Translate this page into:
Hemosuccus Pancreaticus in Chronic Pancreatitis: An Uncommon Cause of Gastrointestinal Bleeding

*Corresponding author: Sunny Qi-Huang, Department of Radiology, Nassau University Medical Center, East Meadow, New York, United States. snny.qi@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Lee W, Qi-Huang S, Ahmed Z, Shah SS. Hemosuccus Pancreaticus in Chronic Pancreatitis: An Uncommon Cause of Gastrointestinal Bleeding. J Clin Imaging Sci 2020;10:72.
Abstract
We present a case of a 69-year-old female who arrived in hemorrhagic shock with symptoms of upper gastrointestinal bleeding. Imaging on admission was diagnostic of a large splenic artery pseudoaneurysm, which was presumed to have bled into the pancreatic duct given clinical symptoms of upper gastrointestinal bleeding. The pseudoaneurysm was successfully treated with coil embolization resulting in resolution of clinical symptoms.
Keywords
Hemosuccus pancreaticus
Splenic artery pseudoaneurysm
Chronic pancreatitis complications
Upper gastrointestinal bleeding
Embolization
INTRODUCTION
Splenic artery pseudoaneurysm formation can occur as a sequela of pancreatitis, trauma, procedural intervention, and peptic ulcer disease.[1] A common life-threatening complication of pseudoaneurysm is massive hemorrhage secondary to rupture, which can occur into any adjacent structures such as the bowel, biliary system, thorax, peritoneum, or the spleen.[2] Given clinical symptoms of upper GI bleeding, the final presumed diagnosis in our case was hemosuccus pancreaticus – hemorrhage from the pancreatic duct into the GI tract – secondary to pseudoaneurysm rupture.
Pseudoaneurysm refers to an arterial wall injury and resulting communication of arterial pressure blood with an adjacent sac. The sac-like structure can be contained by the arterial media, adventitia, or simply the surrounding soft-tissue structures.[2] Pancreatitis is the most common underlying cause of splenic artery pseudoaneurysm, the leading proposed mechanism being pancreatic enzyme autodigestion of surrounding vasculature.[3] Other less common etiologies include trauma and iatrogenic etiologies such as ERCP, surgery, percutaneous biopsy or drainage, and peptic ulcer disease.[1,2] Mortality associated with the rupture of pseudoaneurysms approaches 100%.[4] On CT angiography (CTA), splenic artery pseudoaneurysms appear as a focus of contained active hemorrhage which follows the blood pool in density and remains contained on delayed phases.[2] Conventional digital subtraction angiography provides confirmation and allows real-time visualization contrast extravasation and collateral blood supply.
CASE REPORT
A 69-year-old female with a history of alcohol abuse and chronic pancreatitis presented to the emergency room with diaphoresis and pallor. On initial evaluation, blood pressure was 81/47 mmHg, heart rate 140 beats/min, and hemoglobin 7.3 g/deciliter.
Shortly following admission to the intensive care unit, she suffered a bout of hematemesis. Enteric tube aspiration revealed coffee ground emesis and emergent upper endoscopy showed multiple clean-based duodenal ulcers with no source of active bleeding. Two days later, the patient developed large amounts of melanotic stool and had another episode of hematemesis.
A CTA scan of the abdomen and pelvis (pre-contrast and arterial phase) was performed to identify the source of bleeding. Pre-contrast CT [Figure 1a-c] identified pancreatic calcifications consistent with chronic pancreatitis and a well-circumscribed hypodensity focus occupying the expected location of the pancreatic tail. Arterial phase CT angiographic images [Figure 1d] revealed hyperattenuating material within the hypodense focus. Close inspection on the arterial phase images demonstrated a linear extravasation of contrast material [Figure 1e and f], and diagnosis of pseudoaneurysm arising from the splenic artery was made. Transabdominal ultrasound showed a 5.3 x 5.7 cm mass in the region of the pancreas [Figure 2a]. The mass was found with strong internal Doppler flow signals as demonstrated by the yinyang sign [Figure 2b] consistent with pseudoaneurysm. Color doppler flow image depicts the pseudoaneurysm and its neck to the splenic artery when chasing caudally [Figure 2c]. Follow up spectral analysis was performed that shows a bidirectional turbulent blood flow of a “to-and-fro” pattern of the pseudoaneurysm [Figure 2d and e].
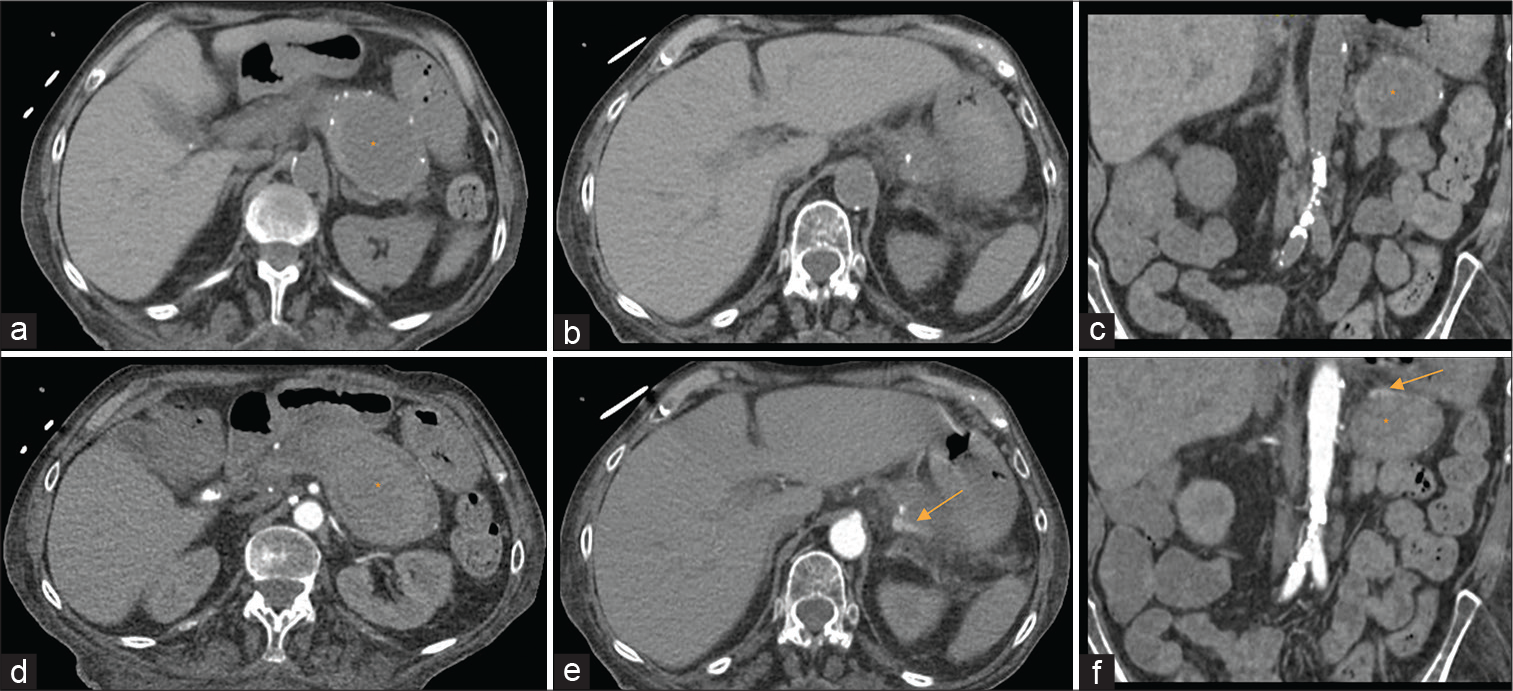
- A 69-year-old female with splenic pseudoaneurysm secondary to a confirmed history of chronic pancreatitis, presenting with hematemesis, hemodynamic instability, and gastrointestinal bleed. Findings: Pre-contrast axial (a and b) and coronal (c) images of CT angiography of the abdomen and pelvis showed a collection (asterisk) inseparable from the pancreatic tail measuring 7.9 × 4.9 × 5.6 cm in three dimensions with peripancreatic inflammation. Small calcifications were detected surrounding the collection reflecting the pancreatic parenchyma consistent with the clinical history of chronic pancreatitis. Contrast-enhanced CT aortogram arterial phase axial (c and d) and coronal (f) images demonstrated shallow pooling of contrast along the superior aspect of the collection as evidenced by axial (e, arrow) and coronal (f, arrow) images, suggested of pseudoaneurysm of the splenic artery.
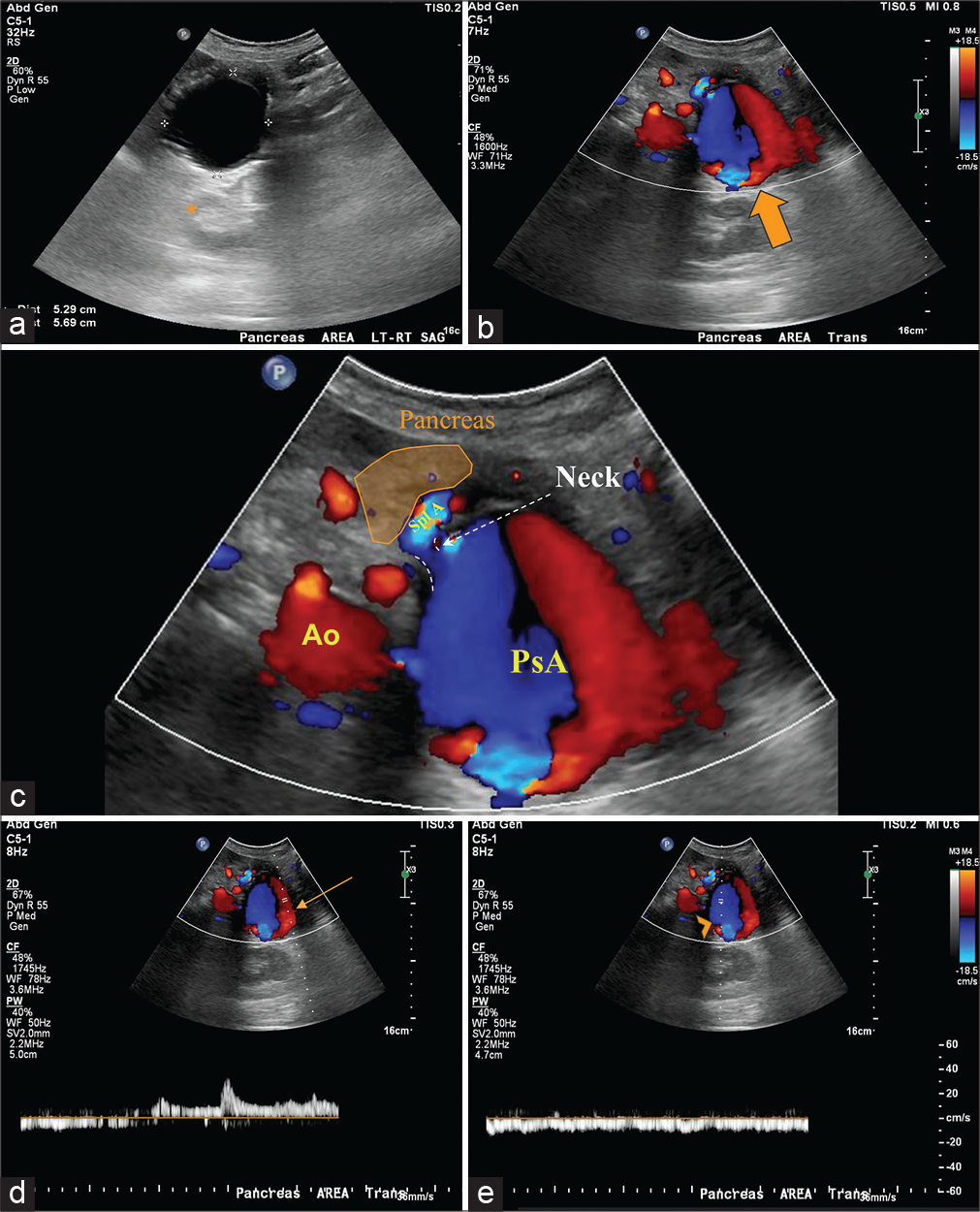
- A 69-year-old female with splenic pseudoaneurysm secondary to a confirmed history of chronic pancreatitis, presenting with hematemesis, hemodynamic instability, and gastrointestinal bleed. Findings: Longitudinal gray scale transabdominal ultrasonography image (a) of the pancreatic region shows an anechoic lesion with posterior acoustic shadowing (asterisk) measuring 5.3 × 5.7 cm. Transverse color Doppler (b) sonogram demonstrated turbulent flow (red-blue) in the lesion. Thus, the structure should be vascular in its nature and is shown with the characteristic “yin-yang” sign (big arrow) compatible with pseudoaneurysm of the splenic artery as depicted by the magnified view (c). Note the pseudoaneurysm neck is illustrated (dashed lines). Color Doppler and spectral analysis (d and e) taken at the site of the pseudoaneurysm shows bidirectional blood flow of arterial (d, arrow) and venous flow (e, arrowhead) consistent with the to-andfro pattern. Ao: Aorta. Spl A: Splenic artery, PsA: Pseudoaneurysm.
In the interventional radiology suite, a conventional diagnostic angiogram of the celiac axis and splenic artery shows a pseudoaneurysm at the junction of the proximal and mid third of the splenic artery [Figure 3a]. At the delayed catheter angiography depicts contrast blush within the pseudoaneurysm without contrast extravasation into the pancreatic duct, which suggest intermittent bleeding [Figure 3b]. The splenic artery distal to the aneurysm was cannulated with a 2.4 French microcatheter. Two detachable coils (4 mm × 13 cm and 3 mm × 8 cm) were deployed within it. Following this, the pseudoaneurysm was accessed into which eight additional coils were deployed (four 20 mm × 40 cm, three 16 mm × 39 cm, and one 18 mm × 36 cm). Further detachable coil placement was attempted to achieve maximal packing density; however, efforts were abandoned given significant tension within the coaxial catheter system. The microcatheter was then retracted to the neck of the pseudoaneurysm, and a coil was deployed from the aneurysm sac across the neck into the narrowed/irregular pre-aneurysmal splenic artery. Three additional microcoils (4 mm × 13 cm) were deployed into the inflow proximal splenic artery [Figure 3c]. Post embolization angiographic images of the splenic artery confirmed the successful exclusion of pseudoaneurysm by coil embolization [Figure 3d].
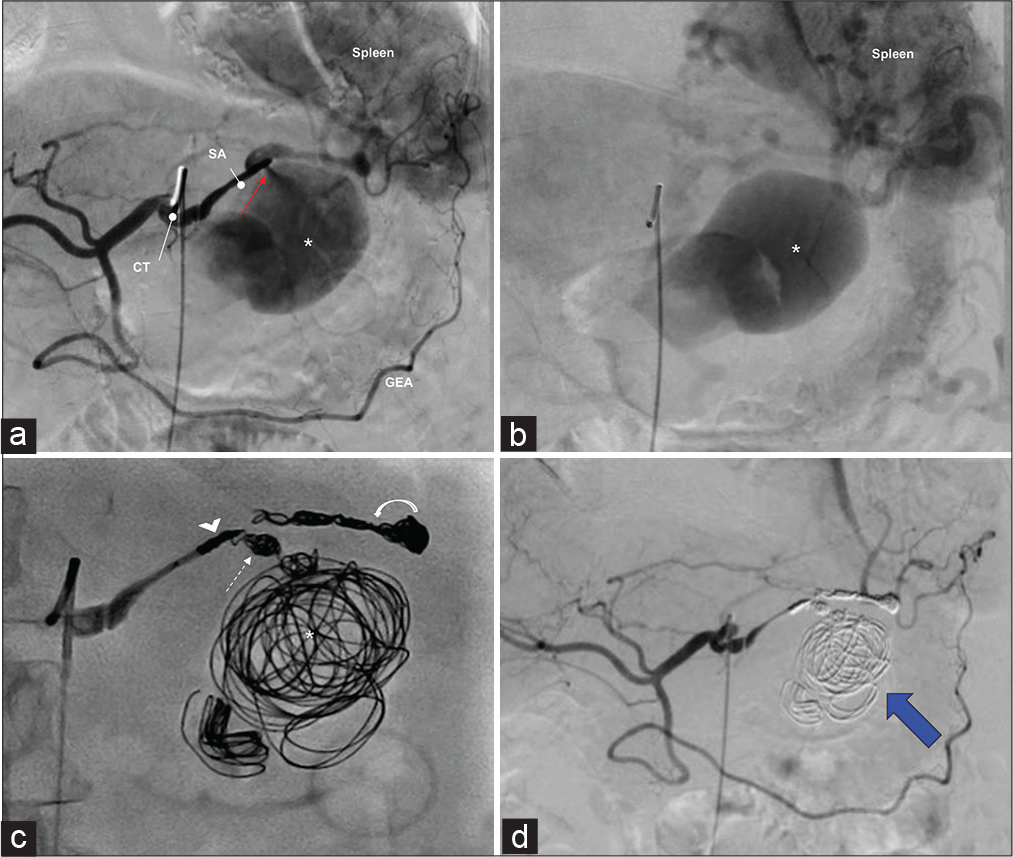
- Selective digital subtraction angiograms showing pre- and post-embolization of a splenic artery pseudoaneurysm. Findings: A pre-intervention angiogram of the celiac trunk (a) shows a large pseudoaneurysm (asterisk) arising from the splenic artery (SA). There is a jet of contrast material from the splenic artery into the pseudoaneurysm that is compatible with extravasation (a, red arrow). Delayed angiogram (b) showing contrast blush suggestive of active bleeding from the pseudoaneurysm (asterisk). Note the spleen parenchyma blush is noted. (c) DSA shows the proximal inflow (arrowhead) and distal outflow (curved arrow) of the splenic artery along with the neck (dashed arrow) and pseudoaneurysm itself (asterisk) is packed with microcoils. (d) A post-intervention arteriogram demonstrates the successful exclusion of pseudoaneurysm by coil embolization (blue arrow). CT: Celiac trunk, GEA: Gastroepiploic artery, DSA: Digital subtraction angiogram.
The “sandwich” technique was elected by the interventional radiology team to embolized the distal and proximal flow to the pseudoaneurysm to avoid anterograde and retrograde perfusion [Figure 4]. Post procedure, the patient remained hemodynamically stable with no evidence of recurrent gastrointestinal bleeding over the rest of the admission.
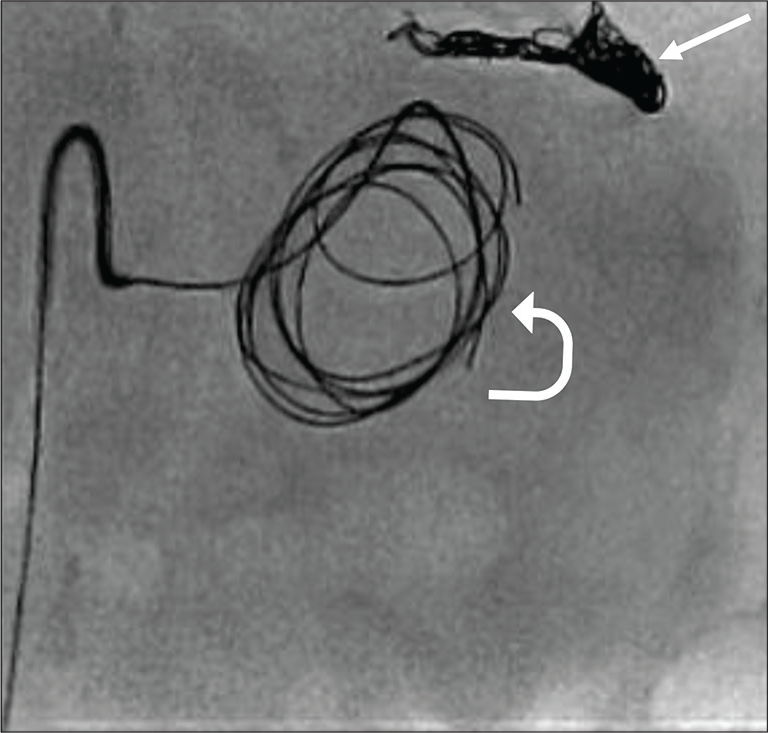
- Findings: Digital subtraction angiogram shows that the neck of pseudoaneurysm is cannulated and detachable coils being deployed into the pseudoaneurysm (curved arrow). Note the distal splenic artery was embolized with coils (arrow) occluding the outflow tract since pseudoaneurysm is arising from splenic artery trunk, which has collateral supply.
DISCUSSION
Hemosuccus pancreaticus is a rare phenomenon, representing only approximately 1/1500 cases of gastrointestinal bleeding,[4,5] but carries an approximate 90% mortality rate with supportive therapy alone.[6] CTA is the initial diagnostic imaging modality of choice for detecting as well accurately identification of active bleeding and potential source. Upper GI endoscopy can provide positive diagnosis with direct visualization of blood products at the ampulla of Vater. A review of literature, in patients with intermittent bleeding in nature, the source of bleeding may not be identifiable. A multiphasic contrast-enhanced CT scan is useful in identification of “sentinel clot” or clotted blood within the pancreatic duct in active bleeding patients and providing the definite diagnosis.[4] In cases of intermittent bleeding such as ours, CT angiography was performed in the arterial phase to identify source of GI bleed, in such active extravasation of contrast into the pancreatic duct was not detected in this case. Alternately, for instance, if multiphasic contrast-enhanced CT were performed (arterial, venous and delayed phases) would have more accurately identified the site of bleeding into the pancreatic duct. However, the identification of predisposing factors such as splenic artery pseudoaneurysm in the background of chronic pancreatitis with no definite source of upper gastrointestinal bleeding is suggestive of hemosuccus pancreaticus.
Significant decrease in morbidity and mortality has been shown with transcatheter embolization of pseudoaneurysms as opposed to traditional surgical techniques.[7,8] A minimal invasive approach for hemostasis in our patient with suspected hemosuccus pancreaticus was reached. Our patient presented with multiple chronic medical comorbidities and tenuous clinical state was determined poor surgical candidate. Overall, endovascular management is a relatively safe procedure with a high overall success rates of 75-100%.[4] Several endovascular techniques are described, the most common methods being coil embolization or treatment with catheter-directed embolic material. In cases where extensive collateral blood supply is present, a “sandwich” coil embolization method is preferred to prevent continued retrograde flow to the pseudoaneurysm which could result in the continued growth and contribute to the risk of rupture. The afferent and efferent blood supplied are embolized, with or without embolization of the lesion itself. In contrast, a pseudoaneurysm in an expendable end artery may be treated by coil embolization of the afferent vessels alone.[9] With regard to catheter-directed embolic material, consideration should be given to the width of the pseudoaneurysm neck, as material outflow and distal embolization pose a risk. In the setting of a wide neck, alternative options including temporary balloon occlusion of the donor artery during material embolization or covered stent placement may be considered.[10] For our patient, we elected to proceed with sandwich coil embolization given the narrow pseudoaneurysm neck and strong collateral circulation. Alternative techniques that were considered were glue embolization or percutaneous thrombin injection, however, these with have pose higher risk of distal embolization.
CONCLUSION
Splenic artery pseudoaneurysm is identified on cross-sectional imaging as a contained focus of contrast extravasation and on ultrasound as the classical yin-yang flow appearance with a “to-and-fro” Doppler waveform. The most common cause of splenic artery pseudoaneurysm is chronic pancreatitis. Pseudoaneurysm rupture is a life-threatening complication with extremely high mortality rate. In the rare setting of rupture into the adjacent pancreatic duct as presented in this case, patients can develop massive GI hemorrhage defined as hemosuccus pancreaticus. Transcatheter embolization remains the treatment of choice in most cases.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Splenic artery aneurysms and pseudoaneurysms: Clinical distinctions and CT appearances. AJR Am J Roentgenol. 2007;188:992-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics. 2005;25(Suppl 1):S173-89.
- [CrossRef] [PubMed] [Google Scholar]
- Potentially fatal bleeding in acute pancreatitis: Pathophysiology, prevention, and treatment. Pancreas. 2003;26:8-14.
- [CrossRef] [PubMed] [Google Scholar]
- Hemosuccus pancreatitis due to a ruptured splenic artery pseudoaneurysm-diagnosis and endovascular management. Radiol Case. 2020;14:7-15.
- [CrossRef] [PubMed] [Google Scholar]
- Successful multimodality treatment for hemosuccus pancreaticus. Am J Gastroenterol. 2009;104:1060.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular management of splenic artery aneurysms and pseudoaneurysms. Cardiovasc Intervent Radiol. 1994;17:179-84.
- [CrossRef] [PubMed] [Google Scholar]
- Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Intervent Radiol. 2003;26:256-60.
- [CrossRef] [PubMed] [Google Scholar]
- Principles used in the management of visceral aneurysms. Tech Vasc Interv Radiol. 2000;3:124-9.
- [CrossRef] [Google Scholar]
- Current treatment methods for postcatheterization pseudoaneurysms. J Vasc Interv Radiol. 2003;14:697-710.
- [CrossRef] [PubMed] [Google Scholar]






