Translate this page into:
Echo Planar Diffusion-Weighted Imaging: Possibilities and Considerations with 12- and 32-Channel Head Coils
Address for correspondence: Dr. John N Morelli, Scott and White Hospital, Texas A and M HSC, 2401 S 31st Street, Temple, TX 76508, USA. E-mail: dr.john.morelli@gmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Interest in clinical brain magnetic resonance imaging using 32-channel head coils for signal reception continues to increase. The present investigation assesses possibilities for improving diffusion-weighted image quality using a 32-channel in comparison to a conventional 12-channel coil. The utility of single-shot (ss) and an approach to readout-segmented (rs) echo planar imaging (EPI) are examined using both head coils. Substantial image quality improvements are found with rs-EPI. Imaging with a 32-channel head coil allows for implementation of greater parallel imaging acceleration factors or acquisition of scans at a higher resolution. Specifically, higher resolution imaging with rs-EPI can be achieved by increasing the number of readout segments without increasing echo-spacing or echo time to the degree necessary with ss-EPI — a factor resulting in increased susceptibility artifact and reduced signal-to-noise with the latter.
Keywords
3 Tesla MRI
diffusion-weighted imaging
echo planar imaging
INTRODUCTION

A clinical 32-channel 3 T phased array head coil was first implemented in 2006, demonstrating up to 3.5-fold gains in signal-to-noise ratio (SNR) relative to an 8-channel head coil.[1] Since that time, studies have focused upon functional and diffusion-weighted magnetic resonance (MR) applications with the coil.[23] A more recent investigation showed the superiority of routine brain MR imaging with such a coil compared to a 12-channel coil at 1.5 T.[4] Diffusion-weighted imaging (DWI) is a highly sensitive and specific sequence for the diagnosis of early brain infarction.[5] A DWI sequence can be implemented in several ways theoretically; however, single-shot echo planar imaging (ss-EPI) is most commonly used as it is relatively robust to phase errors from patient motion. With ss-EPI image, blur and susceptibility artifacts are often severe, especially at 3 T, secondary to the long EPI readout and low bandwidth per pixel in the phase-encoding direction. Alternative implementations of DWI, such as readout-segmented EPI (rs-EPI) reduce artifact from susceptibility, but often suffer from shot-to-shot phase variations due to patient motion during diffusion encoding. More recent rs-EPI implementations employing navigator-based phase corrections and reacquisition have, however, shown to be more robust to such errors.[67]
The present study examines implementation of both ss-EPI and rs-EPI with 12- and 32-channel head coils, the latter sequence using a 2-dimensional navigator-based phase correction and reacquisition strategy to account for phase errors.
MATERIALS AND METHODS
Theory
In ss-EPI, k-space is sampled one line at a time from the signal generated by one radiofrequency pulse. With rs-EPI approaches, k-space is sampled on the basis of signal generated from multiple excitation pulses. The method used for rs-EPI herein is discussed in detail in reference 6. Briefly, this sequence includes a pre-phasing gradient pulse, which is modified for each shot. In this way, the position of each acquired readout segment along the frequency encoding axis is defined. During the second echo for each shot, a 2D navigator readout segment is acquired. The frequency encoding dimension is covered less extensively for a given shot, and, thus, echo spacing, and, therefore, image blur and susceptibility artifact, is theoretically reduced. The 2D navigator-based echo is repeatedly acquired at k-space center; this accounts for shot-to-shot non-linear phase differences arising from non-rigid motion of the head. When such motion is severe, the approach fails (i.e., signal voids appear in navigator images). Such errors correspond to the signal distribution of the navigator readout segment becoming broader, and these areas of broadening are preferentially reacquired. In this study, such re-acquisitions comprise at most 20% of scan time. Corrections near the center of k-space are performed more frequently, as such errors have more severe effects.
Methodology
This study was approved by the Institutional Review Board and is in compliance with the ethical principles of the Helsinki Declaration of 1975, as revised in 2000. For the study, 11 volunteers were evaluated, using both a 12- and a 32-channel head coil (Siemens Medical Solutions USA, Malvern, PA, USA) with both rs- and ss-EPI DWI as shown in Table 1. The 12-channel head coil was also used to scan three patients with acute ischemia. In its present implementation, the rs-EPI sequence used in conjunction with the 32-channel head coil requires a long period of time for image reconstruction, during which the patient would have to remain on the scanner. The time for reconstruction was nearly equal in duration to the scan itself, and 32-channel scans were thus not performed in patients due to concerns with keeping them in the scanner for prolonged periods of time. An additional fast-spin echo sequence was performed to serve as a control by which to evaluate image distortion. Pontine distortion (i.e., variance in the AP dimension of the pons) was chosen as a means to quantify susceptibility artifact involving the rs- and ss-EPI sequences as in previous publications.[8] An offline work station was used to perform SNR measurements (Leonardo; Siemens Medical Solutions USA). In doing so, signal intensity region of interest analysis was performed in both supra- and infra-tentorial locations within the right anterior forceps and mid-pons, respectively, as performed in previous works.[89] Evaluation of the latter region is particularly important, as one recent clinical evaluation noted decreased central infra-tentorial signal intensity with a similar 32-channel head coil.[4] The subtraction method was utilized to account for the implementation of parallel imaging[12]. An image difference was computed from two consecutively acquired b = 1000 s/mm2 scans. The signal intensity (SI) was calculated for each region of interest for each scan. The noise level taken as the standard deviation (SD) from the same regions of interest on the image difference. SNR was then computed as SImean/√2 × SD.
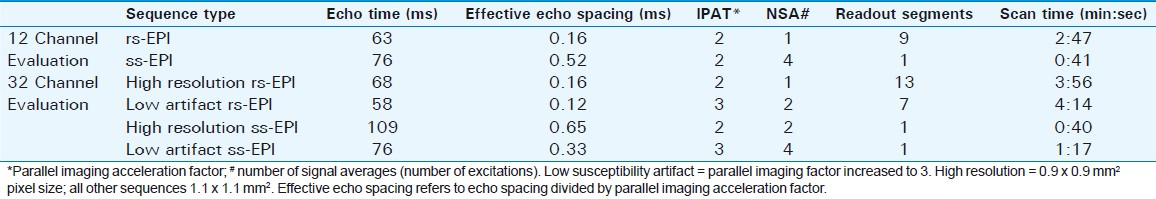
SNR measurements and measures of pontine distortion were compared among the sequences using a one-way ANOVA followed by the Games Howell test for multiple comparisons (SPSS v 16). The scans were grouped in terms of statistical differences, as illustrated in Figures 1–3.
- Supra-tentorial signal-to-noise ratio of the acquired scans obtained using 12- and 32-channel head coils. With the latter coil, both high resolution and low susceptibility artifact (IPAT 3) sequences were obtained. Based on statistical analysis described in the methods, the scans were placed into groups. Scans which are not members of the same group demonstrate statistically significant differences (P<0.05).
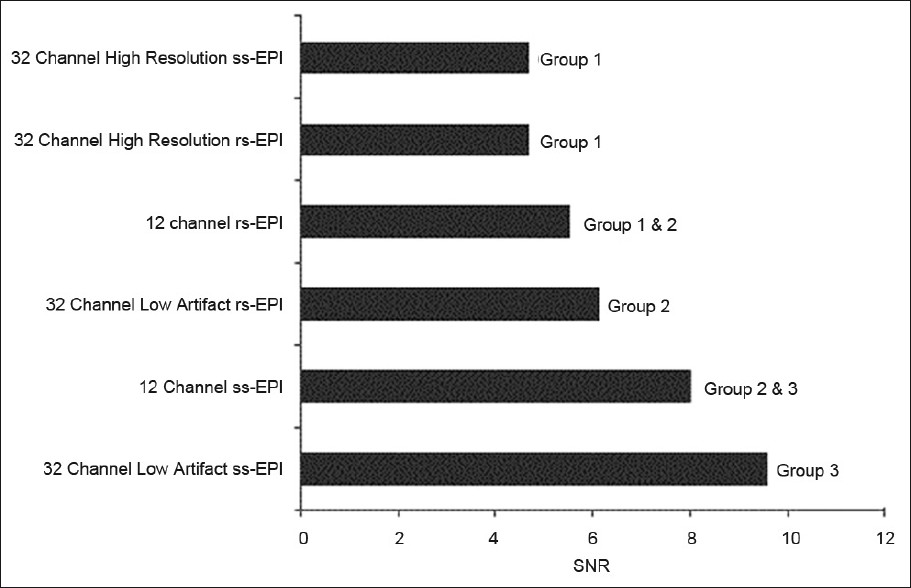
- Infra-tentorial signal-to-noise ratio of the acquired scans obtained using 12- and 32-channel head coils. With the latter coil, both high resolution and low susceptibility artifact (IPAT 3) sequences were obtained. Based on statistical analysis described in the methods, the scans were placed into groups. Scans which are not members of the same group demonstrate statistically significant differences (P<0.05).
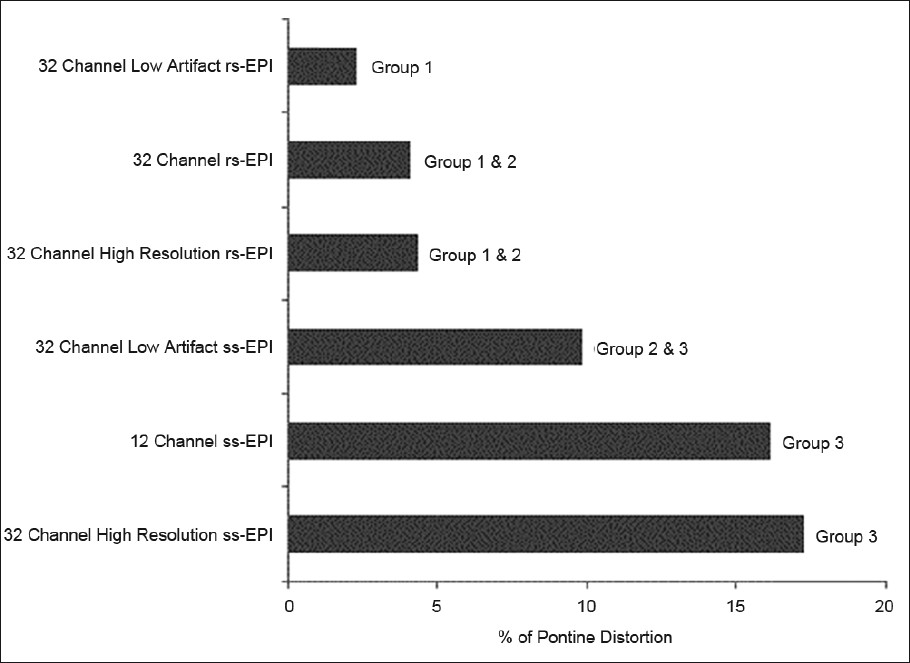
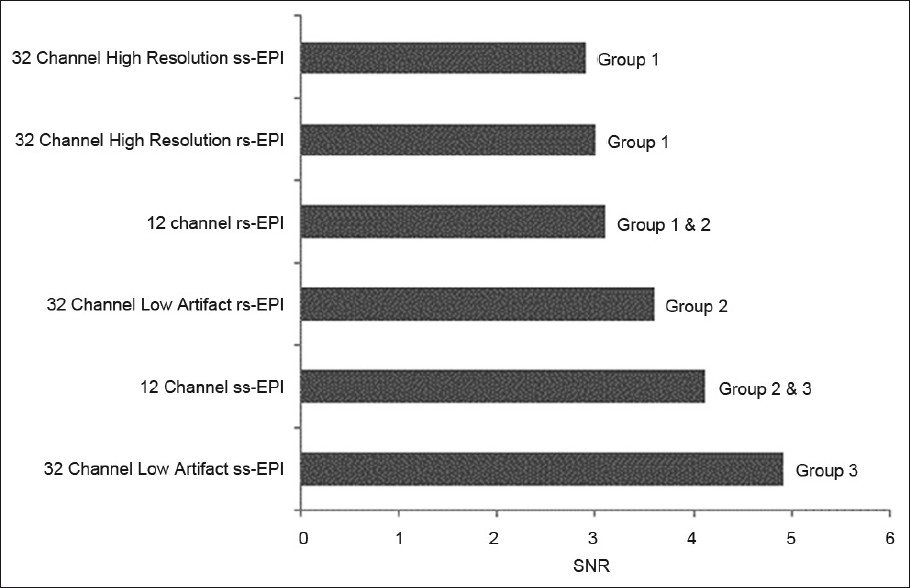
- Percentage differences in pontine length, as compared with a standard fast-spin echo scan, among the various acquired scans obtained using 12- and 32-channel head coils. With the latter coil, both high resolution and low susceptibility artifact (IPAT 3) sequences were obtained. Based on statistical analysis described in the methods, the scans were placed into groups. Scans which are not members of the same group demonstrate statistically significant differences (P<0.05).
All performed studies were evaluated by a fellowship trained member of the American Society of Neuroradiology with 7 years’ experience. The reader was blinded to subject data, study rationale, acquisition parameters, and the specific type of sequences evaluated. All acquired images were displayed on a picture archiving and communication system workstation, and were placed in random order on two 4-quadrant display monitors. The reader ranked the sequences in terms of bulk susceptibility artifact (manifest as pontine stretching and high signal intensity artifact at sinus–parenchymal interfaces), blur, and overall preference. The reader was asked to rank the top three scans for each volunteer overall, to name the least preferred scan, and to name the preferred scan for each patient.
Sequence parameters
The sequence parameters for all scans are provided in Table 1. Slice thickness for all scans was 4 mm. For the 12-channel head coil scan, parameters reflect those used in clinical practice with ss-EPI and used in preliminary investigations with rs-EPI. Although previous studies have employed similar scan parameters for 32-channel head coil evaluations as used with 12-channel coils at 1.5 T,[4] our group's initial experience with the former coil at 3 T was that image quality was more strikingly improved when the greater SNR was used to increase spatial resolution or alternatively to increase parallel imaging acceleration factors so as to reduce susceptibility artifact. This corroborates findings in previous studies whereby beyond a certain point, SNR is found to be a poor predictor of a study's diagnostic value.[1112] Thus, this was the approach chosen with the caveat that the acquisition time for all sequences be kept within the clinical guidelines of our institution. Low susceptibility artifact scans (obtained with a parallel imaging acceleration factor - IPAT - of 3, 1.1 × 1.1 mm2 pixel size) and high-resolution scans (IPAT 2, 0.9 × 0.9 mm2 pixel size) were implemented and performed with both ss- and rs-EPI. In a single volunteer, these scans were likewise performed using the 12-channel head coil; however, results with the low artifact scan were disappointing with respect to SNR with the rs-EPI sequence. Image quality with the high-resolution ss-EPI sequence was also markedly degraded. All ss-EPI scans were performed with 6/8 partial Fourier acceleration, without which a separate preliminary investigation showed the degree of image blur to be unacceptable. The minimum allowed echo time was chosen for each sequence within the constraints of the given parameters, but even with partial Fourier acceleration, the high-resolution ss-EPI scan still required a relatively high echo time (TE = 109). For the IPAT 3 rs-EPI, echo-spacing was increased from 0.32 ms to 0.36 ms, which allowed the number of readout segments to be reduced from 9 to 7, so that overall the scan time with two scan averages was not prohibitive. A greater number of scan averages was likewise used with IPAT 3 ss-EPI to account for SNR loss from the greater IPAT factor.
RESULTS
Details of SNR and pontine distortion comparisons are provided in Figures 1–3. In general, both supra- and infra-tentorial SNR were greater with the ss-EPI sequences than with rs-EPI DWI, despite the longer scan acquisition times with the latter. Bulk susceptibility artifacts, as measured by pontine distortion, were markedly reduced through use of rs-EPI.
The assessment of the blinded reader agreed with quantitative rankings, both in terms of high signal intensity susceptibility artifact and pontine distortion severity. Representative images are provided in Figures 4 and 5. When asked to rank the top three sequences from the 10 volunteer groups overall (both 12- and 32-channel scans), in every instance, the blinded reader selected the 32-channel rs-EPI sequence with a parallel imaging factor of 3, followed by the high-resolution 32-channel rs-EPI sequence, and the 12-channel rs-EPI sequence. The high-resolution ss-EPI scan was the least preferred in every case. Among the patient scans, the rs-EPI scan was preferred in all (3/3) cases. An example patient scan is provided in Figure 6.
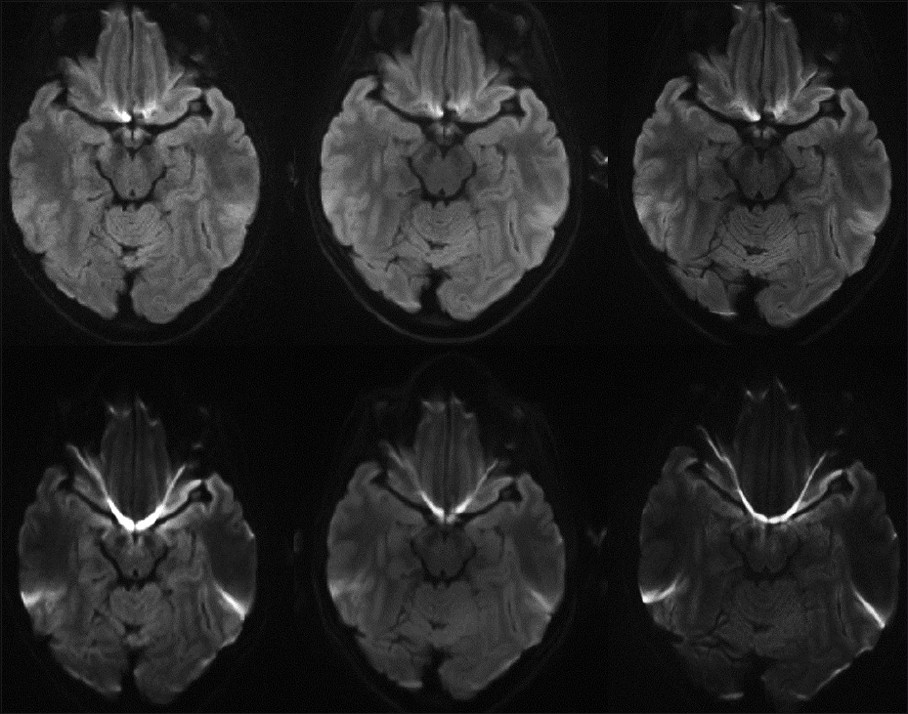
- Representative images at 3 T including: single-shot echo planar imaging (ss-EPI) (bottom left) and readout-segment echo planar imaging (rs- EPI) obtained with a 12-channel head coil (top left), as well as low artifact (IPAT 3) 32-channel coil scans with rs-EPI (top middle) and ss-EPI (bottom middle), in addition to high resolution rs-EPI (top right) and ss-EPI scans (bottom right).
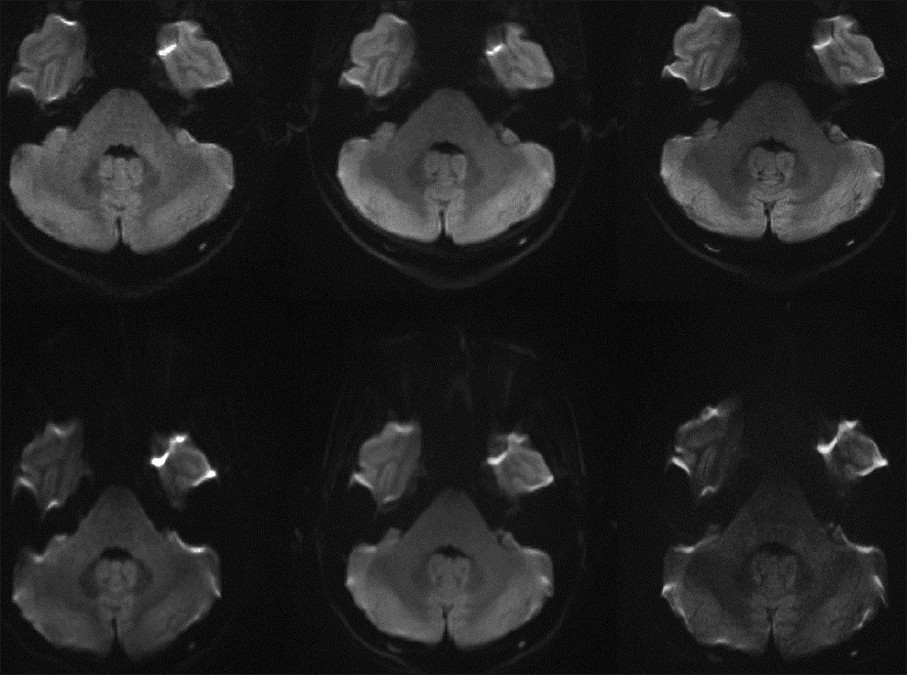
- Representative images at 3 T including: single-shot echo planar imaging (ss-EPI) (bottom left) and readout-segment echo planar imaging (rs-EPI) obtained with a 12-channel head coil (top left), as well as well-as low artifact (IPAT 3) 32-channel coil scans with rs-EPI (top middle) and ss-EPI (bottom middle), in addition to high resolution rs-EPI (top right) and ss-EPI scans (bottom right). Note the marked pontine stretching present with the ss- EPI scans, particularly with the high-resolution image.
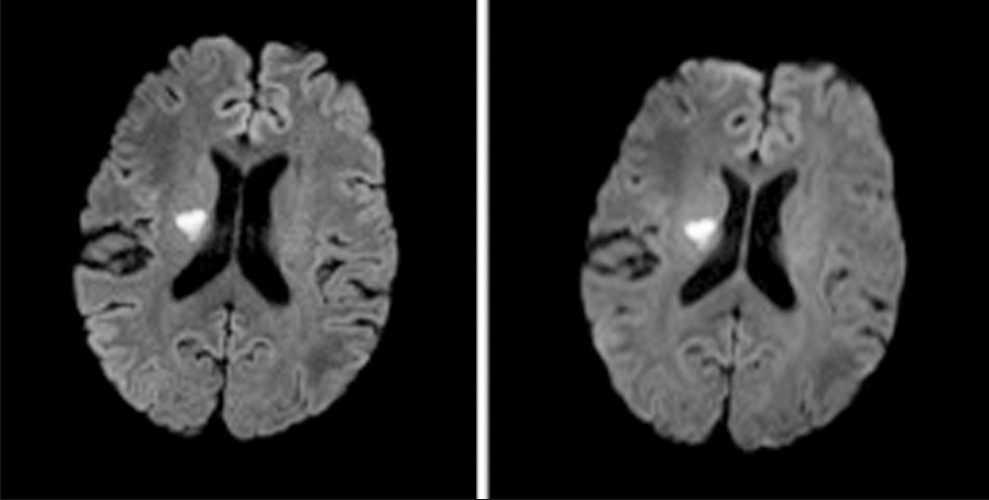
- A 63-year-old man with a focus of acute ischemia illustrated on 12-channel readout-segment echo planar imaging (rs-EPI) (left) and single-shot echo planar imaging (right) diffusion-weighted imaging scans. Note the reduction in susceptibility artifact at the junction of the frontal lobes and frontal sinus with rs-EPI and also the improved sulcal definition, reflecting the higher real spatial resolution of rs-EPI.
DISCUSSION
The SNR gains achievable through use of phased array coils with a greater number of channels are typically employed to decrease scan times through image acquisition with a higher parallel imaging acceleration factor or to improve image spatial resolution. In this study, scan time reduction was not the focus, but rather use of higher acceleration factors to diminish artifacts from bulk susceptibility. Both rs-EPI and ss-EPI were evaluated with 12- and 32-channel head coils. Readout-segmented EPI was found to be superior regardless of the head coil utilized, although scan times with the rs-EPI sequences were longer. The benefits, in terms of image quality, of the rs-EPI sequence appeared to outweigh any benefit obtainable from use of the 32-channel head coil, as the 12-channel rs-EPI scans were preferred to the 32-channel ss-EPI images in every case.
As found in a previous study with T1-weighted images,[4] gains in SNR or spatial resolution alone obtainable with a 32-channel head coil did not correspond with greater reader scan preference. For example, the high-resolution 32-channel ss-EPI and 12-channel ss-EPI scans demonstrate the greatest overall SNR in our study, but were not ranked as the preferred scan by the blinded reader in any case. Likewise, the high-resolution ss-EPI scan was also never preferred, and the high-resolution rs-EPI scan was only second most preferred overall. It seems likely that reader preferences in this case were closely related to the degree of susceptibility artifact present, an artifact which can pose a major clinical detriment to ss-EPI DWI, particularly with respect to visualization of infra-tentorial ischemia.[89]
The major highlight of this work is the versatility of rs-EPI relative to ss-EPI when viewed in light of the increased SNR provided by the 32-channel head coil. As opposed to the ss-EPI sequence, higher spatial resolutions can be achieved with the rs-EPI by increasing the number of readout segments (or shots) without increasing the EPI echo-spacing. This means that the degree of spatial distortion and susceptibility artifacts either remain unchanged or improve when the spatial resolution is increased. In distinction, increasing the spatial resolution in the readout direction with ss-EPI requires an increase in echo-spacing and, thus, spatial distortion and susceptibility artifacts. Greater parallel imaging acceleration factors can be employed to diminish effective echo-spacing (defined as echo spacing/parallel imaging factor), but at the cost of SNR in a one-to-one ratio. Overcoming the corresponding SNR loss through increasing scan averages is thus difficult, as SNR is proportional only to the square root of this parameter.
In addition, in ss-EPI there is a more substantial increase in Time to Echo TE (and corresponding SNR loss) with higher resolution imaging because both the EPI echo-spacing and the number of echoes increases, whereas it is only the number of echoes that increases with the rs-EPI sequence. The high-resolution 32-channel ss-EPI scan was quantitatively no different and qualitatively worse than even the 12-channel ss-EPI overall. In distinction, with rs-EPI scan quality at 3 T for diffusion-weighted imaging in the brain approaches that of conventionally acquired T2-weighted and FLAIR scans, providing a marked improvement in this widely employed application. With the trend in clinical MR towards greater SNR achieved through improved imaging coils, integrated redesigned hardware, and higher field strength, this sequence may be of increased utility in the future relative to conventional ss-EPI in both diffusion-weighted and diffusion tensor imaging.
The major drawback of the rs-EPI sequence remains increased scan times and, potentially, patient motion. With respect to the former, rs-EPI may be most useful in clinical practice as an additional, more definitive sequence, in cases where ss-EPI is non-diagnostic or exhibits equivocal findings. The rs-EPI navigator correction/reacquisition accounts for motion during the diffusion preparation, but not between readout segment acquisitions. Patient motion can also affect ss-EPI image quality; however, shorter scan durations make this less likely. Further, clinical experience will dictate whether additional experience will dictate whether further motion correction with rs-EPI is necessary as has been implemented in other sequences.[9] An additional drawback of rs-EPI is the prolonged image reconstruction times when implemented with the 32-channel head coil, although this may be obviated with faster imaging processing and improved hardware systems. A previous report noted that the 32-channel head coil used herein decreased central, infra-tentorial SNR.[4] In this report, infra-tentorial SNR was indeed less than supra-tentorial SNR for both utilized coils, but not disproportionately so with the 32-channel coil.
CONCLUSION
Readout-segmented EPI is an appealing alternative to ss-EPI in context of 32-channel head coil imaging. This is due to the ability to increase spatial resolution with rs-EPI without significantly increasing echo time or associated artifacts. In distinction, high-resolution 32-channel imaging with ss-EPI does not improve image quality over standard 12-channel ss-EPI.
Source of Support: Nil
Conflict of Interest: None declared.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2012/2/1/31/96548
REFERENCES
- 32-channel 3 Tesla receive-only phased-array head coil with soccer-ball element geometry. Magn Reson Med. 2006;56:216-23.
- [Google Scholar]
- Arterial spin labeling for motor activation mapping at 3T with a 32-channel coil: Reproducibility and spatial accuracy in comparison with BOLD fMRI. Neuroimage. 2011;58:157-67.
- [Google Scholar]
- Diffusion weighted imaging: A comprehensive evaluation of a fast spin echo DWI sequence with BLADE (PROPELLER) k-space sampling at 3 T, using a 32-channel head coil in acute brain ischemia. Invest Radiol. 2009;44:656-61.
- [Google Scholar]
- Evaluation of image quality of a 32-channel versus a 12-channel head coil at 1.5T for MR imaging of the brain. AJNR Am J Neuroradiol. 2011;32:365-73.
- [Google Scholar]
- Sensitivity and interrater agreement of CT and diffusion-weighted MR imaging in hyperacute stroke. AJNR Am J Neuroradiol. 2003;24:878-85.
- [Google Scholar]
- High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med. 2009;62:468-75.
- [Google Scholar]
- Diffusion imaging in humans at 7T using readout-segmented EPI and GRAPPA. Magn Reson Med. 2010;64:9-14.
- [Google Scholar]
- Evaluation of a modified Stejskal-Tanner diffusion encoding scheme, permitting a marked reduction in TE, in diffusion-weighted imaging of stroke patients at 3 T. Invest Radiol. 2010;45:29-35.
- [Google Scholar]
- Diffusion-weighted imaging in patients with acute brain ischemia at 3 T: current possibilities and future perspectives comparing conventional echoplanar diffusion-weighted imaging and fast spin echo diffusion-weighted imaging sequences using BLADE (PROPELLER) Invest Radiol. 2009;44:351-9.
- [Google Scholar]
- Practical approaches to the evaluation of signal-to-noise ratio performance with parallel imaging: Application with cardiac imaging and a 32-channel cardiac coil. Magn Reson Med. 2005;54:748-54.
- [Google Scholar]
- Evaluation of measures of technical image quality for intracranial magnetic resonance angiography. Comput Biomed Res. 1999;32:530-56.
- [Google Scholar]






