Translate this page into:
Different facets of intracranial central nervous system lymphoma and its imaging mimics

*Corresponding author: Hoi Ming Kwok, Department of Diagnostic and Interventional Radiology, Princess Margaret Hospital, Kowloon, Hong Kong, China. hmkwok15@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kwok HM, Li KY, Chan RL, Chan CH, Wong SK, Lee CM, et al. Different facets of intracranial central nervous system lymphoma and its imaging mimics. J Clin Imaging Sci 2022;12:4.
Abstract
Lymphomas of the central nervous system (CNS) are broadly classified into primary CNS lymphoma (PCNSL) and secondary CNS lymphoma (SCNSL). PCNSL refers to lymphoma restricted to the brain, leptomeninges, spinal cord, or eyes without evidence of it outside the CNS at primary diagnosis, while SCNSL refers to secondary CNS involvement by systemic lymphoma. The brain is the most common site of involvement and intracranial CNS lymphoma has a highly variable imaging appearance and varies according to the patient’s clinical profile and immunity. This pictorial essay aims to illustrate the different facets of intracranial CNS lymphomas on neuroimaging. This enables radiologists to be familiarized with their key diagnostic features and differentiate from their differential diagnoses, leading to early diagnosis, and treatment.
Keywords
Primary central nervous system lymphoma
Secondary central nervous system lymphoma
Primary dural lymphoma
Mimics
INTRODUCTION
Neuroimaging especially MRI and CT form the cornerstone for making the diagnosis most of the time. Contrast-enhanced MRI is more sensitive than CT and therefore is the imaging-of-choice not only for initial diagnosis but also for response assessment.[1,2] However, CT and conventional MRI sequences are often the initial modalities for evaluation, and differentiating the main diagnosis from other causes (such as other brain tumors and infection) is of paramount importance. Imaging serves as the surrogate for pathology and thorough understanding would allow radiologists to suspect central nervous system (CNS) lymphoma in the correct clinical setting. Missing the diagnosis might lead to inadvertent use of steroids that would be planned for controlling vasogenic edema in other brain tumors, therefore compromising the yield of primary CNS lymphoma (PCNSL) leading to false-negative biopsies and delaying treatment.[3,4] It is worth mentioning that CNS lymphomas are also termed “vanishing tumors” as they show significant shrinkage if a steroid is given due to the combined cytotoxic effect and anti-edema effect. It has been advocated that corticosteroids should not be prescribed if there is a suspicion of PCNSL, unless in case of a deteriorating clinical condition.[4] PCNSL requires definitive histopathological diagnosis by stereotactic biopsy, instead of primary tumor resection as in other brain tumors.
However, the final diagnosis does not stop at the stage of MRI characterization or stereotactic biopsy. Looking for extracranial involvement, CT of chest, abdomen, and pelvis or 18-FDG PET-CT should be arranged.[5] Ultrasound of testis has been recommended to men as there is insufficient evidence PETCT can reliably exclude testicular involvement. To exclude occult systemic manifestation, bilateral bone marrow biopsy should also be arranged.[2,3] Additional evaluation includes cerebrospinal fluid (CSF) analysis, slit lamp examination, and/or vitreous biopsy, especially when there are ocular symptoms.[5]
The purpose of this pictorial review is to depict the various imaging findings of all the pathologically proven intracranial CNS lymphoma that were identified through the Radiology Information System of Princess Margaret Hospital, Hong Kong from 2010 to 2020. Imaging differentials that might mimic intracranial CNS lymphoma will also be discussed. Other very rare subtypes of intracranial CNS lymphomas (such as T-cell PCNSL, intravascular lymphoma, and intracranial Hodgkin’s lymphoma) are beyond the scope of discussion in this article.
OVERVIEW OF PATHOLOGIES
PCNSL
PCNSL is uncommon and accounts for 1–5% of all brain tumors, and approximately 1% of all Non-Hodgkin lymphoma (NHL). It can involve the brain, eye leptomeninges, and rarely spinal cord without evidence of systemic disease.[1] It originates from the immunoblasts and centroblasts surrounding the intracranial blood vessels where carcinogenic cells are formed in the lymphatic tissue. Most cases of PCNSLs are diffuse large B-cell lymphomas (DLBCL) (90–95 %).[1,2] Age of presentation for PCNSL in immunocompetent patients is approximately 50 years old and is more common in men (male-to-female ratio of 1.5:1),[2,3] while that for immunocompromised patients is earlier at the fourth decades from studies in the Acquired Immunodeficiency Syndrome (AIDS) population.[2] There is an increasing incidence rate of PCNSL among immunocompetent patients.[1] PCNSL affects 6% of the AIDS population.[2] The declining incidence in the AIDS population is believed to be due to the introduction of HAART in past decades.[1,2]
PCNSL in immunocompetent patients
In the immunocompetent patients, the typical imaging appearance of PCNSL in immunocompetent patients most commonly presents as a solitary homogeneously enhancing parenchymal mass in the supratentorial compartment.[1-3] There is a predilection for the periventricular and superficial regions, often in contact with ventricular or meningeal surfaces.[1] The most commonly involved region is the cerebral hemisphere (38%), in particular the frontal or parietal lobes [Figure 1]. Other involved regions in descending order of frequency include the basal ganglia/ thalamus (16%), corpus callosum (14%), ventricular region (12%), and cerebellum (9%) [Figure 2].[2] Another classic presentation is the lesion that crosses the corpus callosum in a butterfly pattern [Figure 3].[2]
- A 65-year-old man presented dizziness. Non-contrast CT revealed a hyperdense left frontal lobe mass (black arrow) with surrounding vasogenic edema. (a).MRI T2-weighted image confirmed that mass had inhomogeneous low signal (black arrowhead) (b). On post-contrast T1-weighted image there is and intense, homogenous enhancement (white arrow) (c). On ADC map, areas of restricted diffusion (white arrow) could be seen (d). Magnetic resonance spectroscopy (MRS) revealed elevated choline (Cho) peak with high Cho-to-NAA ratio (e). No significant elevated cerebral blood volume was noted (white arrowhead) (f). This illustrated typical presentation of PCNSL in immunocompetent patient.

- A 66-year-old-man presented with headache. Non-contrast CT showed a heterogenous but hyperdense mass was seen at right thalamus (white arrowhead) (a). MRI post-contrast T1-weighted image revealed the right thalamic mass to be intensely enhancing (white arrow) (b). On T2-weighed image it showed low signal on (white arrowhead) (c). On ADC map it showed low signal (black arrow) (d) reflecting underlying hypercellular nature of lymphoma. This again illustrated typical presentation of PCNSL in immunocompetent patient.

- A 67-year-old woman with presented with seizure and delirium. Contrast enhanced CT showed a large intra-axial left frontal lobe enhancing mass (white arrowhead) crossing the genu of corpus callosum with expansion and affected the right frontal lobe (a). MRI T2-weighted image showed that the mass had isointense signal (white arrow) with surrounding vasogenic edema (b). On post-contrast T1-weighed image it demonstrated intense and homogenous enhancement (white arrowhead) (c). Stereotactic biopsy was performed confirming DLBCL with post-operative changes at left frontal region (d). This illustrated the classic butterfly pattern in PCNSL.
The characteristic findings of PCNSL on both CT and MRI are due to its hypercellularity. Lymphomas are small round blue cells tumors demonstrating a high nuclear/cytoplasmic ratio and therefore giving rise to imaging features of hypercellularity. They are characteristically iso- to hyper-attenuating on non-contrast CT, with T2-weighted iso- to hypo- intense signal with restricted diffusion on MRI. Homogenous, marked contrast enhancement is typically observed on both CT and MRI due to disruption of the blood-brain barrier.[6,7] Hemorrhage, calcification, necrosis, and cyst formation are rare [Figure 4].[2,6] The low ADC values are not only valuable for diagnosis but also pretreatment assessment as it is predictive of shorter progression-free survival and overall survival as it is correlated with proliferative index Ki-67.[8] Furthermore, increasing ADC values during treatment suggests a positive response to therapy and vice versa.[9] PCNSL is associated with mildly increased or occasionally decreased perfusion that is usually higher than that in most focal brain inflammatory, demyelinating, and infectious diseases but lower than that in high-grade gliomas.[9] On MR spectroscopy (MRS), there are characteristic elevated lipid and lactate peaks at 0.9–1.3 ppm besides high Cho/Cr ratios, decreased N-acetylaspartate (NAA) levels, and high Cho/NAA ratios.[9] It is important to watch out for extension to leptomeninges (15–20%) and eyes (25%) in cases of PCNSL.[4,6] Subependymal infiltration is characteristic in lymphoma. Orbital infiltration would suggest secondary intraocular lymphoma which could be diagnosed by slit-lamp examination or vitreous cytology.[4] It has a poor prognosis (median survival of 9 months).[4]

- A 57-year-old man presented with seizure. Non-contrast CT showed a right medial parietal lobe intra-axial isodense mass with cystic area (white arrow) and vasogenic edema (a). MRI T1- and T2-weighted images showed evidence of hemorrhage and cystic change (arrowheads) (b and c). On T2-weighed image the solid part of this lesion demonstrated low signal, and on post-contrast T1-weighed image there was intense homogenous enhancement (black arrow) (d). On MR perfusion study there was no elevated rCBV (white arrow) (e). On ADC map, restricted diffusion of the solid part was evident (white arrow) (f). The corresponding MR spectroscopy demonstrated elevated Cho-to-Cr ratio with low NAA (black arrow) (g). The major differential diagnoses of these imaging appearances include glioblastoma, high grade glioma, or lymphoma. Stereotactic biopsy confirmed diffuse large B-cell lymphoma. This illustrated an uncommon presentation of PCNSL.
PCNSL in immunocompromised patients
Classically, well-known causes of immunocompromised states associated with PCNSL include AIDS and post-transplant individuals. Autoimmune diseases and iatrogenic causes of immunodeficiency (e.g., by medications) should also be taken into consideration. Immunodeficiency-associated CNS lymphoma as defined by the World Health Organization in 2016 to incorporate these groups. There are reported cases in the literature with the use of MycophenolateMofetil in various autoimmune diseases with an association of PCNSL, possibly due to Epstein-Barr Virus (EBV) driven B-cell lymphoproliferative disease mechanism.[10-13] Kaulen et al. performed a 15-year retrospective cohort study for these cases and found that the majority (87%) of cases are DLBCL and are largely (78%) EBV positive.[14]
The imaging features of PCNSL in immunocompromised patients significantly differ from the above and are characterized by irregular margins, heterogeneity, and ring enhancement.[6,13] The lesions are typically showing heterogeneous peripheral enhancement with central non-enhancement (due to necrosis) [Figure 5].[6,13,15] This is in contrast to solid homogeneous enhancement from that of immunocompetent patients. Besides, they tend more to be multifocal and surrounded by a greater degree of vasogenic edema as well.[14] There is restricted diffusion at the margins of PCNSL lesions probably corresponding to the highly proliferative, densely packed lymphoma cells whereas the center consists of necrotic tissue.[13] The basal ganglia and the corpus callosum are frequently observed, and spontaneous hemorrhage is more frequent.[6,13] There is no standardized treatment due to the rarity of the disease.[11,13] These would be responsive to treatment including withdrawal of offending drugs, in addition to conventional treatment, including chemotherapy, targeted therapy (Rituximab), and radiotherapy.[10,11]

- A 65-year-old female with dermatomyositis on multiple immunosuppressants (Mycophenolate Mofetil, cyclosporin, and hydroxychloroquine) presented with bilateral lower limb weakness. Contrast-enhanced CT performed 1 year after treatment revealed bifrontal lobe intra-axial heterogeneously enhancing masses (black arrow) abutting anterior falx with irregular ring and nodular enhancement (white arrow head), central necrosis, and surrounding vasogenic edema (a). MRI T1-weighted pre-and post-contrast images showed corresponding lesions were associated with hemorrhage (black arrow) (b), irregular ring and nodular enhancement (white arrow head) and central necrosis (c and d). Corpus callosum was being compressed inferiorly without definite infiltration by tumor. On ADC map, low signal areas maybe partly explained by the presence of blood products besides restricted diffusion (e). On T2-weighed image, large amount of vasogenic edema was seen surrounding the tumors (white arrow) (f). PET-CT revealed the hypermetabolic part corresponding to the solid enhancing tumor and no extracranial involvement was noted. (g and h) Stereotactic biopsy confirmed diffuse large B-cell lymphoma. It is worthwhile to remember PCNSL in immunocompromised patients tends to be multifocal, heterogenous peripheral enhancement and with greater degree of vasogenic edema. Brain abscesses due to bacteria or atypical agents are the most important imaging differentials in this case.
Secondary CNS lymphoma (SCNSL)
SCNSL refers to CNS involvement by systemic lymphoma.[6] The reported overall risk of CNS relapse in aggressive NHL is in order of 2–27%, while in patients with Hodgkin lymphoma is very low (≤0.5%).[1] The risk of CNS relapse depends on lymphoma histology (low vs. high grade), advanced disease (Stage III-IV), primary site (e.g., testis, orbits, and paranasal sinuses are high risk), involvement of more than one extranodal site, increased serum lactate dehydrogenase level, and whether CNS prophylaxis was given.[7] Summarized findings of older literature suggested two-thirds of the cases involve the leptomeninges and one-third involve the brain parenchyma [Figure 6].[1] Malikova et al. performed a retrospective analysis of a smaller cohort of 21 biopsy-proven cases and reported up to 85.7% of the parenchymal lesion and combined parenchymal lesion with leptomeningeal involvement up to 47.6%, while solitary leptomeningeal involvement was only present in 4.8%.[16] Corpus callosum involvement and cranial nerves involvement were also reported (each up to 38.1%).[16] Leptomeningeal spread can also involve the cranial nerves, spinal cord, or spinal roots, leading to cranial, or spinal neuropathies [Figure 7].[1,2] Metastatic obstruction of CSF flow or absorption would lead to a headache.[1] Key diagnostic features on MRI include leptomeningeal, subependymal, dural, or cranial nerve enhancement, superficial brain lesions as well as communicating hydrocephalus.[1] Parenchymal metastases from NHL often appear as single or multiple periventricular and/or superficial enhancing lesions and can be accompanied by leptomeningeal metastases [Figure 8]. It is important to remember that it is impossible to differentiate PCNSL versus SCNSL based on MRI alone, especially when parenchymal lesions are solely seen on neuroimaging.[7,16] Systemic imaging (such as 18-FDG PET-CT) and bone marrow biopsies are the keys to looking for systemic involvement beyond the CNS. SCNSL requires contrast MRI of the whole neural axis to define total tumor burden before initiation of therapy.[7] Both PCNSL and SCNSL are treated similarly, apart from systemic therapy for controlling for system disease for SCNSL. The main treatment regimen consists of high-dose methotrexate-based chemotherapy with or without radiation therapy.[7] SCNSL carries a very poor prognosis (median overall survival only 4–5 months).[16]

- A 59-year-woman with history of colonic T-cell lymphoma presented with dizziness and headache. Contrast-enhanced CT demonstrated leptomeningeal enhancement (white arrows) at right parieto-occipital region with a large area of hypodensity (a). Corresponding MRI post-contrast T1-weighed image showed leptomeningeal enhancement and enhancing right tentorial thickening (white arrows). Ependymal enhancement at temporal horn (white arrow head) and occipital horn of right lateral ventricle were also seen (b and c). On ADC map, associated restricted diffusion (black arrowhead) was also evident (d). On T2-weighed images, parenchymal infiltration with vasogenic edema at right parieto-occipital lobe and splenium of corpus callosum with expansion (black arrowheads) were evident (e and f). CSF cytology was positive for lymphoma cells confirming the diagnosis of CNS recurrence.

- A 59-year-old man with history of nasopharyngeal lymphoma in remission presented with facial pain. MRI post-contrast T1-weighed image revealed perineural spread of lymphoma with thickening and enhancement of cranial nerve V at left Meckel’s cave (white arrowhead) suggesting CNS relapse (a and b).

- A 68-year-old man presented with left sided weakness. Non-contrast CT revealed a hyperdense right basal ganglia mass (white arrow) (a). On MRI post-contrast T1-weighed image, the corresponding lesion showed homogenous and intense enhancement (black arrow) (b). On T2-weighed image it was inhomogeneously low signal (black arrowhead) with mild perifocal edema (c). On ADC map it showed restricted diffusion with low signal (white arrowhead) (d). Stereotactic biopsy confirmed diffuse large B-cell lymphoma. Staging PET-CT revealed multiple mediastinal masses suggesting lymphoma and bone marrow biopsy confirmed lymphoma involvement.
Primary dural lymphoma (PDL)
PDL is a rare subtype of PCNSL (accounting for about 7% of it).[17] It arises from the dura mater and is characteristically seen without parenchymal or systemic disease.[6] It typically affects middle-aged females.[2] Most of the cases are caused by marginal zone B-cell lymphoma.[16] They differ biologically from other PCNSL in which they are indolent and low-grade, lacking invasion of brain parenchyma.[16] They can present with multifocal symptoms including cranial nerve palsies, spinal neuropathies, headache, ataxia, and encephalopathy.[16] The key diagnostic features include diffusely enhancing single or multiple extra-axial masses,[1,2,6] which often mimic meningiomas or subdural hematoma.[18] They are most commonly found in cerebral convexities, also in falx, tentorium, sellar/parasellar regions, or spine.[17] Imaging features again follow its hypercellular nature, being iso- to hyper-attenuating on CT, iso- to hypo- intense on T2-weighted images with restricted diffusion [Figure 9].[19] Vasogenic edema may be seen in adjacent brain parenchyma.[19] It is important to exclude parenchymal or systemic involvement because leptomeningeal involvement of lymphoma can occur as SCNSL or synchronously with PCNSL, which carries a much poorer prognosis.[17] Definitive diagnosis can be established by detection of malignant lymphocytes in cytology, a monoclonal population by flow cytometry or gene rearrangement studies, or a combined approach.[17] Contrast MRI of the whole neuroaxis to define total tumor burden should be performed before initiating treatment and is an important tool in evaluating treatment response in these patients.[3] They carry a more favorable prognosis compared with PCNSL (median overall survival 24 months).[17]

- A 78-year-old woman presented with headache without history of head trauma. Non-contrast CT showed incidental finding of the left tentorial thickening and left cerebral convexity dural thickening (black arrows) which persisted for 3 months (follow-up CT not shown) (a and b). Post-contrast T1-weighed MRI showed the left tentorial and left cerebral convexity dural thickening with enhancement. Extension to left orbit with enhancing extraconal orbital mass with involvement of the left ethmoid sinus (white arrowheads) were also evident. On T2-weighed image and ADC map, both lesions showed T2W hypointensity and restricted diffusion (white arrows) (c-f). On coronal T2W, T1W pre-and post-contrast images, the ethmoidal mass (white arrow), and enhancing left cerebral convexity dural thickening (white arrowhead) were also seen (g-i). Biopsy by left ethmoidectomy revealed extranodal marginal zone lymphoma.
[Table 1] summarizes the above entities and highlights the key features for comparison.
| PCNSL | SCNSL | PDL | |
|---|---|---|---|
| Definition | Lymphoma restricted to the brain, leptomeninges, spinal cord, or eyes, without evidence of it outside the CNS at primary diagnosis. 90% Diffuse large B-cell Lymphoma |
CNS involvement by systemic lymphoma | Rare subtype of PCNSL Does not involve brain parenchyma Most frequent histology: marginal zone B-cell lymphoma |
| Primary site of CNS involvement | Brain parenchyma~100% Usually solitary lesion; multiple in 20–40% |
Brain parenchyma~1/3, leptomeninges~2/3 |
Originate from dura mater, can involve epidural or subdural space |
| Typical location | Periventricular and superficial brain regions, often in contact with ventricular or meningeal surfaces Cerebral hemisphere (38%) in particular frontal lobe>basal ganglia/thalamus (16%) > corpus callosum (14%) > ventricular region (12%) > cerebellum (9%) |
Leptomeninges | Cerebral convexities (most common), falx, tentorium, sellar/parasellar regions, or spine |
| Key diagnostic features | Typically a solitary homogeneously enhancing supratentorial parenchymal mass Marked contrast enhancement on CT or MRI |
Leptomeningeal, subependymal, dural or cranial nerve contrast enhancement |
Dural-based enhancing single or multiple extra-axial masses |
| Features of hypercellularity iso- to hyper-attenuating on CT iso- to hypo-intense to grey-matter on T2W and restricted diffusion on MRI |
|||
| Other imaging features | Immunocompetent: Homogenous marked enhancement Immunocompromised: Irregular or ring enhancement, tend to be multifocal and surrounded by a greater degree of vasogenic edema. Spontaneous hemorrhage are more frequent Classic pattern crossing the corpus callosum with butterfly pattern Hemorrhage or calcification are rare. Watch out for extension to leptomeninges (15-20%), eye (25%) (suggesting secondary intraocular lymphoma) |
Cranial nerve enhancement Superficial brain lesions Communicating hydrocephalus | Dural tail sign May seen vasogenic edema in adjacent brain parenchyma |
| Mimic/DDx | Other primary and secondary brain tumors (especially gliomas), infection (e.g., toxoplasmosis and tuberculosis) | Infective meningitis, granulomatous disease | Subdural hematoma, meningioma |
| Prognosis | Very poor prognosis | Very poor prognosis | More favorable prognosis due to indolent and low grade disease |
IMAGING DIFFERENTIALS
Given the highly variable imaging presentations of intracranial CNS lymphomas, some of those may mimic other differentials such as high-grade gliomas (HGGs), metastases, or infectious and granulomatous diseases. Some of the examples are discussed and illustrated.
HGG
The pattern of enhancement can be helpful for differentiation of PCNSL versus HGG, that is, homogeneous in PCNSL in immunocompetent patients and heterogeneous with necrotic areas in HGGs [Figure 10].[4] The location of lesions may also be helpful, as PCNSL tends to infiltrate the optic tracts and basal ganglia more while cerebral cortex involvement is more observed in HGGs.[4] Both of them can show a “butterfly pattern” crossing the corpus callosum as well [Figure 11]. Both entities can show restricted diffusion with a low ADC value. For PCNSL, there is an inverse relationship between cellularity and ADC values. They often have more restricted diffusion and therefore lower ADC values than gliomas and metastases. Restricted diffusion with an ADC threshold value ≤1.1 × 10−3 mm2/s has been recommended to differentiate PCNSL from other intracranial focal lesions.[9] Susceptibility weighted imaging can also be helpful for differentiation as microhemorrhage is rarer in PCNSL than in gliomas.[7] Advanced MRI techniques including cerebral perfusion and MRS can also serve as adjuncts for differentiation. The relative cerebral blood volume (rCBV) of lymphomas is higher than normal brain tissues but lower than that in high-grade glioma such as glioblastoma, due to low induction of neovascularization.[8,14] For MRS, both tumors usually show reduced NAA peaks (neuronal damage), elevated choline to creatine ratio (elevated membrane turnover), lipid peaks (due to release of fatty moieties by transformed lymphocytes in PCNSL and due to necrosis in GBM), and lactate peaks (anaerobic metabolism).[4] It has been suggested that an increase in lipid peak in the absence of necrosis and low perfusion with marked enhancement are characteristic of PCNSL.[2,4] Moreover, PCNSL in immunocompetent patients typically appears as hypermetabolic lesions with increased FDG uptake on PET studies. They show more pronounced and homogenous metabolic activity than metastases and high-grade glioma.[4]

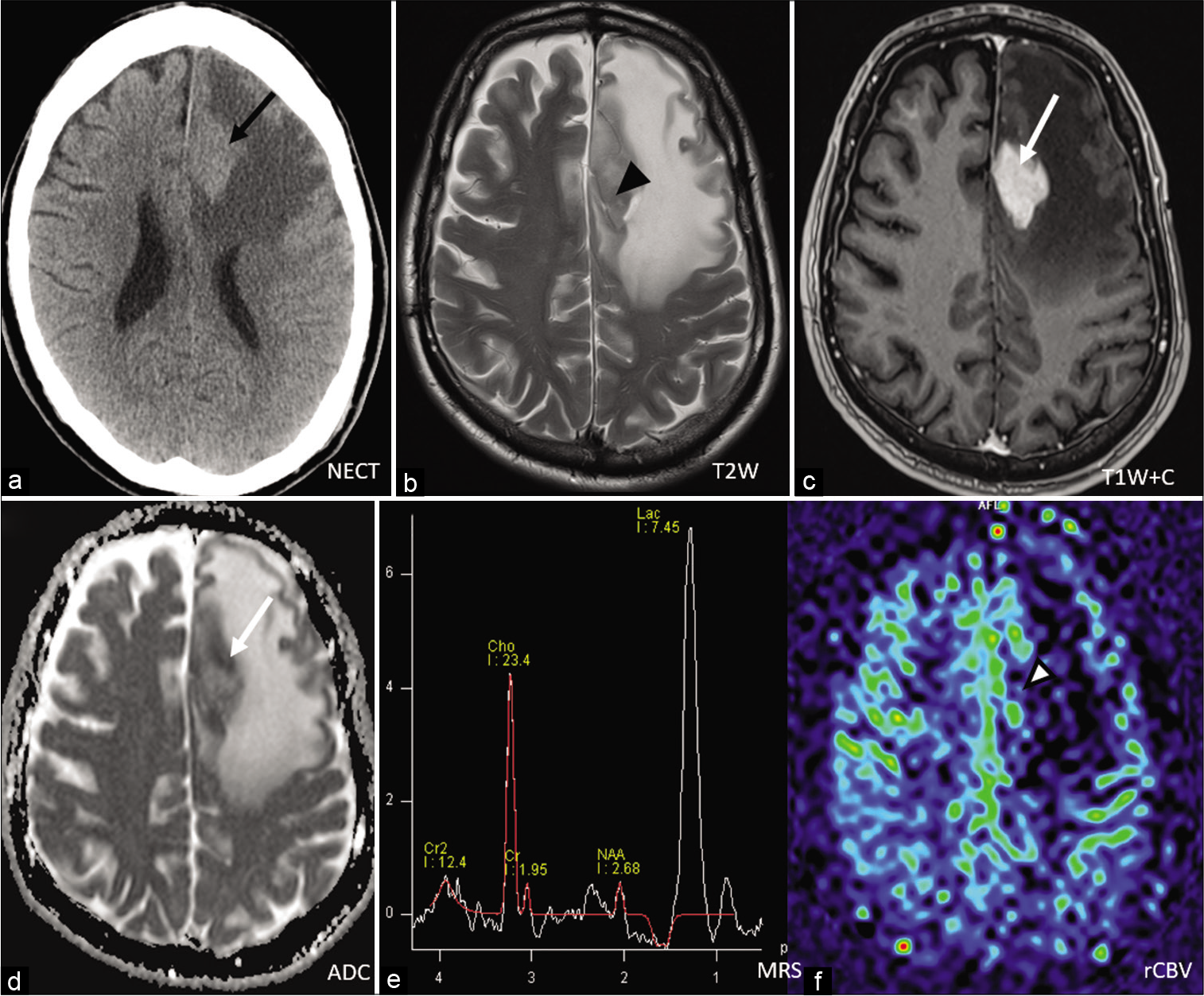
- A 43-year-old man presented with seizure. Non-contrast CT showed a heterogenous mass (white arrowhead) is seen at the anterior part of interhemispheric fissure with mass effect and perifocal edema at both frontal lobes (a). The mass seen to be centered at the body of corpus callosum and crossed midline. On MRI T1-weighed image, it was heterogenous with areas of hemorrhage (white arrow) (b). On T2-weighed image, it was heterogeneously hyperintense (white arrow) (c) On ADC map, low ADC areas maybe partly explained by the presence of blood products besides restricted diffusion (d). On post-contrast T1-weighed image, there was peripheral enhancing component with central necrosis (black arrow) (e and f). Stereotactic biopsy confirmed Glioblastoma Multiforme.

- A 57-year-old female presented with subacute deterioration of general condition. On non-contrast CT, an ill-defined hypodense intra-axial mass is seen at the left frontal lobe (black arrow) crossing the genu of corpus callosum with expansion. The right frontal lobe was involved (a). MRI T1-weighed image showed areas of hemorrhage (arrowhead) (b) and on post-contrast T1-weighed image the lesion showed heterogeneously peripheral enhancing areas (white arrow) with central necrosis (c). On T2-weighed image, the corresponding mass was seen to be heterogenous with intermediate signal component (white arrow), together with central necrotic area and surrounding hyperintense white matter signal (d). On DWI and ADC map, areas of restricted diffusion (white arrows) could be seen (e and f). Stereotactic biopsy confirmed GBM.
BRAIN METASTASES
Common primaries that metastasize to the brain include lung, breast, skin, colon, pancreas, testes, ovary, cervix, renal cell carcinoma, and melanoma.[20] They tend to affect the grey-white junction and show a predilection to the cerebral hemisphere. They can be solitary or multiple and have highly variable imaging appearance on conventional CT and MR. Occasionally their imaging appearance can mimic that of PCNSL when they uncommonly affect deep grey nuclei or infiltrate the corpus callosum [Figures 12 and 13]. However, they tend not to show restricted diffusion with a higher ADC value compared with PCNSL.[20] MR perfusion for metastases generally shows increased rCBV as they are highly vascular lesions, and this may help distinguish from lymphoma which has low perfusion.[20] MRS findings generally show an increased choline-to-creatinine peak ratio, with reduced NAA. Lipid and lactate peaks may also be observed due to necrosis and anaerobic respiration, respectively.[20]
![A 67-year-old woman with lung cancer on targeted therapy presented with left sided numbness. Non-contrast CT showed a heterogeneously hyperdense mass was seen at right thalamus compressing the third ventricle (white arrow) (a). On MRI T2-weighed image, the mass was hyperintense (black arrow) (b) and showed heterogeneous enhancement (black arrow) (c). On ADC map, no restricted diffusion (arrowhead) was noted (d). Differential diagnoses of metastasis or lymphoma were made in this patient with known lung primary. Although lymphoma may also be hyperdense on CT with thalamic location, the characteristic hypercellularity features on MRI were lacking in this case [compare with Figure 3]. Biopsy confirmed metastasis from lung origin.](/content/12/2022/12/1/img/JCIS-12-4-g012.png)
- A 67-year-old woman with lung cancer on targeted therapy presented with left sided numbness. Non-contrast CT showed a heterogeneously hyperdense mass was seen at right thalamus compressing the third ventricle (white arrow) (a). On MRI T2-weighed image, the mass was hyperintense (black arrow) (b) and showed heterogeneous enhancement (black arrow) (c). On ADC map, no restricted diffusion (arrowhead) was noted (d). Differential diagnoses of metastasis or lymphoma were made in this patient with known lung primary. Although lymphoma may also be hyperdense on CT with thalamic location, the characteristic hypercellularity features on MRI were lacking in this case [compare with Figure 3]. Biopsy confirmed metastasis from lung origin.

- A 43-year-old female with breast cancer on treatment presented with left sided weakness. MRI post-contrast T1-weighted image revealed an intra-axial heterogeneously enhancing mass with central necrosis was seen at the right parieto-occipital lobe (white arrow). There was infiltration to the rostrum of corpus callosum crossing the midline (a). On T2-weighted image, the solid part of this lesion was inhomogeneous (white arrow) (b) and on ADC map areas of low signal (arrow head) was seen suggesting restricted diffusion (c). Differential diagnoses of metastasis, glioblastoma and lymphoma were made in this patient with known breast cancer, operative biopsy confirmed metastasis from breast origin.
PYOGENIC BRAIN ABSCESSES
Pyogenic brain abscesses often present as ring-enhancing brain lesions which overlap with those of PCNSL in immunocompromised patients.[11] In the context of immunocompromised patients presenting with ring-enhancing brain lesions, the differentiation would be of utmost importance as the management differ significantly, that is, high dose intravenous antibiotic with surgical drainage in case of brain abscess, while PCNSL requires early stereotactic biopsy for subsequent chemotherapy and radiotherapy. Pyogenic brain abscesses typically show restricted diffusion of the central fluid content (i.e., pus) instead of the peripheral solid component in PCNSL. Pyogenic brain abscesses, also typically demonstrate a lactate peak with reduced choline and NAA, while PCNSL typically demonstrates a lipid peak with raised choline to NAA ratio.[11]
TUBERCULOMAS
It is caused by Mycobacterium tuberculosis. Intracranial tuberculous infections are divided into meningeal form and parenchymal form.[21] There are “different facets” of presentation of tuberculous infection in the brain, broadly divided into meningeal tuberculosis and parenchymal tuberculosis and tuberculoma is the most common form of parenchymal tuberculosis.[21] It is the inflammatory reaction mounted by the body against the tuberculous bacilli, pathologically consisting of epithelioid cells, Langhans giant cells, and a peripheral rim of lymphocytes.[21,22] They can be solitary or multiple, appearing in a supratentorial compartment, infratentorial compartment, or both. Most commonly they occur at grey-white junctions, due to the arrest of the hematogenously spread microbes caused by a reduction in the caliber of vessels in that region.[20] These may also occasionally develop in the brain parenchyma secondary to the spread of CSF infection through the perivascular spaces.[21] Different stages exist including non-caseating granuloma, caseating granuloma, caseating granuloma with central liquefaction, and calcified granuloma. A characteristic T2W hypointense signal can be observed in caseating granulomas [Figure 14], together with lipid peaks at 0.9 or 1.3 ppm on MRS mimicking lymphoma, but an absence of restricted diffusion will favor tuberculoma over lymphoma.[23] It is also useful to look for leptomeningeal enhancement in basal cisterns which can be commonly observed in intracranial tuberculous infection.

- A 52-year-old man presented with the left-sided numbness. Non-contrast CT showed a heterogenous isodense right thalamic mass (white arrow) was noted on non-contrast CT (a). On MRI post contrast T1-weighted image, the mass showed intense and homogenous enhancement (white arrow) (b). On T2-weighed image it was heterogeneously hyperintense with some central low signal with surrounding edema (c). ADC map showed hyperintense signal therefore no definite restricted diffusion was evident (d). Biopsy of the lesion confirmed tuberculoma.
TOXOPLASMOSIS
In immunocompromised patients (especially those who have been infected with HIV), the main differential diagnosis of lymphoma is toxoplasmosis. For cerebral toxoplasmosis, the lesions show a propensity to affect the basal ganglia, thalami, and grey-white junction, with lots of surrounding vasogenic edema.[4] They are usually hypointense on T1- weighted images, but they may show peripheral hyperintensity indicating subacute hemorrhage, a feature that may help distinguish toxoplasmosis from lymphoma. The lesions usually have high or mixed signal intensity on T2-weighted and FLAIR images.[23] A dilemma exists when both entities present with multiple ring-enhancing lesions on CT or MRI. A pathognomonic sign called “eccentric target sign” (25% sensitive and 95% specific) on post-contrast CT or MRI has been described, which aids in the differentiation. It consists of a ring-shaped zone of peripheral enhancement with a small eccentric nodule along the wall.[24,25] DWI cannot reliably differentiate CNS lymphomas from cerebral toxoplasmosis because they show overlapping ADC values [Figure 15]. Thallium-SPECT or PET can aid in this setting, as lymphoma will show increased uptake, while toxoplasmosis will show decreased or minimal uptake.[1,2] However, it is important to note that both entities can occur at the same time.

- A 43-year-old man with known HIV on anti-viral treatment presented with fever. Non-contrast CT showed two heterogeneous, iso-tohypodense lesions at the left basal ganglia and right thalamus (white arrow) with surrounding vasogenic edema (a and b). On MRI T2-weighed imaged, they showed intermediate signals (white arrowheads) (c). On T1-weighed image, mild intralesional hyperintense signals (black arrow) without susceptibility artifact on gradient-echo sequences (not shown) could be due to proteinaceous content (d). On post-contrast T1-weighed image, solid, and ring-like enhancement (white arrow) were seen (e). On ADC map, the low signal area (white arrowheads) suggested restricted diffusion (f). The differential diagnoses of CNS lymphoma and cerebral toxoplasmosis were made. He was put on empirical Pyrimethamine. Stereotactic biopsy was planned but delayed due to profound hyponatremia, early reassessment MRI later showing regressing lesions and the treatment was continued. Follow-up MRI 2 years later confirmed resolved lesions and the diagnosis was presumed to be cerebral toxoplasmosis.
CONCLUSION
Intracranial CNS lymphoma has highly variable appearances in different clinical contexts but in general, the imaging features follow those of hypercellularity. Taking all those into account would be helpful to narrow down the likely differentials in a specific clinical situation. None of its imaging appearances will unequivocally differentiate them from other brain lesions. A visible tumor on imaging is essential to raise the suspicion of CNS lymphoma, which then can lead to an early histologic diagnosis based on cytology of the CSF or stereotactic brain biopsy.
[Table 2] summarizes the distinguishing imaging features of various imaging mimics versus PCNSL.
| Disease entities | Characteristic Features |
|---|---|
| High grade glioma |
|
| Brain metastases |
|
| Pyogenic brain abscess |
|
| Tuberculoma |
|
| Cerebral toxoplasmosis |
|
Declaration of patient consent
Institutional Review Board (IRB) permission was obtained for the study (KW/EX-21-073).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2011;32:984-92.
- [CrossRef] [PubMed] [Google Scholar]
- Multimodality imaging of primary CNS lymphoma in immunocompetent patients. Br J Radiol. 2014;87:20130684.
- [CrossRef] [PubMed] [Google Scholar]
- CNS lymphoma: A practical diagnostic approach. J Neuropathol Exp Neurol. 2014;73:478-94.
- [CrossRef] [PubMed] [Google Scholar]
- Primary CNS lymphomas: Challenges in diagnosis and monitoring. Biomed Res Int. 2018;2018:3606970.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for the diagnosis and management of primary central nervous system diffuse large B-cell lymphoma. Br J Haematol. 2019;184:348-63.
- [CrossRef] [PubMed] [Google Scholar]
- CT and MRI findings of intracranial lymphoma. AJR Am J Roentgenol. 2005;184:1679-85.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of nervous system involvement in hematologic malignancies: What radiologists need to know. AJR Am J Roentgenol. 2015;205:604-17.
- [CrossRef] [PubMed] [Google Scholar]
- Associations between apparent diffusion coefficient (ADC) and KI 67 in different tumors: A meta-analysis. Part 1 ADCmean. Oncotarget. 2017;8:75434-44.
- [CrossRef] [PubMed] [Google Scholar]
- Modern techniques of magnetic resonance in the evaluation of primary central nervous system lymphoma: Contributions to the diagnosis and differential diagnosis. Rev Bras Hematol Hemoter. 2016;38:44-54.
- [CrossRef] [PubMed] [Google Scholar]
- Diffuse large B-cell lymphoma of the central nervous system in mycophenolate mofetil-treated patients with systemic lupus erythematosus. Lupus. 2010;19:330-3.
- [CrossRef] [PubMed] [Google Scholar]
- Primary lymphoma of CNS, mycophenolate mofetil and lupus. Lupus. 2006;15:886-8.
- [CrossRef] [PubMed] [Google Scholar]
- Development of primary central nervous system lymphoma in a systemic lupus erythematosus patient after treatment with mycophenolate mofetil and review of the literature. Lupus. 2017;26:1224-7.
- [CrossRef] [PubMed] [Google Scholar]
- Post-transplantation primary central nervous system lymphoma in a patient with systemic lupus erythematosus and prolonged use of immunosuppressant. Hong Kong Med J. 2014;20:541-4.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic markers for immunodeficiency-associated primary central nervous system lymphoma. J Neurooncol. 2019;144:107-15.
- [CrossRef] [PubMed] [Google Scholar]
- Primary central nervous system lymphoma in AIDS: A wider spectrum of CT and MRI findings. Neuroradiology. 2001;43:29-35.
- [CrossRef] [PubMed] [Google Scholar]
- Secondary central nervous system lymphoma: Spectrum of morphological MRI appearances. Neuropsychiatr Dis Treat. 2018;14:733-40.
- [CrossRef] [PubMed] [Google Scholar]
- Primary leptomeningeal lymphoma: International primary CNS lymphoma collaborative group report. Neurology. 2013;81:1690-6.
- [CrossRef] [PubMed] [Google Scholar]
- Primary central nervous system marginal zone B-cell lymphoma arising from the dural meninges: A case report and review of literature. Clin Case Rep. 2020;8:491-7.
- [CrossRef] [PubMed] [Google Scholar]
- From the radiologic pathology archives: Mass lesions of the dura: Beyond meningioma-radiologic-pathologic correlation. Radiographics. 2014;34:295-312.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of brain metastases. Surg Neurol Int. 2013;4(Suppl 4):S209-19.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic resonance imaging spectrum of intracranial tubercular lesions: One disease, many faces. Pol J Radiol. 2018;83:e524-35.
- [CrossRef] [PubMed] [Google Scholar]
- Central nervous system tuberculosis: An imaging-focused review of a reemerging disease. Radiol Res Pract. 2015;2015:202806.
- [CrossRef] [PubMed] [Google Scholar]
- MRI in intracranial tuberculosis: Have we seen it all? Clin Imaging. 2020;68:263-77.
- [CrossRef] [PubMed] [Google Scholar]
- Eccentric target sign in cerebral toxoplasmosis: Neuropathological correlate to the imaging feature. J Magn Reson Imaging. 2010;31:1469-72.
- [CrossRef] [PubMed] [Google Scholar]
- Best cases from the AFIP: Cerebral toxoplasmosis. Radiographics. 2009;29:1200-5.
- [CrossRef] [PubMed] [Google Scholar]






