Translate this page into:
Correlation of patient characteristics with peak enhancement time for pediatric cardiac computed tomography in congenital heart disease

*Corresponding author: Koteshwar Prakashini, Department of Radiodiagnosis and Imaging, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India. prakashini.k@manipal.edu
-
Received: ,
Accepted: ,
How to cite this article: Visakh T, Priya PS, Panakkal NC, Banga G, Prakashini K. Correlation of patient characteristics with peak enhancement time for pediatric cardiac computed tomography in congenital heart disease. 2024;14:50. J Clin Imaging Sci. doi: 10.25259/JCIS_153_2024
Abstract
Objectives:
Cardiac computed tomography (CCT) plays a key role in diagnosing congenital heart disease (CHD), emphasizing the need for specialized protocols in newborns due to CHD’s complexity. The aim is to explore the relationship between peak enhancement time (PET) and various parameters during CHD assessment with CCT.
Material and Methods:
The study involved 38 CHD patients undergoing CCT, with observations made on their heart rate, respiratory rate, saturation, cardiac output, weight, height, and age. The PET for each case was determined, and Spearman’s rank correlation was employed to evaluate the association between these parameters and the PET.
Results:
The median PET was 20.63 s, with a mean aortic Hounsfield unit of 512.16 ± 160.56. A moderate negative correlation was found between PET and both heart rates (r = −0.42, P = 0.009) and respiratory rates (r = −0.41, P = 0.01), whereas a negligible positive correlation (r = 0.19, P = 0.25) was observed with SpO2. A moderate positive correlation was noted between PET and both weight (r = 0.44, P = 0.005) and height (r = 0.40, P = 0.01). In addition, there were significant differences in median PET across different age categories.
Conclusion:
The findings conclude that PET during CCT is significantly associated with heart rate and respiratory rate. An increase in these rates corresponds to a notable decrease in PET. Consequently, the study recommends minimizing scan delays in CCT for patients exhibiting higher heart rates.
Keywords
Cardiac computed tomography
Congenital heart disease
Peak enhancement time
Cardiac parameters
INTRODUCTION
Computed tomography (CT) is becoming a rapidly growing imaging tool, which has become highly beneficial in the diagnosis of complex congenital heart disease (CCHD). Advancements in imaging technology allow good temporal resolution and improved spatial resolution, shorter duration of scan, multiplanar reconstruction techniques, and improved contrast. There is a dramatic increase in CT scans for diagnosis and follow-up of congenital heart disease (CHD).[1-3] It is a common malformation in children and the leading cause of death with a fatality rate of 19.23% in India.[4,5] Therefore, early detection of CHD is essential for initiating therapy that leads to prolonged survival of the patients. Although echocardiography is a widely used imaging tool to diagnose and follow-up assessment of CHD, it is insufficient for CCHD. Mainly due to limitations in accurately assessing distal pulmonary arteries, complex systemic anatomy, and cavopulmonary anastomoses also limited reproducibility in quantifying single or right ventricular size and function.[1,2,6]
CT is beneficial in terms of visualizing anatomy in 3D volume along with its relative structures. It will help a surgeon while planning surgical intervention.[1-3] However, the related radiation exposure and the usage of iodinated contrast media are a cause of concern. This issue is of more concern while doing pediatric scans because of their higher radiosensitivity and potential biological effects in the future.[1] Furthermore, there are studies that report a 10.3% incidence of contrast agent-induced nephrotoxicity in pediatrics. Therefore, to obtain maximum diagnostic information during a CT scan, we should take into account the difference in anatomy and physiology between adult and pediatric patients. Rapid circulation in pediatric patients needs dedicated protocol while administering contrast media to attain the peak and homogeneous enhancement.[7] The time should be defined after considering patient-specific cardiovascular and technical parameters to get peak enhancement. Most of the available literature correlates peak enhancement time (PET) with cardiac parameters in adults. As per our knowledge, no studies have been done to correlate the features of pediatric participants with PET, especially in patients with CHD. Heart defects can influence the cardiac output which in turn affects the contrast opacification timing.[3] Therefore, keeping this in mind, the study aims to correlate parameters such as heart rate, respiratory rate, cardiac output, weight, height, and age with PET.
MATERIAL AND METHODS
Study population
The prospective study was conducted in a tertiary care hospital in South India from 2021 to 2022. The study was approved by the Institutional Ethics Committee (IEC688/2020). The informed consent was obtained from the parent or guardian. The study comprised 38 participants, younger than 5 years of age with CHD referred for CT cardiac scan.
Cardiac CT (CCT)
All the scans were acquired in a Philips 128 slice incisive CT Scanner with post-processing software ISP (Intelli Space Portal, Version: 10.1.3.32808). All the patients followed a free breathing protocol and scans ranged from the apex of the lung to the liver in the craniocaudal direction. Technical parameters used for the study were 80 kVp, 70–170 mAs, pitch of 1, rotation time: 0.40 s, and collimation of 64 × 0.625.
Contrast media
Iodinated contrast media (Iopromide 300 mgI/mL) were injected through the peripheral vein using a double-headed power injector (MEDRAD Stellant) with a volume of 2 mL/kg followed by a saline chaser of 5–10 mL. The flow rates were based on age; pediatric patients below the age of 1 month were given at 0.8 mL/s, those above 1 month to 1 year were 1 mL/s, and those above 1 year were 1.5 mL/s. Visual bolus was used to trigger the scan. Post-threshold delay for each scan was decided by a radiologist based on the indication and ranged from 10 to 12 s from the injection of the contrast.
Clinical data
All the clinical data such as age, gender, weight, indication, cardiac output, heart rate, and echo findings were collected and tabulated. PET was calculated from the time of injection to the time of image acquisition after observing visual bolus at the level of the descending aorta and the image was processed to find a Hounsfield unit (HU) after drawing a circular region of interest in the descending aorta using CT application in IntelliSpacePortal (ISP). The PET and HU value was then correlated with cardiac parameters. The collected data were categorized into two groups. Any CHD case affecting the pulmonary artery or right ventricular outflow tract was classified as a right-sided pathology. Conversely, any CHD case without involvement of the pulmonary artery or right ventricular outflow tract was classified as left-sided pathology.
Statistical analysis
All statistical tests were performed using the Statistical Package for the Social Sciences 16. The time to peak enhancement and HU for the aorta was summarized using descriptive statistics. Data that did not follow the normality assumption were summarized using median and interquartile range (IQR) (Q1, Q3) and which followed the normality assumption was summarized using mean and standard deviation. Spearman’s rank correlation analysis was used to determine the relationship between PET with heart rate, cardiac output, SpO2, respiratory rate, weight, height, and contrast volume. Kruskal–Wallis test was done to compare the median PET across different age categories.
RESULTS
Among the 38 participants, there was an equal distribution of males and females with ages ranging from 1 day to 3 years. The mean weight was found to be 5627 ± 3197 g and the median height was 59.5 (48.74) cm.
The median PET was found to be 20.63 s (IQR: 14–40) with a mean aortic HU of 512.16 ± 160.56.
Correlation of PET with cardiac parameters
The study reported a moderately negative correlation between PET with heart rate (r = −0.42, P = 0.009) and respiratory rate (r = −0.41, P = 0.01), respectively. However, there was a negligible positive correlation between SpO2 and PET (r = 0.19, P = 0.25). The scatter plots for the various cardiac parameters are demonstrated in Figures 1 and 2.
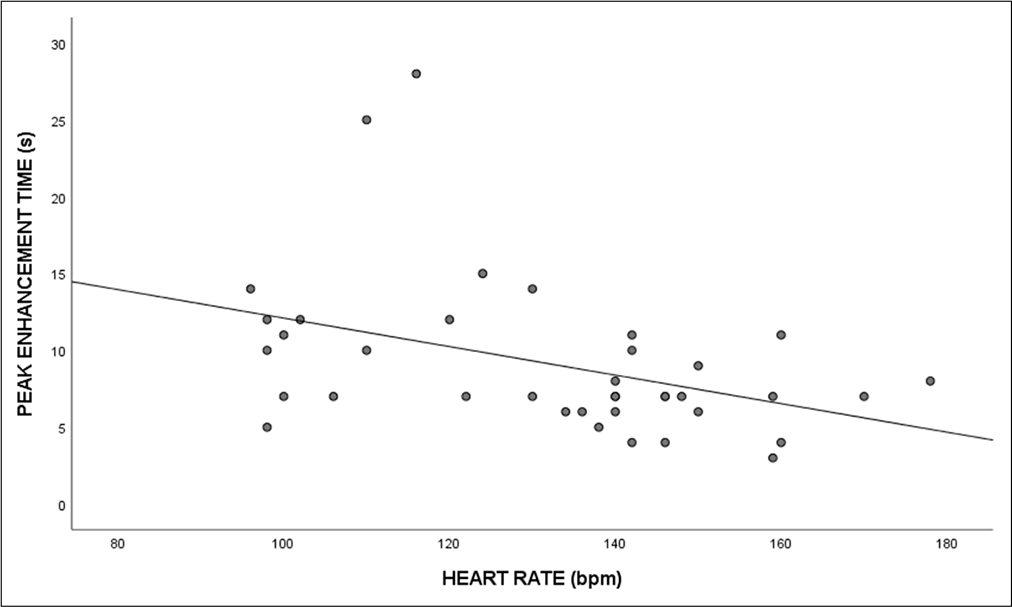
- Scatterplot depicting the correlation between peak enhancement time and heart rate.
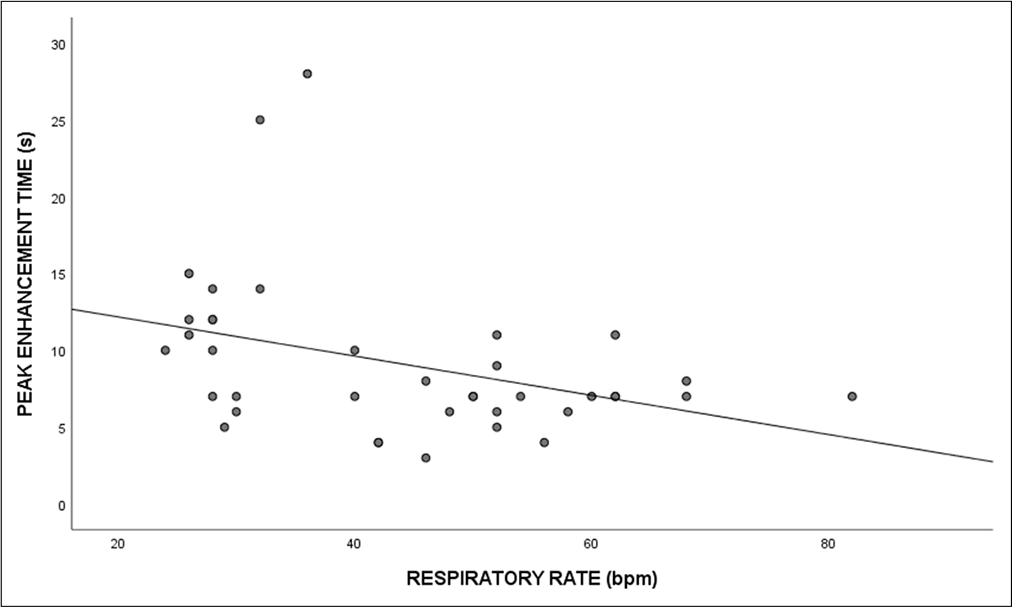
- Scatterplot depicting the correlation between peak enhancement time and respiratory rate.
The correlation of PET with cardiac output of the right ventricle (RV) and left ventricle (LV) is shown in Table 1.
| Peak enhancement time | Spearman’s rank correlation coefficient (rs) | 95% Confidence interval |
P-value |
|---|---|---|---|
| Cardiac output right | 0.18 | (−0.48, 0.71) | 0.61 |
| ventricle (n=11) | |||
| Cardiac output left | −0.25 | (−0.73, 0.39) | 0.43 |
| ventricle (n=12) |
An increase in right ventricle (RV) cardiac output was associated with a decrease in PET, whereas an increase in left ventricle (LV) cardiac output was associated with an increase in PET. However, the correlations were not statistically significant.
Correlation of PET with patient-related parameters
A moderate positive correlation was observed between PET and weight (r = 0.44, P = 0.005). Similarly, a moderate positive correlation was found between PET and height (r = 0.40, P = 0.01).
A Kruskal–Walli’s test revealed significant differences in median PET across different age categories as shown in Table 2.
| Age categories | Peak enhancement time in seconds (Median and interquartile range) |
|---|---|
| Categories 1: Below | 7 (6.8) |
| 6 months (n=21) | |
| Categories 2: 6–3 years (n=13) | 10 (7.12) |
| Categories 3: More | 13 (9.15) |
| than 3 years (n=4) |
The median PET was found to be significantly different between different age categories, P = 0.025. In the post hoc comparison adjusted for Bonferroni corrections, a significant difference was observed in the PET between age categories 1 and 2 (P = 0.04) and 1 and 3 (P = 0.02).
DISCUSSION
CT protocol of CHD should be adequately planned before the acquisition as multiple factors affect the protocol. Most of the complex diseases will have altered physiology of flow dynamics. Patient parameters and technical parameters need to be selected to get good contrast opacification. The present study correlated the average PET with clinical parameters such as heart rate, saturation, and respiratory rate and patient-related parameters such as height, weight, and age group.
The ability to predict the PET before scanning can help in adjusting the duration of contrast injection and the optimization of the dose. Our study reported a negative correlation between heart rate and PET (r = −0.42), meaning PET decreased with an increase in heart rate. This observation leads to adjusting the delay time after the heart rate of the patient is obtained. It is very important to note that the heart rate of newborns will have a value of 120–190 bpm compared to infants who will have 70–100 bpm, whereas in the pediatric group, heart rate varies from 70 to 190 bpm depending on their age requiring delay time to be adjusted accordingly. Since the decrease in heart rate leads to an increase in enhancement time, time delay must be reduced in neonates compared to infants.[8,9] Tang et al. demonstrated that the delay time and density can be predicted by factors such as heart rate and reported a negative correlation between delay time and heart rate. While the study conducted by Tang et al. included adults, our population consisted of neonates, infants, and children whose heart rate is generally higher than adults (60–100 bpm). A higher heart rate results in a higher flow rate which in turn results in immediate contrast washout from the circulation. Moreover, we included children with CHD for our study, where there could be potential changes in the hemodynamics of each patient depending on the complexity of their disease.
Similarly, we noticed a small positive correlation of saturation with PET (r = 0.19). So, higher the saturation level, longer will be the PET. There was a negative relationship between PET and respiratory rate (r = −0.41).
Patient-specific factors such as height and weight also had an influence on the PET. We noticed a positive correlation between weight and height with PET among the participants. PET increased with an increase in height (r = 0.40) and weight (0.44) of the participants. This reading is consistent with the findings of Tang et al. The study reported a positive trend with these patient-related factors in CCT and the delay time with the height and weight of the patient.[10]
PET varied across different age groups. Since our patient population was children, we divided the age group as below 6 months, 6 months to 3 years, and more than 3 years. We noticed that PET increased with increasing age. The PET of the group below 6 months, 6 months to 3 years, and above 3 years was 7, 10, and 13 s subsequently. There were some limitations noted while conducting the study. First, the sample size for the study was small. As it was a time-bound study, we could only incorporate 38 participants for the study. Second, we could not determine the cardiac output time in all the cases due to no previous echo reports. Furthermore, the study included patients who had various types of CHD. Therefore, it was not feasible to study this variation in detail as the number of cases in each type was less in number to statistically analyze. In light of this, the present study recommends that each type of CHD should be evaluated independently to determine whether there is a correlation between PET and cardiac parameters.
CONCLUSION
PET during CCT is significantly correlated with heart rate and respiratory rate. A significant decrease in PET is associated with an increase in these rates. In light of the study’s findings, CCT scan delays should be minimized in patients exhibiting higher heart rates. The knowledge of the effect of these patient-specific factors can decide while imaging cardiac cases with respect to contrast dosage, volume, duration of injection, and flow rate in CHD.
Ethical approval
The study was approved by the Institutional Ethics Committee, number IEC688/2020, dated January 21, 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- A prospective evaluation of contrast and radiation dose and image quality in cardiac CT in children with complex congenital heart disease using low-concentration iodinated contrast agent and low tube voltage and current. Br J Radiol. 2017;90:20160669.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular CT angiography in neonates and children: Image quality and potential for radiation dose reduction with iterative image reconstruction techniques. Eur Radiol. 2013;23:1306-15.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in cardiac CT contrast injection and acquisition protocols. Cardiovasc Diagn Ther. 2017;7:439-51.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiac CT in the preoperative diagnostics of neonates with congenital heart disease: Radiation dose optimization by omitting test bolus or bolus tracking. Acad Radiol. 2020;27:e102-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence, profile, and pattern of congenital heart disease in Central India : A prospective, observational study. Niger J Cardiol. 2018;15:45-9.
- [CrossRef] [Google Scholar]
- Computed tomography imaging in patients with congenital heart disease part I: Rationale and utility. An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT). Endorsed by the Society of Pediatric Radiology (SPR) and the North American Society of Cardiac Imaging (NASCI) J Cardiovasc Comput Tomogr. 2015;9:475-92.
- [CrossRef] [PubMed] [Google Scholar]
- Chest CTA in children younger than two years-a retrospective comparison of three contrast injection protocols. Sci Rep. 2019;9:18109.
- [CrossRef] [PubMed] [Google Scholar]
- Heart rate during the first 24 hours in term-born infants. Arch Dis Child Fetal Neonatal Ed. 2021;106:489-93.
- [CrossRef] [PubMed] [Google Scholar]
- Relating normal pulse rate with dandruff in hair cells. Int J Res Stud Biosci. 2019;7:24-5.
- [CrossRef] [Google Scholar]
- Factors influencing delay time and coronary arterial density during coronary angiography with DSCT. Acta Radiol. 2011;52:59-63.
- [CrossRef] [PubMed] [Google Scholar]







