Translate this page into:
Desmoplastic (collagenous) fibroma of the parietal bone: Case report and review of the literature

*Corresponding author: Jining Sun, Department of Radiology, Weifang People’s Hospital, Weifang, China. jiningsun22@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Zhang B, Yu H, Pylypenko D, Sun J. Desmoplastic (collagenous) fibroma of the parietal bone: Case report and review of the literature. J Clin Imaging Sci. 2024;14:48. doi: 10.25259/JCIS_136_2024
Abstract
Desmoplastic fibroma (DF) is an uncommon benign bone tumor that typically affects the facial bones, with cerebral cranium involvement being extremely rare. We report a unique case of DF in the parietal bone of a 28-year-old woman, notable for its rapid growth during pregnancy-a phenomenon not previously documented. The imaging features of this case also differ from all but one previously reported case. The patient underwent surgical removal, and histopathology confirmed the diagnosis of DF (collagenous fibroma). After 17 months of follow-up, no local recurrence was observed. We also provide a comprehensive review of 32 cases involving DF of the cerebral cranium, analyzing clinical features, imaging findings, treatment methods, and recurrence patterns. This case highlights the importance of considering DF in the differential diagnosis of cranial lesions, particularly in pregnant patients with rapid tumor growth. Complete surgical resection with a wide margin remains the recommended treatment to minimize recurrence risk.
Keywords
desmoplastic fibroma
cranium
parietal bone
computed tomography
magnetic resonance imaging
INTRODUCTION
Desmoplastic fibroma (DF), also known as collagenous fibroma, represents a rare yet locally aggressive primary bone tumor. Its occurrence in the cerebral cranium is particula scarce. This report presents a case of DF located in the parietal bone of a 28-year-old woman, highlighting its rapid growth during pregnancy and unique imaging features. A comprehensive literature review, encompassing 32 cases from 28 articles [Table 1],[1-28] is presented to better understand the clinical presentation, imaging characteristics, treatment options, and recurrence patterns of cranial DF.
| Author (ref. no.) | Gender Age (yr) | Location | Size (cm) | Presentation | Imaging appearance | Treatment | Recurrence Follow-up |
|---|---|---|---|---|---|---|---|
| Gardini et al. (1978)[1] | F/7 | Frontal bone | NA | Tender mass for1 month | NA | Complete resection, cranioplasty | NA, NA |
| Hufnagel et al. (1987)[2] | F/22 | Parietal bone | 2×1 | Headache for 6 months | X-ray: A lytic lesion without sclerosis or bone expansion CT: A lytic area with irregular borders showing destruction of both skull tables and a central soft-tissue component |
Surgical resection, cranioplasty | NA, NA |
| Ovul et al. (1988[3] | M/3 month | Parietal bone | 4×5×0.8 | 3 month history of growing mass (present at birth) | X-ray: A hypodense mass in the diploic with enlargement external | Complete resection | NR, 2 years |
| Okuno et al. (1990)[4] | F/86 | Temporal bone | 2×3 | Ear drainage for 1 month and stenosis of the ear canal | a mass | Surgical resection | NR, 2 years |
| Goldberg et al. (1995)[5] | F/42 | Frontal bone | NA | Left-sided headache for 3 months | CT: An abnormal trabecular pattern with expansion | En bloc resection | NR, 6 years |
| Selfa-Moreno et al. (1995)[6] |
F/28 | Parietal bone | 2 | Continuous right-sided headache for several weeks | X-ray: A round lytic lesion without sclerosis CT: A lytic area involving both tables with bone fragments and a central soft-tissue component |
Surgical removal | NR, 3 years |
| Pensak et al. (1997)[7] | F/21 | Temporal bone | NA | Aural fullness over 6 months and a mild decrease in hearing | CT: An expansive bony lesion | Temporal craniotomy and petrosectomy | NR, 4 years |
| F/28 | Temporal bone | 3.5 | 1 year history of aural fullness and intermittent suppuration, progressive decrease in hearing | CT: A destructive mass causing a bubbling expansive destruction of bone MR: A enhancing extraaxial mass |
Temporal craniotomy and petrosectomy with tympanoplasty | NR, 18 months | |
| Celli et al. (1997)[8] | F/64 | Parietal bone | 2×3 | Incidental finding | MR: A diploic lesion, isointense on T1WIs and hyperintense on T2, with moderate enhancement | Complete resection and cranioplasty | NR, 1 years |
| Horiuchi et al. (1998)[9] | F/29 | Frontal bone | 2 | Right frontal headache for 2 months | X-ray: An osteolytic lesion CT: A lytic, non-sclerotic lesion with a soft tissue in the diploic MR: A well-demarcated enhancing tumor |
Complete resection and cranioplasty | NA, NA |
| Dutt et al. (2000)[10] | F/72 | Temporal bone | 2.3 | Painless swelling for nearly 10 years | CT: A large triangular bony expanding the diploic space and involving predominantly the outer tableof the skull with soft tissue MR: An enhancing soft-tissue mass with an intracranial extension |
Complete resection and cranioplasty | NR, 6 months |
| Kim et al. (2001)[11] | M/21 | Parietal bone | 4 | Persistent right-sided headache that had lasted several weeks. a painful mass | X-ray: A round lytic lesion without bone expansion or sclerosis CT: A expensive lytic skull lesion with destruction of both skull tables and a central soft-tissue component |
Complete resection with wide margin and cranioplasty | NR, 35 months |
| Rabin et al. (2003)[12] | F/43 | Temporo-parietal bone | (CT: 6×5) (MR: 8×5×10) | Progressively enlarged swelling for 12 years | CT: A large, isodense mass associated with complete bone destruction MR: A mass infiltrating through the diploic space and involving the adjacent galea and soft tissue |
Complete resection, cranioplasty | NA, NA |
| Wolfe et al. (2005)[13] | M/3 | Frontal bone | 3 | Mildly tender bone prominence enlarged slightly over 2 years | CT: Focal calavarial thickening and expansion of the diploic space with a ground-glass appearance | Complete resection with negative margins | NR, 1 year |
| F/7 | Temporal bone | NA | Non-tender bone mass for 14 months | X-ray: A lytic lesion with irregular borders and a trabeculated pattern CT: A lytic lesion eroding the outer tablewith enlarged diploic space and enhancement of an intralesional soft-tissue mass |
Resection and curettage | NR, 3 months |
|
| M/22 month | Frontal bone | NA | Non-tender, enlarging mass surrounded by engorged vessels | CT: A focal, ill-defined lesion with enlarged diploic space, thinned cortices and internal trabeculations MR: An intradiploic soft tissue lesion with prominent contrast enhancement |
Complete resection | NA, NA | |
| Yoon et al. (2006)[14] | F/1 | Frontal bone involving temporoparietal bone | 8×7×1 | Progressive swelling for 10 months | X-ray: A large round lytic lesion without a sclerotic margin CT: A slightly expansile mass in the dipole involving partial destruction of the outer table MR: A mass of low signal intensity on T1WI, and profoundly low signal intensity on T2WI. e mass enhanced heterogeneously with gadolinium, with strong enhancement along the dural and bony margins of the mass, but weak enhancement of the center of the mass |
Complete resection, aggressive curettage, cranioplasty | NR, 1 year |
| Lath et al. (2006)[15] | M/18 | Frontal bone | NA | Headaches and a scalp swelling for 1 month |
CT: A iso to hypodense mass involving the outer and inner tablewith intracranial extension and enhancement of intradural component | Complete resection and duroplasty | NR, 1 year |
| Kim et al. (2006)[16] | F/53 | Temporal bone | 3×2.5 | Continuous right side headache for 1 year | X-ray: A lytic lesion with mild sclerotic margin CT: A lytic lesion in the diploic eroding the outer tableof skull MR: High signal intensity lesion on T2WI |
En bloc resection with wide margins | NR, 15 months |
| Hwang et al. (2007)[17] | F/1 | Frontal bone | 2 | Non-tender, fixed, progressive protuberance for 3 months | X-ray: A round osteolytic lesion with sclerotic margin CT: A well-defined osteolytic mass and diploic space enlarged with thinned cortices MR: An intradiploic soft-tissue lesion with prominent contrast enhancement |
Complete resection | NR, 6 months |
| Deniz et al. (2008)[18] | M/21 | Parietal bone | 2.5×3 | Non-tender mass with progressive increase for 3 years | CT: A osteolytic intradiploic lesion | Complete resection | NR, 15 months |
| Bahri et al.* (2011)[19] | M/3 | Parietal bone | 2.9×2.0 | a mass | CT: A expensive lytic mass with brain compression and cortical destruction | Complete resection | NR, 6 months |
| Lee et al. (2012)[20] | M/20 | Frontal bone | 3×3.5 | Worsening headache and a swelling for 1 year | X-ray: A lytic lesion with a sclerotic margin CT: A focal calvarial thickening and expansion of the diploic space by a hypo-attenuated mass with a sclerotic margin and ground-glass appearance MR: Heterogeneous signal intensity on T2WI and intermediate signal intensity on T1WI with multifocal enhancement |
Complete resection with sufficient safety margins | NA, NA |
| Cho et al. (2013)[21] | F/11 | Parieto-occipital bone | 1.6×1.4 | Intermittent pain and a slowly increasing soft mass | CT: A well-defined expansile lytic bone lesion with erosion through the outer and inner tables of skull | Complete resection,autologous reconstruction | NR, 4 months |
| Dadlani et al. (2014)[22] | F/20 | Frontal bone | 18×17.5×14 | Progressive swelling for 15 years | X-ray: A large sclerotic lesion with presence of ‘‘sun ray’’ spicules with significant soft tissue component CT: Speculations arising from the outer table MR: A giant heterogeneous lesion with markedly hypointense on both T1WI and T2WI; a soft tissue component with isointense on T1WI and heterointense on T2WI; multiple hypointense bony “sun ray” spicules extending into the soft tissue component |
Subtotal resection | Died 9 days after surgery due to pulmonary embolism |
| Majumder et al. (2015)[23] | M/13 | Temporal bone | NA | 9-month history of right aural fullness, progressive hearing loss, and pulsatile tinnitus | CT: A large fibro-osseous lesion MR: An enhancing mass |
Complete resection | NR, 1.5 years |
| Koiso et al. (2016)[24] | F/33 month | Parietal bone | 4×1×3.5(27 month),4.3×1×4(32 month) | Recurrent afebrile seizures | CT: A osteolytic mass in the intradiploic space eroding the outer and inner tables of the calvarium MR: A hypointense spindle-shaped mass with hypointense centrally on T1WI, and hyperintense peripherally on T2WI. Contrast-enhanced MRI showed a well-defined homogeneous mass |
Complete resection, the adjacent dura was coagulated but not removed | R, 4 months After second operation: NR, 1 year |
| Garov et al.* (2016)[25] | NA | Temporal bone | 3×4 | NA | CT, MR: A expansile lytic mass in the diploic space with destruction of the inner table and thinning of the outer table | Surgical treatment | NA, NA |
| Lucke-Wold et al. (2020)[26] | F/10 months | Frontal bone | 3 | 1 month history of progressive swelling | CT: An expansile osteolytic mass in the intradiploic space eroding the outer table of the skull | Completely resection | NR, 1 year |
| Kato et al. (2021)[27] | F/50 | Temporal bone | 3.2×2.8×2.1 | Hearing loss and otalgia for 6 months | CT: Destructive changes centered with mastoid opacification MR: A destructive, contrast-enhancing soft tissue lesion with a cystic component |
Complete mastoidectomy | NR, 6 months |
| Elbadawy et al. (2021)[28] | F/33 | Occipital bone | 4×4 | Swelling for 2 years | CT: A extracranial heterogenous hyper-dense well circumscribed lesion MR: Heterogenous isointense signal on T1WI without contrast enhancement, and hypo-intense signal on T2WI |
Complete resection and curettage | NA, NA |
| This present case | F/28 | Parietal bone | 5×5 | 8 year history of progressive swelling | CT: A extracranial heterogeneous mass with a soft-tissue component and hyper-dense calcification without invading the diploe MR: Isointense on T1WI and slightly hypointense on T2WI, and inhomogeneously progressive enhanced on postcontrast T1WI |
Complete resection, curettage | NR, 1year |
M: Male, F: Female, NA: No available, NR: No recurrence, R: Recurrence, CT: computed tomography, MR: Magnetic resonance, MRI: Magnetic resonance imaging, T1WI: T1-weighted image T2WI: T2-weighted image, *Non English literature (information obtained from titles, abstracts, and images)
CASE REPORT
A 28-year-old woman presented with swelling over her left parietal region about 2 × 2 cm 8 years ago. During pregnancy about 2 years ago, the swelling obviously increased to a size of about 5 × 5 cm, and it became soft despite being firm initially. She denied any headache or other neurological symptoms that could be attributed to the lesion. The patient sought further treatment and was admitted to the neurosurgery department as a scalp tumor. Physical examination demonstrated a firm, immobile swelling over the left parietal region. The overlying scalp appeared intact. Neurological examination revealed normal.
Imaging
Computed tomographic (CT) brain and bone window with 3D reconstruction showed a 2.5 × 5 × 5 cm extracranial heterogeneous mass with a soft-tissue component and hyperdense calcification based on the left parietal bone. The lesion did not invade the diploe, as shown in Figure 1a-g. Cerebral CT angiography (CTA) showed blood supply from temporal artery branch [Figure 1h].
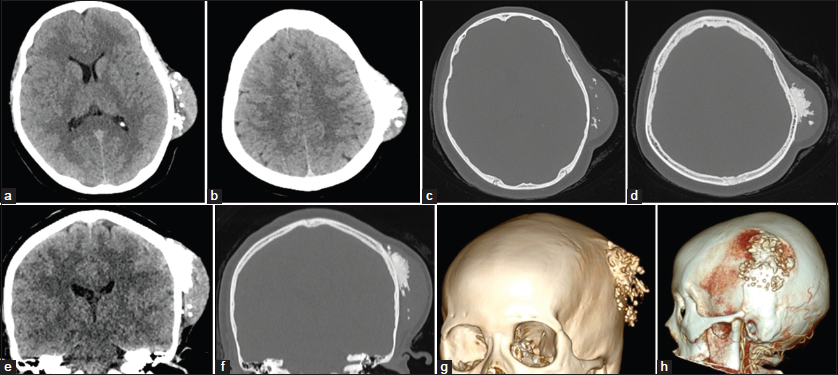
- Axial and coronal computed tomography (CT) brain and bone window with 3D reconstruction showing a 2.5 × 5 × 5 cm extracranial heterogeneous mass with a soft-tissue component and hyperdense calcification based on the left parietal bone. The lesion did not invade the diploe. Brain CT angiography (CTA) showing blood supply from temporal artery branch. (a and b) Axial CT brain window. (c and d) Axial CT bone window. (e and f) Coronal CT brain and bone window. (g) 3D reconstruction. (h) CTA.
Magnetic resonance imaging (MRI) of the brain revealed a heterogeneous mass based on the left parietal bone that was isointense on T1-weighted image and slightly hypointense on T2-weighted image, and inhomogeneously progressive enhanced on postcontrast T1-weighted image. The multiple hypointense areas in the central regions of the lesion on T1- and T2-weighted images suggested calcification [Figure 2a-m].
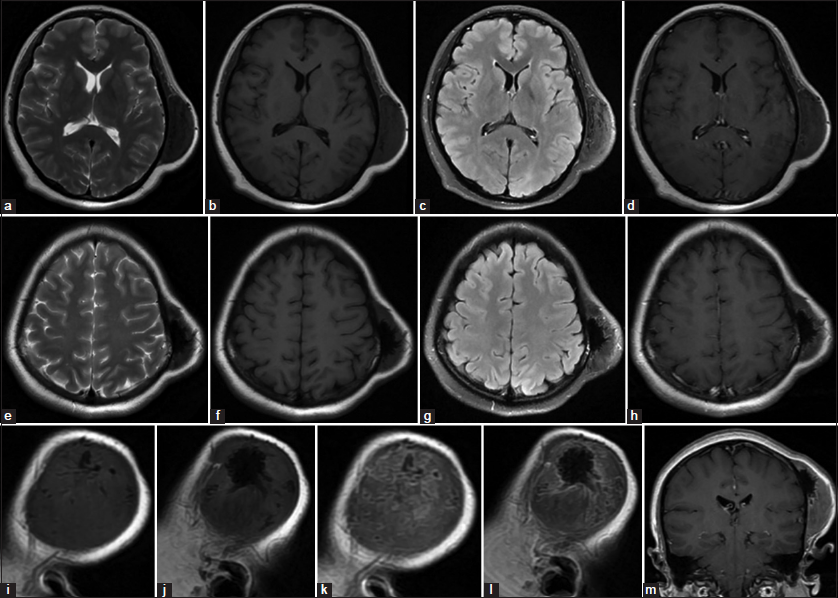
- Magnetic resonance imaging of the brain revealing a heterogeneous mass based on the left parietal bone that was isointense on T1-weighted image and slightly hypointense on T2-weighted image, and inhomogeneously progressive enhanced on postcontrast T1-weighted image. The multiple hypointense areas in the central regions of the lesion on T1 and T2-weighted images suggested calcification. (a-d) T2W, T1W without contrast, T2 weighted-Fluid-attenuated inversion recovery (T2-FLAIR) and T1W with gadolinium mid-axial image. (e-h) T2W, T1W without contrast, T2-FLAIR and T1W with gadolinium upp-axial image. (i and j) T1W sagittal image without contrast. (k and l) T1W sagittal image with gadolinium. (m) T1W coronal image with gadolinium.
The radiological differential diagnoses included hemangioma, hemangiopericytoma, invasive meningioma, and metastasis.
Management
The patient underwent surgical removal of the tumor. During the operation, a white, tough texture, locally hard and ossified tumor with insufficient blood supply was found to be adherent to the outer table of the skull. The tumor was separated from the surrounding tissues and cut into blocks. The remaining part attached to the skull was removed with a bone chisel and ground with a drill [Figure 3]. The surgery went smoothly, and the patient returned to the ward safely after the surgery.
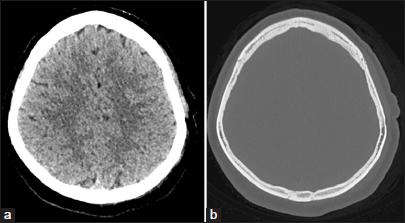
- Post-operative axial computed tomography (CT) brain and bone window showing complete excision of the lesion and curettage of the attached part of skull. (a) Axial CT brain window. (b) Axial CT bone window.
Pathology
The gross specimen consisted of multiple irregular pieces of firm, gray-white tissue, with areas of ossification. After partial decalcification of the tissue, routine histological examination was performed. Microscopic examination revealed that spindle and star shaped fibroblasts within abundant collagen or collagen myxoid stroma background, with sparse blood vessels and no obvious cellular atypia or nuclear division. There was not clear between the lesions with bone tissue. The final diagnosis was DF (collagenous fibroma) [Figure 4].
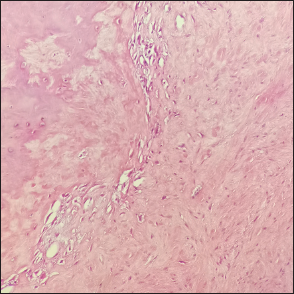
- Hematoxylin and eosin staining showing spindle and star shaped fibroblasts within abundant collagen or collagen myxoid stroma background, with sparse blood vessels and no obvious cellular atypia or nuclear division (magnification, ×200).
Regular follow-up over 17 months revealed no local recurrence.
DISCUSSION
Clinical perspective
DF stands as an exceptionally rare tumor, accounting for <0.1% of all primary bone tumors.[10,29-31] While it can manifest in any bone, the long bones, particularly the distal femur and proximal tibia, are commonly affected; however, its predilection for the mandible in the skull context is noteworthy.[32,33] The occurrence of DF in the cerebral cranium is extremely rare, with Gardini et al. first describing it in 1978,[1] and only 31 cases have been reported to date. This study presents the inaugural primary case featuring a rapid increase during pregnancy, accompanied by distinct imaging findings not typical in prior cases.
The literature review highlights the temporal bone as the most frequently affected site, followed by the frontal and parietal bones.[18] In this series, the occipital bone exhibited the least impact (1 case). There is no consistent preference among frontal (11 cases), parietal (10 cases), and temporal bones (10 cases).
Divergent perspectives exist in the literature concerning the incidence rate across genders and ages of DF in bone. Initial research suggested no gender predilection,[34] while subsequent studies, such as Kamata et al.,[35] reported a male predominance. DF of the mandible showed a slight female predilection.[36,37] Notably, DF of the cerebral cranium exhibited a marked female predilection in this review (22 cases in women vs. nine cases in men, with gender unavailable in one case).
Despite the elderly woman in the previously cited publication,[35] DF of the bone typically occurs in the 3rd decade.[29,34,38] In the 32 cases of DF in the cerebral cranium [Table 1], ages ranged from birth to 86 years old. The majority (24/31, 77.4%, with no available age in one case) occurred in the first three decades, consistent with prior research.[39] The frontal and parietal bones exhibited a higher predilection before the age of 30 (10/11, 91%, mean age 11.2-years-old, and 9/10, 90%, mean age 23.1 years old, respectively). Temporal bone lesions manifested at a relatively older age,[27] with 5 cases (5/9, 55.5%, mean age 41.4 years old, with no available age in one case) reported after 40 years old.
Clinical presentations of DF in the cerebral cranium often include a palpable mass on the head, colloquially referred to as “a lump in the scalp,”[24] and/or headaches. Temporal bone lesions may cause additional symptoms such as aural fullness, hearing loss, and ear drainage.[7,10,12,20] While there have been no reports of permanent facial paresis or paralysis, transient facial paresis has been documented.[23] Uncommon symptoms involve recurrent afebrile seizures in a child,[24] and some cases are discovered incidentally.[8] The duration of symptoms varies widely, ranging from several weeks to 15 years, with one case being congenital.[15] Triantafyllou et al. suggested a potential hormonal dependence of DF and reported a case exhibiting rapid tumor proliferation during pregnancy.[40] In our case, the notable increase in the lesion during pregnancy further supports the potential hormonal influence on DF progression.
Imaging
Exploring the imaging features of DF within the cerebral cranium reveals a domain still awaiting widespread recognition due to the limited corpus of published studies. Radiographically, DF in the cerebral cranium typically manifests as a solitary, lytic lesion, occasionally with a mild sclerotic margin[17,20] or without a margin.[6,11,14] CT and MRI play key roles in determining the extent of bone destruction.[31]
On CT, DF in the cerebral cranium often presents as a well-defined, solitary, lytic lesion, and with or without diploic space expansion.[5,12,17,18,25] The outer and inner skull tables may show thinning or even cortical breakthrough,[16,21,24,26] sometimes creating a distinctive beveled edge due to uneven destruction of the inner and outer tables.[2] A central soft tissue with mild, heterogeneous enhancement is frequently observed.[13] Notably, these tumors can extend into the soft tissues of the scalp and underlie the dura.[15] In our case, a unique extracranial, heterogeneous hyper-dense lesion with a soft-tissue component based on the outer tables is evident, without involvement of the diploic space and inner tables, a pattern seldom reported in the literature.[28] A notable case by Dadlani et al. shares resemblance.[22] The extended 15-year history showed a giant sclerotic lesion with “sun ray” spicules and a substantial soft-tissue component, causing erosion of the diploic space and inner tables, ultimately inducing compression of the brain parenchyma.
MRI findings are less specific, with only 16 of the 31 cases providing MR data. However, these features exhibit considerable heterogeneity. Commonly described are intradiploic lytic masses with soft-tissue involvement. T1-weighted images usually depict hypointensity[9] or isointensity,[8,20] while T2-weighted images demonstrate heterogeneous hypointensity[12,14] to slightly hyperintensity.[8,16] Contrast-enhanced MRI reveals diverse enhancement patterns, including moderate enhancement[8] and homogeneous or heterogeneous significant enhancement.[9,12-14,17,24] Our case aligns with a CT appearance reported by Elbadawy et al. yet diverges in MR findings.[28] In our instance, the soft-tissue component appears slightly hypointense on T2-weighted images with heterogeneous progressive moderate enhancement. Notably, this contrasts with marked hypointensity on T2-weighted images without enhancement. Importantly, none of the previously reported cases have shown invasion into the brain parenchyma.
Given the non-specific imaging characteristics, radiological diagnosis alone is challenging, emphasizing the need for pathological confirmation.[6]
Treatment approaches and recurrence patterns
In an insightful exploration by Böhm et al.,[29] a meticulous investigation into DF recurrence rates following various treatment modalities was conducted. The outcomes underscore a notable disparity, revealing a higher recurrence rate (55%) in patients subjected to curettage. In stark contrast, patients undergoing resections exhibited a substantially lower recurrence rate (17%). Impressively, among those who underwent wide resections with a minimal follow-up of 3 years (mean 6.1 years), no recurrences were reported in 11 cases.
The findings strongly advocate for the indispensability of complete resection with a wide margin in averting recurrence, particularly when juxtaposed with DF occurrences in other anatomical sites. Within the purview of our review, surgical resection emerges as the predominant and favored treatment paradigm for nearly all patients. Follow-up durations ranged from 3 months to 6 years, revealing a reassuringly low recurrence incidence. Remarkably, only a singular patient exhibited recurrence merely 4 months post-surgery[24] in contrast to the comparatively elevated recurrence rates observed in DF affecting long bones.[41] In this exceptional case, the recurrent mass was successfully eradicated at 10 months postoperatively, with coagulation of the surrounding dura mater. Encouragingly, no signs of recurrence surfaced even 12 months after the second craniotomy.
Regarding the dura, prior cases in our review delineate a prevalent trend wherein most patients did not receive specific treatment for the dura,[1-9,11,13,16-23,26,27] with exceptions for four lesions where treatments included extensive dural resection and partial dural coagulation, electric and chemical cauterization, and dural excision and duroplasty.[10,12,14,15]
The realm of potential adjuvant therapies for DF remains underexplored, with endocrine therapy employing tamoxifen showing promise, particularly in young patients.[7] However, the broader efficacy of adjuvant therapies, encompassing radiotherapy or chemotherapy, remains inconclusive.[20] Notably, radiotherapy assumes significance as a plausible alternative therapy if en bloc resection is not feasible.[42]
CONCLUSION
DF of the cerebral cranium is a rare entity, with only 32 cases reported in the literature. This case is unique due to the rapid tumor growth observed during pregnancy, and the imaging features diverge from those commonly reported. Although rare, DF should be considered in the differential diagnosis of cranial lesions, particularly in pregnant women presenting with rapidly growing masses. Complete surgical resection with wide margins remains the preferred treatment to reduce the risk of recurrence.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Desmoplastic fibroma of frontal bone. Report of a case (author’s transl) Pathologica. 1978;70:575-9.
- [Google Scholar]
- Desmoplastic fibroma of parietal bone simulating eosinophilic granuloma. Case report. J Neurosurg. 1987;67:449-51.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital desmoplastic fibroma of the cranium. Childs Nerv Syst. 1988;4:45-6.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the skull: A case report. Otolaryngol Head Neck Surg. 1995;112:589-91.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the skull. Case report. J Neurosurg. 1995;82:119-20.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the skull. Case report and review of the literature. Neurochirurgie. 1997;43:260-4.
- [Google Scholar]
- Desmoplastic fibroma of the calvarium. J Clin Neurosci. 1998;5:102-5.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the temporal bone. J Laryngol Otol. 2000;114:314-7.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the skull; a case report, review of the literature, and therapeutic implications. J Korean Neurosurg Soc. 2001;30:1037-41.
- [Google Scholar]
- Desmoplastic fibroma of the cranium: Case report and review of the literature. Neurosurgery. 2003;52:950-4.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the pediatric skull. Report of three cases. J Neurosurg. 2005;103:362-5.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the skull in an infant. Childs Nerv Syst. 2006;22:176-81.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the frontal bone. Neurol India. 2006;54:314-5.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the skull. Acta Neurochir (Wien). 2008;150:285-90.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the skull in an infant. Neurochirurgie. 2011;57:39-41.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the cranium in a young man. J Korean Neurosurg Soc. 2012;52:561-3.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the pediatric cranium: Case report and review of the literature. Childs Nerv Syst. 2013;29:2311-5.
- [CrossRef] [PubMed] [Google Scholar]
- Giant calvarial desmoplastic fibroblastoma. J Clin Neurosci. 2014;21:696-9.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the temporal bone. Otol Neurotol. 2015;36:e119-20.
- [CrossRef] [PubMed] [Google Scholar]
- desmoplastic fibroma of the pediatric cranium: An aggressive skull tumor with local recurrence. Neurol Med Chir (Tokyo). 2016;56:85-8.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the temporal bone. Vestn Otorinolaringol. 2016;81:81-3.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the skull in an infant: A case report. Neurol Sci Neurosurg. 2020;1:108.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the temporal bone. Otolaryngol Case Rep. 2021;20:100326.
- [CrossRef] [Google Scholar]
- Desmoplastic fibroma of the occipital bone in adult female: A case report. Interdiscip Neurosurg Adv Tech Case Manag. 2021;24:101124.
- [CrossRef] [Google Scholar]
- Desmoplastic fibroma of the bone. A report of two patients. review of the literature and therapeutic implications. Cancer. 1996;78:1011-23.
- [CrossRef] [Google Scholar]
- Enzinger and weiss's soft tissue tumors (4th ed). St Louis: Mosby; 2001. p. :320-8.
- [Google Scholar]
- WHO classification of tumors In: Pathology and genetics of tumors of soft tissue and bone. Lyon: IARC Press; 2002. p. :288-432.
- [Google Scholar]
- Desmoplastic fibroma with perineural extension. AJR Am J Roentgenol. 2005;185:1498-9.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the mandible: A case without recurrence after enucleation. Cureus. 2023;15:e42213.
- [CrossRef] [Google Scholar]
- Desmoplastic fibroma of bone: Radiographic analysis. Radiology. 1989;172:827-32.
- [CrossRef] [PubMed] [Google Scholar]
- Natural evolution of desmoplastic fibroblastoma on magnetic resonance imaging: A case report. J Med Case Rep. 2011;5:139.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the jaw: A case report and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:82-94.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the mandible: A series of three cases and review of literature. Head Neck Pathol. 2015;9:196-204.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of bone: A report of 18 cases. Skeletal Radiol. 1994;23:283-8.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma of the mandible: Review and report of two cases. J Oral Maxillofac Surg. 1996;54:1249-54.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic (collagenous) fibroma of the femur: A case report and review of the literature. Oncol Lett. 2013;6:1285-8.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic fibroma: A role for radiotherapy? South Med J. 1995;88:1267-9.
- [CrossRef] [PubMed] [Google Scholar]






