Translate this page into:
MRI and PET/CT of multiple primary hepatic angiosarcomas in 25-year-old man: A case report

*Corresponding author: Zhuldyz Kuanysh, Department of Science, National Research Oncology Center, Astana, Kazakhstan. zhuldyzkuanysh@icloud.com
-
Received: ,
Accepted: ,
How to cite this article: Jakipov M, Karimov A, Khamitova Z, Kochiev B, Bolatov D, Spatayev Z, et al. MRI and PET/CT of multiple primary hepatic angiosarcomas in 25-year-old man: A case report. J Clin Imaging Sci. 2024;14:32. doi: 10.25259/JCIS_69_2024
Abstract
This study aims to provide a comprehensive understanding of primary hepatic angiosarcoma, a rare and aggressive malignancy, focusing on its diagnostic challenges and unique imaging characteristics. The objective is to delineate the distinctive features of angiosarcoma through computed tomography and magnetic resonance imaging modalities, contributing to improved diagnostic precision and adding valuable insights to the scientific literature. We present the case of a 25-year-old male with primary hepatic angiosarcoma, emphasizing the challenges in distinguishing it from other vascular tumors.
Keywords
Hepatic angiosarcoma
Liver cancer
Epithelioid hemangioendothelioma
Imaging characteristics
INTRODUCTION
Angiosarcoma is a high-grade (grade 3) sarcoma characterized by the proliferation of atypical endothelial cells. This malignancy primarily affects adults, with peak incidence in the sixth and seventh decades of life.[1] It constitutes <2% of all primary hepatic malignancies, with approximately 200 cases diagnosed globally each year.[2] The estimated incidence of this high-grade hepatic sarcoma is between 0.14 and 0.25/million.[3] Clinical presentations often include nonspecific symptoms such as abdominal pain, weakness, or weight loss and may also involve hepatomegaly, ascites, jaundice, thrombocytopenia, hemoperitoneum, and liver failure.[4]
Computed tomography (CT) imaging has been extensively studied for its ability to reveal hepatic angiosarcoma, typically showing nonspecific hypoattenuating masses with heterogeneous enhancement on contrast-enhanced images. These features make it challenging to distinguish hepatic angiosarcoma from other invasive malignant primary or metastatic liver tumors.[5] On magnetic resonance imaging (MRI), primary hepatic angiosarcomas exhibit hyperintensity on T2-weighted images and hypointensity on T1-weighted images, with a heterogeneous structure in both sequences. Dynamic contrast magnetic resonance (MR) images reveal heterogeneous contrast accumulation in the arterial and venous phases, with progressive enhancement in the delayed phase.[6]
Macroscopically, angiosarcoma demonstrates varied growth patterns, ranging from massive tumors to diffuse micronodular lesions.
Due to the low frequency and aggressive nature of this tumor, there are no established and effective treatment guidelines. However, surgery remains the preferred treatment for selected patients, and long-term survival may be achieved in cases involving solitary tumors.[7]
The prognosis for hepatic angiosarcoma is poor, with a mean (median) survival ranging from 5 to 17 months, even when treated with surgical excision combined with adjuvant chemotherapy.[4] While 70% of hepatic angiosarcomas are sporadic, the remaining 30% are associated with environmental carcinogens, including vinyl chloride monomer, pesticides, arsenicals, and ionizing radiation.[4,5]
We present a case of hepatic angiosarcoma in a 25-year-old patient, highlighting the diagnostic and treatment challenges encompassed by this rare malignancy.
CASE REPORT
A 25-year-old male patient presented with intermittent episodes of abdominal pain and overall fatigue. The patient had no history of chronic diseases or prior surgeries, no family history of cancer, and denied contact with individuals carrying infectious diseases.
On local examination, the abdomen was non-enlarged, symmetrical, and moved synchronously with breathing. Abdomen was soft without pain. Liver function tests were normal.
Alpha-fetoprotein level was 2.58 mg/dL (upper limit: 40 mg/dL). Blood enzyme-linked immunosorbent assay for viral hepatitis and HIV markers was negative.
An outpatient abdominal ultrasound revealed masses within the liver. MRI of the abdomen enhanced with an extracellular contrast agent showed multiple well-defined nodules (14 in number) in both liver lobes, ranging from 1.3 to 3.3 cm. These nodules were predominantly peripherally located, with rounded and oval shapes. They exhibited moderate hyperintensity on T2, with the largest ones displaying a “target sign” appearance (hyperintense center and moderately hyperintense outer part, which was hypointense on T1) [Figure 1]. On post-contrast imaging, the liver lesions were hypovascular, showing mild-to-moderate centrifugal enhancement on arterial phase to delayed (4 min) phase [Figure 2]. Diffusion-weighted imaging (DWI) at b-value of 800, showing slightly hyperintense signal of the lesion [Figure 3a] corresponding apparent diffusion coefficient (ADC) map showing hyperintense signal of the lesion, ADC value of the lesion: 1.25 × 10−3 mm2/s [Figure 3b]. Capsular retraction in the affected region was also noted [Figure 4]. There was no intralesional lipid, background liver was normal. Portal and hepatic veins are not involved by lesion, biliary ducts are not dilated. Colonoscopy and esophagogastroduodenoscopy revealed no tumors. Considering the patient’s age, MRI findings indicative of vascular tumors, and absence of immunocompromised status, these findings suggested hepatic epithelioid hemangioendothelioma.
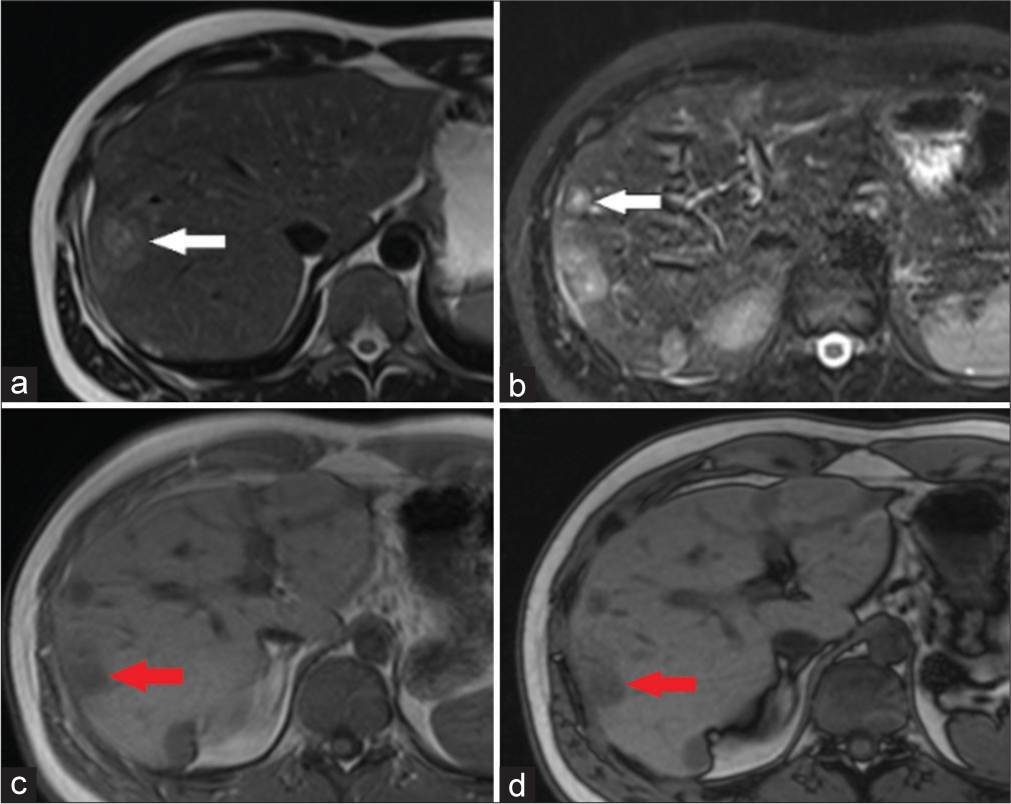
- Axial T2-weighted (a) and fat-saturated T2-weighted (b) magnetic resonance (MR) imaging scans show a multiple round hyperintense masses by “target sign” appearance with a more hyperintense central part (white arrows in a and b). In (c) and out (d) of phases of T1-weighted MR images reveal multiple hypointense masses in both lobes of the liver (red arrows in c and d).

- On native (a) and arterial (b) phases of contrast-enhanced magnetic resonance imaging liver nodules are hypointense (white arrows in a and b), predominantly with mild-to-moderate central enhancement (red arrows in c and d) on portovenous (c) and delayed (d) (4 min) phases.

- Diffusion-weighted imaging in b-value of 800 s/mm2 (a) shows slightly hyperintense mass (white arrow) on the right lobe of the liver. The apparent diffusion coefficient (ADC) value of the lesion was 1.25 × 10−3 mm2/s. ADC map (b) of the liver lesion (white arrow).
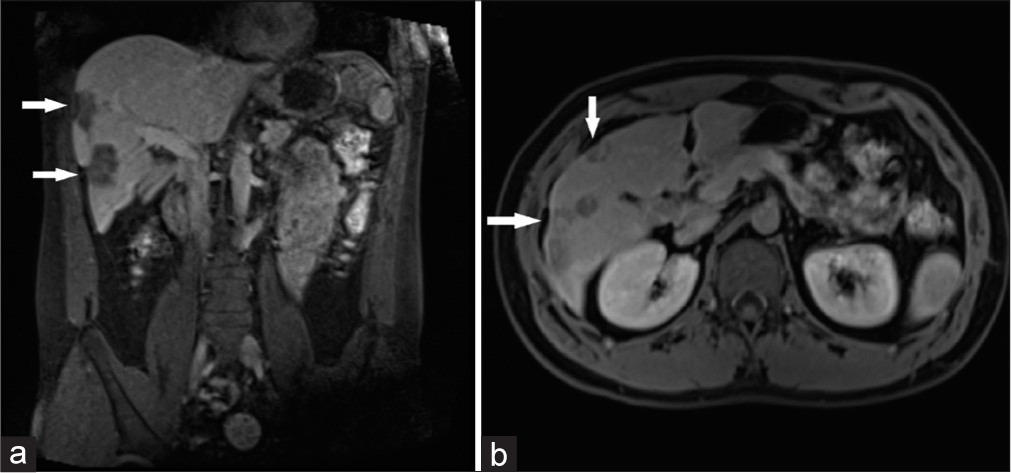
- Coronal (a) and axial (b) venous phase magnetic resonance imaging demonstrates capsular retraction (white arrows in a and b).
Percutaneous core biopsies of the liver were negative. However, fludeoxyglucose 18F (18-FDG) positron emission tomography/computed tomography (PET/CT) imaging revealed multiple metabolically active liver nodules with an SUVmax of 3.8 [Figure 5]. No extrahepatic tumors or distant metastases were detected on PET/CT.

- The Fluorodeoxyglucose 18F (FDG)- Positron emission tomography/Computerised tomography (PET/CT) with axial fused PET/CT (a), axial (b) and coronal (c) PET scans revealed multiple metabolically active liver nodules (white arrows in a, c), predominantly involving the right lobe of the liver, with moderate heterogeneous FDG uptake of standardized uptake value (SUV) max 3.8 (b). No distant metastases were revealed.
Given the negative biopsy results and radiological data suggesting malignancy, a preliminary diagnosis of multiple liver masses with suspicion of malignancy was established. Cytoreductive surgery with resection of accessible nodules was performed.
Multisegmental atypical liver resection revealed multiple grayish-white nodules, 2.5–5 cm in diameter, with capsular retraction. Remaining nodules in segments II, IVa, VII, and VIII were not resected due to their deep anatomical location and high risk of hepatic insufficiency.
Histological examination showed tumors composed of atypical, anastomosing vessels and strands of immature endothelial cells with large, bizarre shapes, and multiple mitoses. The stroma had sparse, polymorphic oval and spindle cells with hyperchromatic nuclei. Neoplastic endotheliocytes were growing into the lumen of liver vessels. Extensive necrosis and hemorrhages were evident [Figure 6]. Histological findings were consistent with hepatic angiosarcoma. Immunohistochemical analysis confirmed tumor cell positivity for endothelial markers CD–31 and CD–34 [Figure 7].

- Hematoxylin-eosin staining of tumor demonstrates (a) irregular anastomosing vascular channels (×40, white arrows) (b) lined by atypical endothelial cells with hyperchromatic, enlarged nuclei (×100, red arrows).

- Immunohistochemical staining: tumor cells were positive for (a) CD–31, ×20 and (b) CD–34, ×20.
The post-operative period was uneventful. Follow-up CT scans revealed four remaining nodules located deeper in the liver [Figure 8a and b]. Showing signs of improvement, the patient was discharged for continued chemotherapy.

- On contrast-enhanced computed tomography images after surgery (a) and 6 months after 4 courses of chemotherapy (b), the remaining undeleted nodules (white arrows in a) are visualized, the size of which increased slightly after 6 months (white arrows in b). Recovery of liver resection zones is noted. Recovery of liver resection zones (asterisks in a, b) is noted.
The patient underwent four courses of chemotherapy with doxorubicin. Control CT images after 6 months showed a slight increase in the size of residual liver nodules, with a total increase of 26%. The residual nodes were surgically extracted, and the patient was subsequently released for outpatient adjuvant chemotherapy and ongoing treatment supervision.
DISCUSSION
Hepatic angiosarcoma, a rare liver tumor, affects males more than females, with a higher prevalence in middle-aged and elderly individuals.[8] Our case is significant due to the patient’s youthfulness, being only 25 years old, and the diagnostic challenge posed by distinguishing radiological findings from other vascular tumors.
Primary liver angiosarcoma exhibits a spectrum of imaging characteristics, from small solitary nodules to multiple and large infiltrating lesions, each manifesting diverse patterns of enhancement on post-contrast imaging.[9-11] Contrast-enhanced CT images typically depict hepatic angiosarcomas as non-specific hypoattenuating masses with heterogeneous enhancement, making differentiation from other invasive malignant primary or metastatic liver tumors challenging.[7] On MRI, primary hepatic angiosarcomas manifest as hyperintense lesions on T2-weighted images and hypointense on T1-weighted images, displaying a heterogeneous structure in both sequences. Dynamic contrast MRI reveals a distinctive pattern of heterogeneous contrast accumulation in both the arterial and venous phases, with progressive enhancement in the delayed phase.[6] In complex cases, early central enhancement and arterioportal shunting on dynamic CT imaging can be valuable indicators for distinguishing hepatic angiosarcoma with a solid growth pattern from benign vascular neoplasms.[12] The differential diagnosis of hepatic angiosarcoma encompasses benign vascular liver diseases, Kaposi sarcoma, liver metastases originating from angiosarcoma in other organs, epithelioid hemangioendothelioma, hepatocellular carcinoma, and fibrosarcoma.[2]
Given the infrequency and aggressive nature of the tumor, there are no established and effective treatment guidelines. However, surgery remains the treatment of choice for selected patients, offering a potential for long-term survival, particularly in cases involving solitary tumors.[7] Due to its aggressive nature and high recurrence rate, the prognosis for liver angiosarcoma is generally bleak, with most untreated patients succumbing within 6 months. Even with treatment, a mere 3% of patients manage to survive beyond 2 years.[2]
Our case presented a significant diagnostic challenge, where pre-operative radiological images initially suggested hepatic epithelioid hemangioendothelioma, but post-operative histological examinations revealed hepatic angiosarcoma. The patient’s health was not adversely affected by the initial misdiagnosis, given the absence of standardized treatment recommendations for either hepatic hemangioendothelioma or angiosarcoma. Surgical resection emerges as a viable treatment method for locally limited hepatic hemangioendothelioma and hepatic angiosarcoma.[13]
Several factors contributed to the misdiagnosis in this case, including the rarity of hepatic angiosarcoma among the young population and the similarity in treatment protocols for these hepatic vascular neoplasms. The primary considerations in the differential diagnosis involved hemangioendothelioma, atypical hemangiomas such as sclerosed hemangioma, and hemangiomas exhibiting centrifugal (inside-out) enhancement. Atypical slow-filling and sclerosed hemangiomas can mimic angiosarcoma due to their slow progressive enhancement and capsular retraction, although they typically feature slow peripheral nodular or ring-like enhancement, unlike the case presented here.[14,15] Hemangiomas with centrifugal (inside-out) enhancement display typical characteristics on T2-weighted MRI, such as markedly hyperintense signal intensity and a homogeneous structure.[16] There are no documented instances of sclerosed hemangiomas with centrifugal enhancement in the existing literature. The previous studies examining liver radiological images depicting multiple peripherally located nodules with a “double-target” sign, central enhancement, and capsular retraction similarly concluded that the presentation was more akin to hepatic epithelioid hemangioendothelioma.[17]
In the primary diagnosis of liver angiosarcoma, incorporating PET in addition to CT and MRI provides a comprehensive assessment of disease prevalence and aids in the differential diagnosis with benign processes. In our case, the SUVmax estimates for these tumors were measured at 3.8, facilitating their detection and evaluation for treatment response. The previous reports have indicated that hepatic angiosarcomas often exhibit increased 18-FDG uptake with SUVmax values ranging from 3.6 to 7.8.[18-22] However, a study by Dong et al. suggested that 18-FDG PET/CT may not be effective in differentiating angiosarcoma from hemangioendothelioma.[23,24] Hypermetabolism on 18-FDG PET/CT for benign liver masses, particularly for atypical hemangiomas, is generally atypical, with rare exceptions noted in cases of giant solitary hemangiomas.[25,26] Therefore, the combination of mild-to-moderate hyperintensity on T2 MRI and hypermetabolism on 18-FDG PET/CT in multiple liver tumors of unknown origin and nature can indicate malignancy, helping to exclude tumors originating from other locations.
Nevertheless, the adoption of PET/CT in routine clinical practice remains a subject of debate in hospitals, primarily due to its associated high cost.[27]
CONCLUSION
This case report highlights the occurrence of hepatic angiosarcoma in a young male and provides a comprehensive review of its imaging characteristics observed through CT and MRI modalities. Our findings emphasize the importance of recognizing the distinct imaging features specific to primary hepatic angiosarcoma, particularly the patterns of tumor enhancement visible in CT and MRI.
Further, exploration of these unique imaging characteristics is recommended to improve diagnostic accuracy in identifying primary hepatic angiosarcoma. By focusing on specific indicators of malignancy in liver lesions on MRI, such as signal characteristics on T2 and DWI sequences, and observing heterogeneous progressive enhancement, clinicians can significantly expedite the differential diagnosis process. This approach has the potential to facilitate prompt identification of hepatic angiosarcoma, enabling timely and targeted clinical interventions when needed.
Continued research aimed at refining these diagnostic criteria is crucial for enhancing early detection and optimizing treatment outcomes in cases of primary hepatic angiosarcoma.
Ethical approval
The research/study was approved by the Institutional Review Board at Local Ethics Committee of National Research Oncology Center, number assigned number 041, protocol No. 24, dated October 31, 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Sleisenger and Fordtran’s gastrointestinal and liver disease: Pathophysiology/diagnosis/management (10th ed). United States: Saunders Elsevier; 2010.
- [Google Scholar]
- Primary hepatic angiosarcoma. Eur J Surg Oncol (EJSO). 2015;41:1137-43.
- [CrossRef] [PubMed] [Google Scholar]
- Mesenchymal tumors of the liver. Clin Liver Dis. 2001;5:219-57.
- [CrossRef] [PubMed] [Google Scholar]
- Angiosarcoma of the liver in a rural population. Four cases diagnosed in a 29-month period. JAMA. 1976;236:1704-6.
- [CrossRef] [Google Scholar]
- Primary hepatic angiosarcoma: findings at CT and MR imaging. Radiology. 2002;222:667-73.
- [CrossRef] [PubMed] [Google Scholar]
- Tumors and tumor-like lesions of the hepatobiliary tract: General and surgical pathology. (1st ed). Switzerland: Springer International Publishing; 2017. p. :965-988.
- [CrossRef] [Google Scholar]
- A pooled analysis of primary hepatic angiosarcoma. Jpn J Clin Oncol. 2020;50:556-67.
- [CrossRef] [PubMed] [Google Scholar]
- The natural history of a hepatic angiosarcoma that was difficult to differentiate from cavernous hemangioma. Intern Med. 2012;51:2899-904.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatic angiosarcoma mimicking cavernous hemangioma on angiography. Hepatogastroenterology. 2002;49:1425-7.
- [Google Scholar]
- Hepatic hemangiosarcoma: Imaging findings and differential diagnosis. Eur Radiol. 2000;10:129-33.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatic hemangioendothelioma: An update. World J Gastrointest Oncol. 2020;12:251-2.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical therapy of primary hepatic angiosarcoma. BMC Surg. 2019;19:5.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging features of sclerosed hemangioma. AJR Am J Roentgenol. 2007;189:67-72.
- [CrossRef] [PubMed] [Google Scholar]
- Atypically enhanced cavernous hemangiomas of the liver: Centrifugal enhancement does not preclude the diagnosis of hepatic hemangioma. J Gastroenterol. 2006;41:1227-30.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatic angiosarcoma with early central enhancement and arterioportal shunt on dynamic CT. Hepatogastroenterology. 2000;47:1717-8.
- [Google Scholar]
- Primary hepatic angiosarcoma on coregistered FDG PET and CT images. AJR Am J Roentgenol. 2007;188:1615-7.
- [CrossRef] [PubMed] [Google Scholar]
- A case of diffuse hepatic angiosarcoma diagnosed by FDG-PET. Ann Nucl Med. 2005;19:519-21.
- [CrossRef] [PubMed] [Google Scholar]
- Co-registered positron emission tomography/computed tomography and gadoliniumethoxybenzyl-diethylenetriamine pentaacetic acid magnetic resonance imaging features of multiple angiosarcoma of the liver. Hepatol Res. 2014;44:E297-303.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatic angiosarcoma with dyskeratosis congenita. Intern Med. 2015;54:2867-72.
- [CrossRef] [PubMed] [Google Scholar]
- Metastasis from a primary hepatic angiosarcoma to the colon: A case report and literature review. Oncol Lett. 2017;13:2765-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatic hemangioendothelioma: CT, MR, and FDG-PET-CT in 67 patients-a bi-institutional comprehensive cancer center review. Eur Radiol. 2020;30:2435-42.
- [CrossRef] [PubMed] [Google Scholar]
- MRI and FDG PET/CT findings of hepatic epithelioid hemangioendothelioma. Clin Nucl Med. 2013;38:e66-73.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value of 18F-FDG PET/CT for atypical hepatic hemangioma. Jpn J Clin Oncol. 2022;52:98-100.
- [CrossRef] [PubMed] [Google Scholar]
- The rare case of FDG-positron emission tomography for giant cavernous hemangioma of the liver. Br J Res. 2017;4:19.
- [Google Scholar]
- Role and cost effectiveness of PET/CT in management of patients with cancer. Yale J Biol Med. 2010;83:53-65.
- [Google Scholar]







