
- Benign and malignant phyllodes tumors: Mammographic and sonographic findings. Radiology. 1996;198:121-4.
- [CrossRef] [PubMed] [Google Scholar]
- Phyllodes tumours of the breast: A consensus review. Histopathology. 2016;68:5-21.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant phyllodes tumors of the breast: A study in clinical practice. Int Surg. 2012;97:95-8.
- [CrossRef] [PubMed] [Google Scholar]
- MR imaging features of pure mucinous carcinoma of the breast. Eur J Radiol. 2006;60:405-13.
- [CrossRef] [PubMed] [Google Scholar]
- Uncommon breast tumors in perspective: Incidence, treatment and survival in the Netherlands. Int J Cancer. 2007;121:127-35.
- [CrossRef] [PubMed] [Google Scholar]
- Mucinous carcinoma of the breast: Mammography and ultrasound appearance with histopathology correlation. JOJ Case Stud. 2019;9:555774.
- [CrossRef] [Google Scholar]
- MRI features of mucinous cancer of the breast: Correlation with pathologic findings and other imaging methods. AJR Am J Roentgenol. 2016;206:238-46.
- [CrossRef] [PubMed] [Google Scholar]
- Mucinous Carcinoma of the Breast: A Diagnostic Challenge in Breast Radiology, ECR No. C-2272; 2014
- [Google Scholar]
- Mucinous Breast Carcinomas: A Pictorial Review of the Typical Imaging Findings and Its Differential Diagnosis, ECR No. C-0756; 2017
- [Google Scholar]
- Mucinous breast cancer: A review study of 5 year experience from a hospital-based series of cases. Maedica (Bucur). 2015;10:14-8.
- [Google Scholar]
- Metaplastic carcinoma of the breast: Report of three cases. Cancer. 1998;82:1082-7.
- [CrossRef] [Google Scholar]
- Review of metaplastic carcinoma of the breast: Imaging findings and pathologic features. J Clin Imaging Sci. 2012;2:21.
- [CrossRef] [PubMed] [Google Scholar]
- Metaplastic carcinoma of the breast. Hum Pathol. 2010;41:960-70.
- [CrossRef] [PubMed] [Google Scholar]
- Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: The experience of the European institute of oncology and review of the literature. Breast Cancer Res Treat. 2007;101:349-53.
- [CrossRef] [PubMed] [Google Scholar]
- Metaplastic carcinoma of the breast: Clinical, mammographic, and sonographic findings with histopathologic correlation. AJR Am J Roentgenol. 2002;178:1421-5.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging differences in metaplastic and invasive ductal carcinomas of the breast. AJR Am J Roentgenol. 2007;189:1288-93.
- [CrossRef] [PubMed] [Google Scholar]
- Multimodality imaging findings of metaplastic breast carcinomas: A report of five cases. Ultrasound Q. 2018;34:88-93.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment options for metaplastic breast cancer. ISRN Oncol. 2012;2012:706162.
- [CrossRef] [PubMed] [Google Scholar]
- Intracystic papillary carcinoma of the breast. Radiographics. 2010;30:2021-7.
- [CrossRef] [PubMed] [Google Scholar]
- Papillary carcinoma of the breast: An overview. Breast Cancer Res Treat. 2010;122:637-45.
- [CrossRef] [PubMed] [Google Scholar]
- Papillary lesions of the breast: MRI, ultrasound, and mammographic appearances. AJR Am J Roentgenol. 2012;198:264-71.
- [CrossRef] [PubMed] [Google Scholar]
- The hyperechoic zone around breast lesions-an indirect parameter of malignancy. Ultraschall Med. 2014;35:547-53.
- [CrossRef] [PubMed] [Google Scholar]
- Patterns of enhancement on breast MR images: Interpretation and imaging pitfalls. Radiographics. 2006;26:1719-34.
- [CrossRef] [PubMed] [Google Scholar]
- Phyllodes tumours of the breast: Radiological presentation, management and follow-up. Br J Radiol. 2014;87:20140239.
- [CrossRef] [PubMed] [Google Scholar]
- Metastatic malignant phyllodes tumor of the breast: An aggressive disease-analysis of 7 cases. Indian J Surg Oncol. 2015;6:363-9.
- [CrossRef] [PubMed] [Google Scholar]
- Breast metastasis from extramammary malignancies. Radiology. 1982;144:309-12.
- [CrossRef] [PubMed] [Google Scholar]
- Non-mammary metastases to the breast and axilla: A study of 85 cases. Mod Pathol. 2013;26:343-9.
- [CrossRef] [PubMed] [Google Scholar]
- Metastatic tumors to the breast: Mammographic and ultrasonographic findings. J Ultrasound Med. 2000;19:257-62.
- [CrossRef] [PubMed] [Google Scholar]
- Breast metastases from extramammary malignancies: Typical and atypical ultrasound features. Korean J Radiol. 2014;15:20-8.
- [CrossRef] [PubMed] [Google Scholar]






