Translate this page into:
Initial Experience of Utilizing Real-Time Intra-Procedural PET/CT Biopsy
Address for correspondence: Dr. Aung Zaw Win, Department of Radiology, San Francisco Veterans Affairs Medical Center, 4150 Clement Street, San Francisco, California - 94121, USA. E-mail: aungzwin@gmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
Nonreal-time Positron Emission Tomography/Computed Tomography (PET/CT) biopsies that use the image co-registration of a prior PET with an intra-procedural CT have been reported. The aim of this study was to report the initial experience of performing real-time intra-procedural PET/CT-guided biopsies.
Materials and Methods:
All patients (n = 4) had a prior PET/CT examination of the concerning lesion and no significant CT correlate. On the day of the biopsy, 5 mCi of 18F-fluorodeoxyglucose (FDG) or NaF18 was intravenously injected. After 60 min of biodistribution of the molecular probe, PET/CT images were obtained in a limited one bed position over the region of the concerning lesion to be biopsied.
Results:
One patient had a mesenteric mass and the other three had bone lesions, one located in the rib and two in the iliac bone. The pathology report revealed that two lesions (50%) were malignant and two lesions (50%) were benign. The results of the biopsy changed management in all cases. There was 0% complication rate.
Conclusions:
No additional software or hardware is required to perform real-time intra-procedural PET/CT-guided biopsies. It can optimize the yield, especially in cases where there are no anatomical abnormalities. Real-time intra-procedural PET/CT biopsy may have benefits over conventional biopsy techniques in terms of accuracy.
Keywords
Biopsy
fluorodeoxyglucose
NaF-18
positron emission tomography/computed tomography
INTRODUCTION

The clinical management of lesions suspicious of malignancy relies not only on diagnosis of benign versus malignant entity but also on tumor grading, immunohistochemical findings, and genetic information. Pathological analysis remains the Gold Standard for definite diagnosis. Hence, a carefully performed biopsy with low risk of complication is crucial. Compared with open biopsy, image-guided biopsies are minimally invasive and confer several advantages including low morbidity, low complication rate, and cost savings.[1] PET/CT has been used in initial staging, treatment response evaluation, and follow-up. PET/CT-guided biopsies may allow early histological diagnosis and staging before morphologic changes are evident.[2] PET/CT biopsy can rule out malignancy and it can correctly stage different types of cancer.[3] Nonreal-time PET/CT biopsies use co-registration of a prior PET image with a procedural CT image. However, this method is inaccurate in time and space, and requires special software. The aim of this study is to report the initial experience of utilizing the real-time PET/CT-guided biopsies, and explore feasibility and technical requirements of the procedure.
MATERIALS AND METHODS
Four real-time intra-procedural PET/CT biopsies were performed at our institution. University of San Francisco institutional review board (IRB) approved the study. Patient records/information was anonymized and de-identified prior to analysis.
FDG-PET/CT imaging
In all cases, the patients had a prior whole body PET/CT scan showing abnormal focal uptake, but no CT correlation, and needed further histological confirmation. Patients underwent overnight fasting before an 18F-fluorodeoxyglucose (FDG)-PET/CT biopsy procedure. Limited one bed PET/CT imaging was performed over the selected lesion following an uptake period of 60 min after intravenous administration of 5 mCi of FDG or NaF. Imaging was performed in a PET/CT scanner (GE STE 64 slice CT scanner, GE healthcare, Waukesha, WI). CT scan was done for attenuation correction and anatomic localization (CTAC). Imaging parameters were 140 kVp, 90–120 mA, 1.25 mm collimation with reconstruction as 3.75 mm thick sections by using a 512 × 512 matrix and a filtered back projection algorithm. Immediately after CTAC, PET images were obtained in two-dimensional mode for 5 min acquisitions for one-bed position. PET images were reconstructed by using a 128 × 128 matrix and an iterative-ordered subset expectation maximization algorithm. PET/CT images therefore were obtained in a limited one-bed position over the region of the lesion. Once the lesion was identified and assessed, a biopsy needle was guided, real time, to the area of focal uptake.
Biopsy procedure
A recent complete blood count (CBC), history of allergies, prothrombin time (PT), and international normalized ratio (INR), co-morbidities and medications were reviewed prior to the procedure. A 12 cm Jamshidi bone biopsy needle was used for bone biopsies (3 cases) and a mesenteric biopsy was performed with an 18 gauge introducer needle and a 20 gauge core biopsy needle (1 case). The coaxial (introducer) needle allows the radiologist obtain several samples with a single needle stick. The patients were not moved in and out of the gantry for each manipulation of the needle. We obtained three samples per biopsy.
Procedure
-
Patients were positioned and immobilized in the correct position, depending on the location of the lesion. Patients were placed in the biopsy position prior to limited PET/CT.
-
Local anesthesia with 1% Lidocaine was administered.
-
The biopsy needle was guided, real time, by using PET/CT imaging to the targetorgan showing the focal uptake. Perfect intra-lesion placement of the tip of the biopsy needle was confirmed by PET/CT imaging.
-
The core of the coaxial needle was pulled out and an 18 gauge biopsy needle was inserted.
-
After removal of the needle, manual compression was performed for 2-3 min at the puncture site.
-
The specimens obtained were fixed in 10% formalin and presented to the pathologist, who was in the same room, for quick histopathological examination.
-
The patients were observed for 2-6 h and then discharged.
RESULTS
All PET/CT positive lesions were successfully biopsied real-time without the need for repeating the biopsies. The alignment was perfect in all cases. Assessing the intra-lesion placement of the needle tip was very easy despite extreme windowing of the transmission images. The pathology report revealed that two lesions (50%) were malignant and two lesions (50%) were benign. The results of the biopsy changed management in all cases. There was 0% complication rate. The characteristics of the patients and the lesions are described in Table 1. PET/CT guided biopsy and stained biopsy samples from patient 3 [Figures 1 and 2] led to the identification of a benign lesion. PET/CT images and stained biopsy sample from patient 4 [Figures 3 and 4] identified the lesion as malignant.
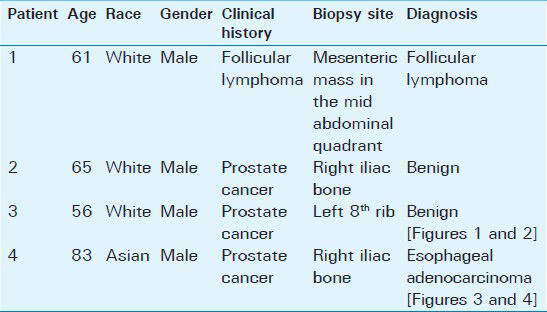
- 56-year-old male with a complaint of difficulty in urinating was diagnosed with prostate cancer. (a) Low power view of the marrow biopsy (×20) shows no abnormalities. Scale bar = 200 microns (b) High power view of the biopsy sample (×200) shows normal hematopoietic cells, including megakaryocytes, visible within the adipose tissue of bone marrow (arrows). Scale bar = 20 microns. No metastasis of the prostate cancer WAS observed. Was = past tense.
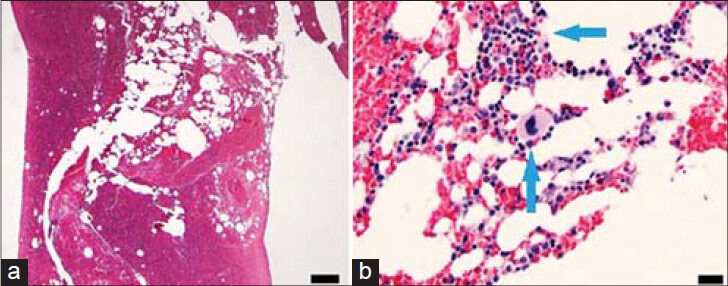
- 56-year-old male with a complaint of difficulty in urinating was diagnosed with prostate cancer. Axial PET/CT images (a) Increased FDG uptake in the left 8th rib (arrows) and (b) 12 cm Jamshidi bone biopsy needle (arrows) in the metabolically active region of the left 8th rib.
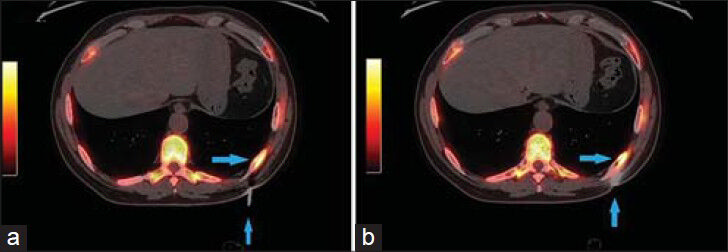
- 83-year-old male with elevated prostate-specific antigen (PSA) was diagnosed with prostate cancer. (a) Low power view of FNA cell block material (×10) shows abundant tumor tissue (arrows). Scale bar = 100 microns (b) High power view (×100) of an area of dyshesive neoplastic cells demonstrates esophageal adenocarcinoma of signet ring cell morphology. Signet ring morphology is described as abundant in cytoplasm with the nucleus pushed to one side (arrows) Scale bar = 20 microns (c) Hematoxylin and eosin stained sample (×50) shows clusters of neoplastic cells (arrows) and (d) immunohistochemical staining of the sample shows cells positive for pancytokeratin (arrows) (d). Scale bars = 50 microns.
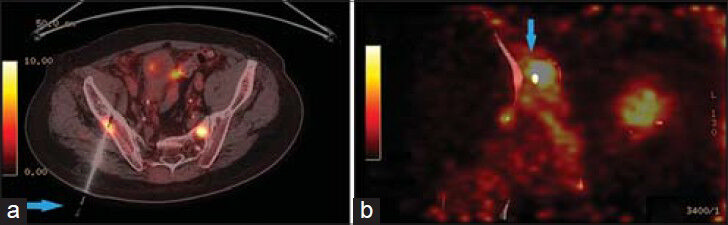
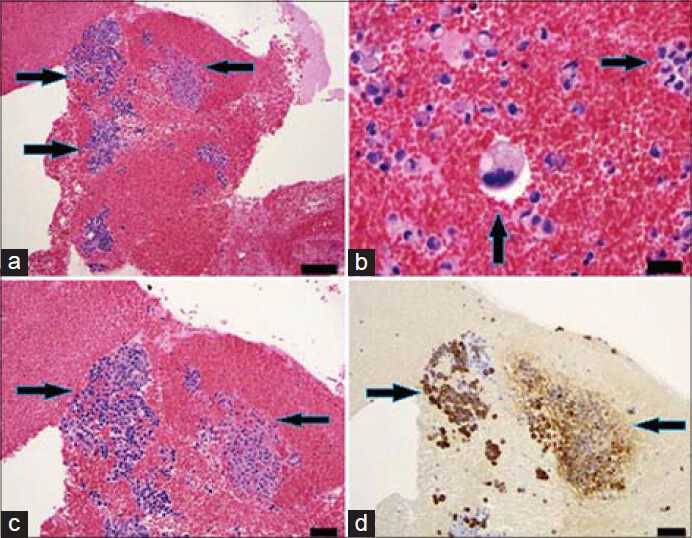
- 83-year-old male with elevated prostate-specific antigen (PSA) was diagnosed with prostate cancer. Axial PET/CT image shows high FDG uptake in the right iliac bone. (a) Hybrid PET/CT guided the needle (arrow) to the exact location of the hypermetabolic lesion in the right iliac bone (b) The tip of the needle (arrow) in the lesion suspected for metastasis.
DISCUSSION
This is the first study to report the intra-procedural real-time PET/CT biopsy procedure and to report the biopsy of a mesenteric mass using PET/CT. Most PET/CT-guided biopsy studies published thus far use prior PET images to fuse with intra-procedural CT to guide the biopsy needle.[4567] Success of biopsy and accurate diagnosing depends upon adequate tissue being obtained at the right location. The inaccuracy of diagnosis prior to surgery can lead to significant morbidity and increased mortality.[8] Most of the time, before performing a biopsy, magnetic resonance imaging (MRI) or CT image is used to identify the mass.[8] On MRI, benign soft tissue lesions are usually small (<5 cm), well circumscribed, and have homogeneous signal intensity, whereas malignant lesions tend to be large, inhomogeneous and have poorly defined margins.[8] Tumors without these features are difficult to classify on MRI and there are many exceptions to this classification. It is difficult to consistently distinguish benign from malignant areas within a heterogeneous mass; more importantly, to define the most malignant area.[8] For FDG avid tumors, FDG-PET can not only detect malignancy but also help to locate the most active areas within the malignancy.[8] Hain et al., found that the PET scan can guide the needle to the best biopsy site.[8]
There are several techniques for performing PET/CT biopsy. One method uses previously acquired PET/CT images to co-register with intra-procedural CT images, using computer software.[2] A rigid image registration algorithm is required to achieve bimodal fusion between a pre-acquired PET scan and an intra-procedural CT scan.[9] Image fusion takes longer time, special hardware, and may require software add-ons.[10] Image fusion is the overlay of two or more imaging data sets together as one display, whereas image registration consists of aligning or matching the two imaging data sets spatially to each other.[11] In the method employed for this paper, the entire biopsy procedure was performed inside the PET/CT scanner. The emission images are therefore in the absence of movement, always matching the intra-procedural CTAC images.[10] The spatial accuracy of this technique is necessary to display clinically relevant image guidance information during biopsy.
The study by Singh et al., has shown that relying on anatomic imaging alone was not adequate to detect cancer.[12] Even with the use of costly 3-T MRI scanner, Singh et al., found cancer in only 3% of the cases.[12] Furthermore, artifacts increase with increasing magnetic field strength.[13] In MRI-guided biopsy, which is complex to perform, patients may have to slide in and out of the scanner several times during the procedure and the biopsy needle may have to be inserted several times until it is in the desired target area.[14] Also, commonly used titanium biopsy needles are not directly visible on MRI and an artifact forms in the neighborhood of the needle.[15] Therefore, the true needle position differs from the artifact position. Larger needles have higher diagnostic yield and accuracy, however, they cause larger artifacts on MRI images.[13] In a study by Johnson et al., MRI-guided biopsy could not visualize 40% of the lesions ≤ 10 mm in size.[16] Some MRI-guided biopsies require contrast injection and consequently, the biopsy time is limited to about 45 min before the contrast washes out.[17] Relative to ultrasound (US)- and CT-guided biopsy, MRI-guided biopsy generally requires longer procedure time.[10]
Prior et al., reported that CT missed small cancerous lesions (10-20 mm) in their study and the lesions were visualized only by PET.[18] In fact, the accuracy of CT-guided biopsy for cancerous lesions (0.7–1.5 cm) in size could be as low as 35%.[19] In a study by Davis et al., the ability of CT-guided biopsy to obtain adequate samples for solid tumors (<1 cm) was 25% and for cystic tumors was 0%.[20]
With the US-guided biopsy, the diagnostic yield can be as low as 46% for lesions less than 20 mm in diameter.[21] US-guided biopsy is not optimal for bone, bowel, and lesions deep inside the body.[22] The field of view of US is also limited and nearby critical structures may not be visualized. The 2-dimentional US images commonly used today for real-time guidance make proper needle placement challenging when dealing with complex 3D targets.[23] US-guided biopsy is operator dependent and it is difficult to visualize the needle tip.[10] Not surprisingly, the specificity of US-guided biopsy can be as low as 67%.[24]
In large tumor masses, most of the tissue can be necrotic and only a small portion can be metabolically active. PET/CT biopsy can pinpoint the most active tissue within the large mass and this decreases the need to re-biopsy. In the case of multiple lesions, PET/CT can guide the operator to the most active lesion. In the present study, there was no need for repeat biopsies and the diagnostic yield was 100%. Venkatesan et al., fused real-time US to co-registered CT and FDG-PET scans.[9] In that study, malignancy was found in 50% (18/36) biopsies and 2 biopsies were not successful. We also found malignancy in 50% of the cases and we have similar or better results compared with the US/PET/CT multimodality fusion study. In an article by Servois et al., 100% of the PET positive lesions are malignant.[25] It is very important to rule out malignancy in suspicious lesions; especially in patients with cancer. PET/CT biopsy is clearly better than CT-guided biopsy because in some cancers, morphological changes are not apparent. Segaert et al., found that integrated PET/CT is superior to both PET and CT in detecting cancerous lymph nodes and it had a sensitivity of 100%.[26] PET/CT biopsy can be utilized for any part of the body including the bone and bone marrow. In addition, FDG dosage injected in our procedures was far less than the dosage for diagnostic PET/CT exams. Combining PET/CT exam and PET-guided biopsy will minimize radiation by requiring only one FDG injection. Tatli et al., used an average of 20.6 mCi of FDG in their PET/CT biopsies.[27] We have successfully performed biopsies with only 5 mCi.
Cerci et al., reported that PET/CT-guided biopsy is feasible and may optimize the diagnostic yield of image-guided interventions.[28] FDG-PET can accurately identify soft tissue sarcomas for biopsy.[8] FDG-PET/CT-guided biopsy has been utilized for soft tissue tumors and for mucormycosis.[829] FDG-PET/CT has much higher diagnostic accuracy than CT or MRI in characterizing tumor.[28] In addition to biopsy, PET/CT has been used in ablation and surgical procedures.[7] Werner et al., wrote that bone scintigraphy cannot reliably detect malignant bone marrow infiltration.[30] Bone scintigraphy shows only bone metabolism, not malignancy, and hence may not be specific enough to separate bone remodeling and healing fractures, which are common after treatment of bone metastases from live tumor cells.[30] According to Werner et al., PET/CT-guided biopsy is the best approach for detecting bone and bone marrow abnormalities.[30]
Limitations
This is a retrospective study with a small study population. There was only one case of soft tissue biopsy [Table 1]. The veteran patients are mostly male and the patients in this study were all males. The tracers used for the real-time PET/CT biopsy were NaF18 and 18F-FDG. Cancers such as thyroid cancer, renal cancer, and skeletal metastasis of prostate cancer can have low FDG avidity. In contrast, NaF18 is not ideal for osteolytic bone lesions such as breast cancer metastases.
CONCLUSION
PET/CT-guided biopsy is feasible and there is rarely a need for repeat biopsies with this technique. The radiation dosage required for PET/CT biopsy is less than routine PET/CT exams. This procedure allows the PET positive lesions with no morphological correlation to be evaluated. PET/CT biopsy requires no additional equipment and can be done with equipment already available for regular examinations. We found that real-time PET/CT biopsy has benefits over conventional biopsy techniques in terms of accuracy of locating the correct biopsy site.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2014/4/1/54/141941
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Radiation dose reduction in pediatric CT-guided musculoskeletal procedures. Pediatr Radiol. 2013;43:1303-8.
- [Google Scholar]
- Image-guided biopsy: What the interventional radiologist needs to know about PET/CT. Radiographics. 2012;32:1483-501.
- [Google Scholar]
- Role and cost effectiveness of PET/CT in management of patients with cancer. Yale J Biol Med. 2010;83:53-65.
- [Google Scholar]
- PET/CT-guided interventions: Personnel radiation dose. Cardiovasc Intervent Radiol. 2013;36:1063-7.
- [Google Scholar]
- Abdominal masses sampled at PET/CT-guided percutaneous biopsy: Initial experience with registration of prior PET/CT images. Radiology. 2010;256:305-11.
- [Google Scholar]
- Minimizing image misregistration during PET/CT-guided percutaneous interventions with monitored breath-hold PET and CT acquisitions. J Vasc Interv Radiol. 2011;22:1287-926.
- [Google Scholar]
- Cone-beam computed tomography fusion and navigation for real-time positron emission tomography-guided biopsies and ablations: A feasibility study. J Vasc Interv Radiol. 2012;23:737-43.
- [Google Scholar]
- Can FDG PET be used to successfully direct preoperative biopsy of soft tissue tumours? Nucl Med Commun. 2003;24:1139-43.
- [Google Scholar]
- Real-time FDG PET guidance during biopsies and radiofrequency ablation using multimodality fusion with electromagnetic navigation. Radiology. 2011;260:848-56.
- [Google Scholar]
- The challenging image-guided abdominal mass biopsy: Established and emerging techniques ‘ if you can see it, you can biopsy it’. Abdom Imaging. 2013;38:672-96.
- [Google Scholar]
- Multimodality image fusion-guided procedures: Technique, accuracy, and applications. Cardiovasc Intervent Radiol. 2012;35:986-98.
- [Google Scholar]
- Patient selection determines the prostate cancer yield of dynamic contrast-enhanced magnetic resonance imaging-guided transrectal biopsies in a closed 3-Tesla scanner. BJU Int. 2008;101:181-5.
- [Google Scholar]
- MRI-guided breast biopsy at 3T using a dedicated large core biopsy set: Feasibility and initial results. Eur J Radiol. 2011;79:257-61.
- [Google Scholar]
- MRI-guided biopsy for prostate cancer detection: A systematic review of current clinical results. Curr Urol Rep. 2013;14:209-13.
- [Google Scholar]
- Accuracy analysis in MRI-guided robotic prostate biopsy. Int J Comput Assist Radiol Surg. 2013;8:937-44.
- [Google Scholar]
- Cancelation of MRI guided breast biopsies for suspicious breast lesions identified at 3.0 T MRI: Reasons, rates, and outcomes. Acad Radiol. 2013;20:569-75.
- [Google Scholar]
- Initial report of PET/CT-guided radiofrequency ablation of liver metastases. J Vasc Interv Radiol. 2007;18:801-3.
- [Google Scholar]
- Predictive factors of diagnostic accuracy of CT-guided transthoracic fine-needle aspiration for solid noncalcified, subsolid and mixed pulmonary nodules. Radiol Med. 2013;118:1071-81.
- [Google Scholar]
- Computed tomography-guided renal tumor biopsies: Tumor imaging features affecting sample adequacy. J Comput Assist Tomogr. 2013;37:171-5.
- [Google Scholar]
- Ultrasound-guided transbronchial biopsy of solitary pulmonary nodules less than 20 mm. Eur Respir J. 2009;34:1284-7.
- [Google Scholar]
- Ultrasound-guided musculoskeletal procedures in children. Pediatr Radiol. 2013;43(Suppl 1):S55-60.
- [Google Scholar]
- Clinical utility of real-time fusion guidance for biopsy and ablation. J Vasc Interv Radiol. 2011;22:515-24.
- [Google Scholar]
- Diagnostic utility of endoscopic ultrasound guided aspiration cytology in evaluation of pancreatic masses. J Coll Physicians Surg Pak. 2013;23:484-6.
- [Google Scholar]
- Preoperative staging of liver metastases from uveal melanoma by magnetic resonance imaging (MRI) and fluorodeoxyglucose-positron emission tomography (FDG-PET) Eur J Surg Oncol. 2010;36:189-94.
- [Google Scholar]
- Additional value of PET-CT in staging of clinical stage IIB and III breast cancer. Breast J. 2010;16:617-24.
- [Google Scholar]
- PET/CT-guided percutaneous biopsy of abdominal masses: Initial experience. J Vasc Interv Radiol. 2011;22:507-14.
- [Google Scholar]
- The impact of coaxial core biopsy guided by FDG PET/CT in oncological patients. Eur J Nucl Med Mol Imaging. 2013;40:98-103.
- [Google Scholar]
- Utility of 18F-FDG PET/CT in diagnosis and management of mucormycosis. Clin Nucl Med. 2013;38:e370-1.
- [Google Scholar]
- FDG-PET/CT-guided biopsy of bone metastases sets a new course in patient management after extensive imaging and multiple futile biopsies. Br J Radiol. 2011;84:e65-7.
- [Google Scholar]






