Translate this page into:
Malignant Tenosynovial Giant Cell Tumor of the Leg: A Radiologic-Pathologic Correlation and Review of the Literature
Address for correspondence: Dr. Danielle M Richman, Department of Radiology, Brigham and Women's Hospital, Boston - 02115, Massachusetts, USA. E-mail: DMRichman@partners.org
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Malignant tenosynovial giant cell tumor (TGCT) is a rare clinical entity that can arise as a recurrent lesion or can co-exist with a benign TGCT lesion. Malignant TGCT most commonly arises in the lower extremity and tends to be clinically aggressive, with most patients developing recurrent lesions or dying. Much of the literature describes the histopathologic features and classifies this broad group of tumors, with little description of the imaging characteristics of this disease. We present the multimodality appearance of a case of malignant diffuse-type TGCT that recurred 2 months after resection with subsequent rapid clinical progression.
Keywords
Diffuse-type tenosynovial giant cell tumor
malignant tenosynovial giant cell tumor
pulmonary metastasis
tenosynovial giant cell tumor
INTRODUCTION

A 55-year-old female presented with 6-month history of progressively worsening right posterior calf pain. On physical exam, the right posterior calf appeared erythematous and warm with decreased range of motion. Core needle biopsy of the right calf mass demonstrated diffuse-type giant cell tumor [also known as diffuse-type tenosynovial giant cell tumor (TGCT)] and the patient underwent surgical resection.
Approximately 7 weeks after surgery, the patient noticed swelling and warmth of the right lower extremity with associated slow growth of friable red soft tissue, approximately 1 cm lateral to the incision. The patient returned to the operating room for resection of suspected recurrent TGCT and debridement of devitalized tissue, ultimately followed by amputation.
The slowing of disease was short-lived and, despite multiple subsequent systemic therapies first with imatinib, followed by adriamycin–ifosfamide, the patient developed extensive metastatic disease with significant tumor burden in the chest and right inguinal region. The patient died 21 months after diagnosis secondary to respiratory failure from metastatic disease.
RADIOLOGIC FEATURES
Radiographs taken on presentation revealed mild osteoarthritis in bilateral knees, with fullness posterior to the right knee [Figure 1]. Post-operative ultrasound performed 7 weeks after initial resection showed a complex cystic and solid mass with hypervascular solid components [Figure 2]. MRI demonstrated a large, well-circumscribed, mixed-density soft tissue 20-cm mass, with both solid and cystic components extending from the popliteal fossa to the mid-calf [Figure 3]. CT of the chest, abdomen, and pelvis showed no evidence of metastatic disease.
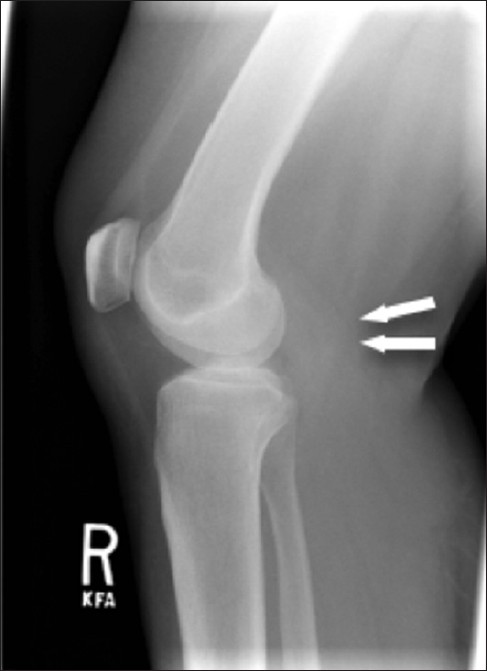
- 55-year-old female with right posterior calf pain diagnosed with tenosynovial giant cell tumor. Lateral radiograph of the knee reveals soft tissue fullness in the popliteal fossa (arrow).
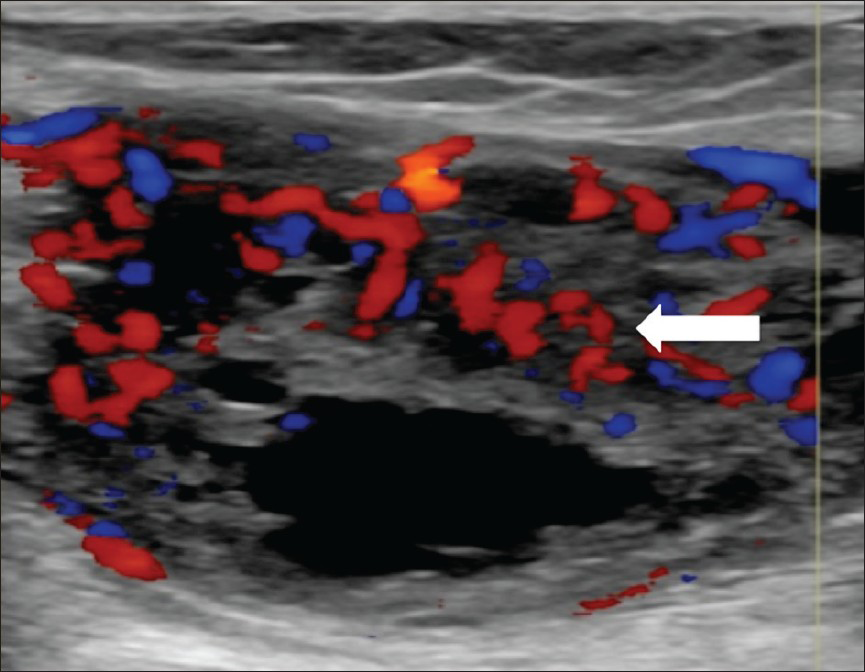
- 55-year-old female with right posterior calf pain diagnosed with tenosynovial giant cell tumor. Ultrasound of the soft tissue mass demonstrates solid and cystic components. Doppler ultrasound shows prominent vascularity within the solid, echogenic components of the lesion (arrow).
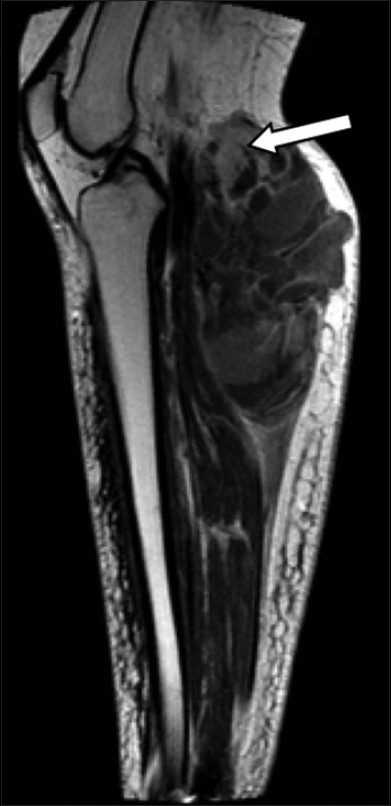
- 55-year-old female with right posterior calf pain diagnosed with tenosynovial giant cell tumor. Sagittal T1-weighted MR post-contrast imaging reveals enhancement of solid areas (arrow).
MRI done 7 weeks after initial resection showed a heterogeneous lobulated lesion with enhancement of the solid components, centered within the gastrocnemius and extending 7 cm superior to the tibiofemoral joint [Figure 4]. Popliteal vessels and nerves were encased by the tumor. Low-volume lymph nodes in the right inguinal region were unchanged. Given the aggressive appearance of the lesion, the patient underwent a below the knee amputation.
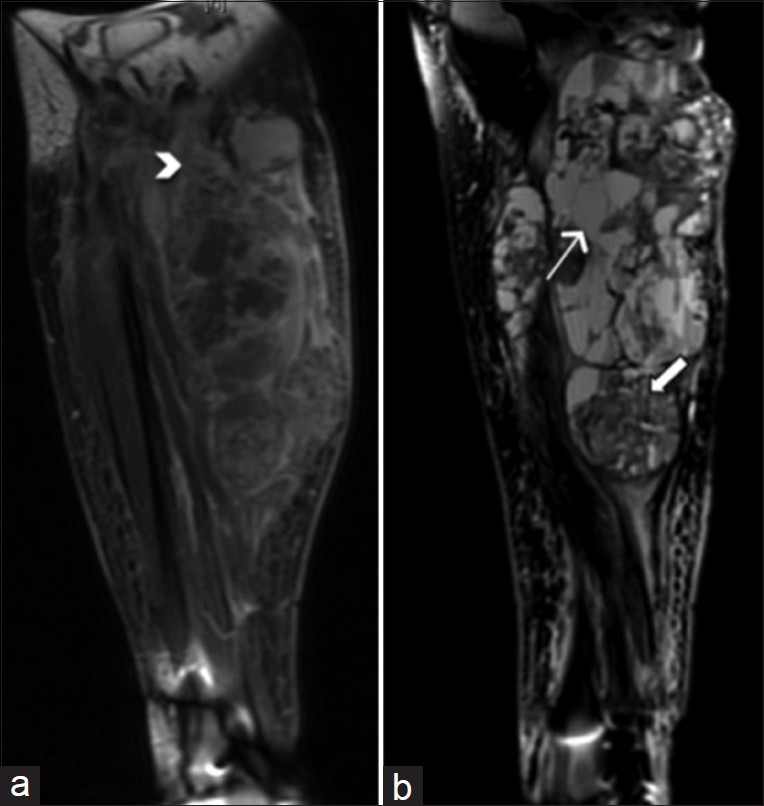
- 55-year-old female 7 weeks post-resection of tenosynovial giant cell tumor with complaints of pain and friable tissue growth at the incision site. (a) Coronal T1-weighted post-contrast MRI of the right knee shows a heterogeneous lobulated lesion extending to the mid-calf. The lesion measures 24.4 cm in craniocaudal dimension. Margins of soft tissue components of the lesion are ill-defined and somewhat infiltrative in some areas (arrowhead). (b) Sagittal T2-weighted MR of the right knee shows both cystic (thick arrow) and solid (thin arrow) areas within the heterogeneous lesion.
PATHOLOGIC FEATURES
Initial pathology
Core needle biopsy of the right calf mass demonstrated diffuse-type giant cell tumor (also known as diffuse-type TGCT) with a heterogeneous cell population consisting of sheets of larger, epithelioid cells with round nuclei and discrete cell borders in a background of histocytes, lymphocytes, and scattered hemosiderin [Figure 5a], the characteristic appearance of diffuse-type TGCT. Numerous giant cells and focal necrosis were also present. Although the cytology of the tumor was atypical with a high mitotic rate of 27 per 10 high-power fields (HPFs), a diagnosis of malignant diffuse-type TGCT was not warranted in this limited sample as the sample did not meet five out of the eight criteria needed for a diagnosis of malignancy (see Discussion). Immunohistochemical stains were negative for smooth muscle actin (SMA), desmin, CD34 (cluster of differentiation protein), Pan-K, and S100 protein in the small amount of tissue present.
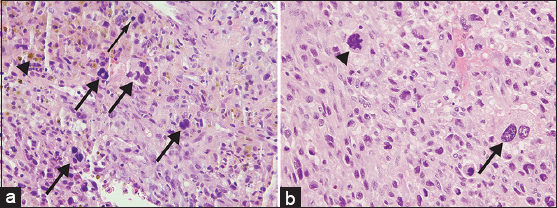
- 55-year-old female with right posterior calf pain initially diagnosed with tenosynovial giant cell tumor with subsequent resection demonstrating malignant tenosynovial giant cell tumor. Pathology of core biopsy and resection specimens is shown. (a) Core biopsy sample of the mass stained with Hematoxylin and eosin, ×400, shows epithelioid cells with round nuclei and a background of lymphocytes, histiocytes, several multinucleated giant cells (thick arrows), and scattered mitoses (thin arrow). Hemosiderin is also present (arrowhead). (b) Although the majority of the resection specimen appears similar to the core biopsy, select areas of the resection stained with Hematoxylin and eosin (×400), display pleomorphic cytology with large, highly atypical cells (arrow) and atypical mitoses (arrowhead), consistent with the diagnosis of malignant diffuse-type TGCT.
The right lower extremity amputation specimen, which was received 7 weeks after the initial resection, revealed a large, cystic and solid mass extending from the popliteal fossa to the mid-calf [Figure 6]. Microscopic examination showed areas of large, bizarre, and anaplastic epithelioid cells, warranting a diagnosis of malignant TGCT. This degree of cytologic atypia was not represented in the original biopsy. Atypical mitoses were also present, and the tumor showed an infiltrative pattern, with invasion of the skin, associated ulceration and necrosis, and a mitotic rate of 23 per 10 HPFs [Figure 5b]. Hemosiderin deposition and characteristic cleft-like spaces were readily apparent, along with widespread areas with relatively bland cytology, the characteristic appearance of benign diffuse-type TGCT.
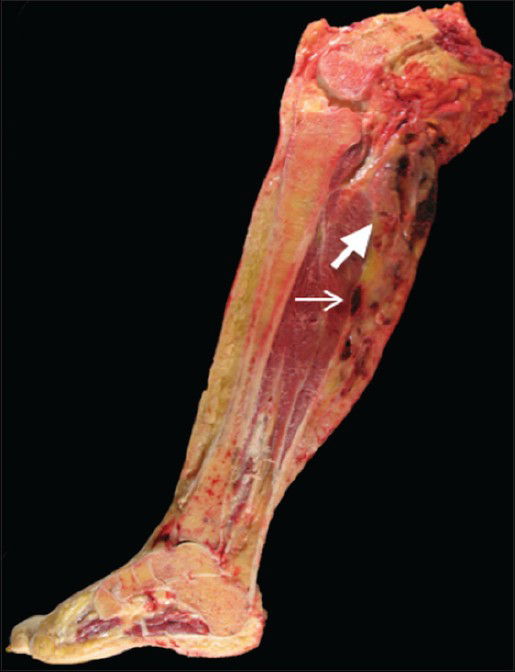
- 55-year-old female who initially presented with progressive right posterior calf pain now status post right leg amputation for malignant tenosynovial giant cell tumor. Gross pathology of the amputated right lower extremity reveals a heterogeneous tumor with cystic (thick arrow) and solid (thin arrow) components extending from the popliteal fossa to the mid-calf.
Immunohistochemical stains for SMA, D2-40 (focal), and desmin (rare) were positive in lesional cells, with CD163-positive histiocytes, while S100, Pan-K, CD34, and leukocyte common antigen (LCA or CD45) immunostains were negative. These differences from the original immunoprofile were minor and also likely due to tumor heterogeneity. This immunohistochemical profile was consistent with malignant diffuse-type TGCT.
DISCUSSION
TGCT was first described in 1852 by Chassaignac as synovial membrane proliferation involving the flexor tendons of the fingers and was later redefined by Jafee et al., to encompass lesions involving the synovium, tendon sheath, bursa, and joint.[12] TGCT can be classified as localized or diffuse. The most common type of localized TGCT is also referred to as giant cell tumor of the tendon sheath, which typically occurs as a localized nodule on the tendon sheaths of the fingers or toes. On the contrary, the diffuse type tends to infiltrate the joint, and more frequently involves large joints, most commonly the knee, followed by the hip. Diffuse-type TGCT tends to be more aggressive, often recurring locally (8–56%), and is capable of malignant transformation.[3]
Fewer than 50 cases of malignant TGCT have been described in the English literature.[45] Enzinger et al., first described malignant TGCT as uncontrolled growth within a benign lesion or as the recurrence of a previously benign lesion.[6] Despite aggressive management, including surgical resection, chemotherapy, and radiation, prognosis in malignant TGCT is poor. Lesions most commonly metastasize to regional lymph nodes and lungs, with a median survival of 22.5 months after diagnosis.[45] Time to metastatic disease ranges from 4 months to 5 years, with a median of 11 months.[4]
Li et al., and Bertoni et al., described the following histologic characteristics as suggestive of malignancy: Nodular and/or solid infiltrative growth pattern, large round or oval cells, cells with large nuclei and nucleoli, areas of necrosis, and atypical mitoses.[47] Criteria for malignant TGCT were also developed at the Armed Forces Institute of Pathology (AFIP) to include five out of eight characteristics including diffuse pleomorphism, prominent nucleoli, high cytoplasmic to nuclear ratios, mitotic ratio greater than 10 per 10 HPFs, necrosis, discohesion of tumor cells, paucity of giant cells, and a diffuse growth pattern.[8] Our case demonstrated features described by both Bertoni et al., and the AFIP to be classified as malignant [Figure 5b] and was confirmed by histologic evaluation to be metastatic to inguinal lymph nodes. The initial biopsy of the lesion was atypical including a mitotic rate greater than 10 per 10 HPFs as well as necrosis; however, it did not fulfill enough of the criteria to be classified as malignant TGCT.
Radiographic appearance of TGCT is non-specific, ranging from soft tissue swelling to soft tissue mass and bony involvement. Radiographs of TGCT lesions most commonly demonstrate joint effusion, soft tissue swelling, bony erosion, and lack of calcification in the context of normal bone mineralization and preservation of the joint space.[3] Similarly, ultrasound is non-specific; however, it is useful for characterizing its solid and cystic components.[9] MRI is superior due to its better delineation of soft tissues and its ability to more accurately determine the extent of synovial proliferation, joint effusion, bone erosion, and deposits of hemosiderin.[310] TGCT lesions generally have hemosiderin deposits, which will appear as dark, low-signal regions on T1-weighted and T2-weighted images, with associated susceptibility artifact on gradient echo sequences. The presence of hemosiderin is characteristic of TGCT lesions; however, it can also be seen in other lesions such as synovial hemangioma or hemophiliac arthropathy and does not correlate with malignant potential.[3] Regions of enhancement can be seen in TGCT in the regions of synovial proliferation, but do not predict the aggressiveness of the lesion.[310] Imaging of malignant TGCT is rarely reported, and the only description of the MRI characteristics suggestive of malignant TGCT is reported in the pathology and rheumatology literature and includes frequent lobulation, infiltrative lesions with poorly defined margins, and close association with tenosynovial structures and/or joint spaces.[410] Murphey et al., suggest that findings most consistent with a malignant lesion are bony invasion and presence of metastases.[3] Our patient's first post-resection MRI included multiple areas of lobulation and close association with the joint; however, these findings are also often seen in benign disease. The most concerning feature of the post-resection MRI was diffuse involvement of the medial and lateral gastrocnemius muscles with an infiltrative appearance and loss of clear tissue planes, a finding that was not seen on the initial MRI.
CONCLUSION
In all reported cases, the diagnosis of malignant TGCT was not made until after histopathologic examination of the biopsied lesion, most likely because of the low incidence of the lesion and the paucity of imaging of malignant TGCT in the literature. This case highlights the appearance of malignant TGCT on multiple imaging modalities. While no single clinical, histopathologic, or radiographic feature separates benign from malignant lesions, a compilation of findings including rapid recurrence, and infiltrative appearance on imaging with invasion of adjacent soft tissues suggest malignancy. Therefore, the current standard of practice remains local wide resection of TGCT lesions with close follow-up and tissue sampling when clinically indicated.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Jyothi Jagannathan of Dana Farber Cancer Institute and Dr. Chris S. Chung of Brigham and Women's Hospital for their contributions to this study.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2015/5/1/13/152343
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Pigmented villonodular synovitis, bursitis and tenosynovitis. Arch Pathol. 1941;31:731-65.
- [Google Scholar]
- Pigmented villonodular synovitis: Radiologic-pathologic correlation. Radiographics. 2008;28:1492-518.
- [Google Scholar]
- Malignant diffuse-type tenosynovial giant cell tumors: A series of 7 cases comparing with 24 benign lesions with review of the literature. Am J Surg Pathol. 2008;32:587-99.
- [Google Scholar]
- Malignant pigmented villonodular synovitis of the temporomandibular joint with lung metastasis: A case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:e30-6.
- [Google Scholar]
- Benign tumors and tumor-like lesions of the synovial tissues. In: Enzinger FM, Weiss SW, eds. Enzinger and Weiss's Soft Tissue Tumors (3rd ed). Philadelphia: Mosby; 1995. p. :769-88.
- [Google Scholar]
- Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis) Am J Surg Pathol. 1997;21:153-63.
- [Google Scholar]
- Primary tumours of the synovium: A report of four cases of malignant tumour. J Bone Joint Surg Br. 2007;89:1504-8.
- [Google Scholar]
- Pigmented villonodular synovitis of the knee: A case report. J Ultrasound. 2011;14:167-9.
- [Google Scholar]
- MRI features of pigmented villonodular synovitis (PVNS) Clin Rheumatol. 2004;23:31-4.
- [Google Scholar]






