Translate this page into:
Anatomical Variations in the Circulus Arteriosus Cerebri with Clinical Importance – Results of an Magnetic Resonance Angiography Study and Review of Literature

*Correspondence author: Sefedin Mucaj, National Institute of Public Health of Kosovo, Prishtina, Kosovo, Albania. sefedin.mucaj@uni-pr.edu
-
Received: ,
Accepted: ,
How to cite this article: Shatri J, Bexheti S, Shatri M, Kabashi A, Mucaj S. Anatomical variations in the circulus arteriosus cerebri with clinical importance – Results of an magnetic resonance angiography study and review of literature. J Clin Imaging Sci 2021;11:8.
Abstract
Objectives:
Anatomy of circulus arteriosus cerebri (CAC) shows wide variation in different individuals, and population groups and has vital clinical significance in causation and presentation of clinical disease. The literature revealed a connection between the variations of CAC and cerebrovascular disease, ischemia, stroke, aneurysms, and atherosclerosis.
Material and Methods:
In this study, 513 patients without clinical manifestation in regard to cerebrovascular diseases, who are considered healthy on CAC anatomy, are included. Patients were instructed by clinicians for head imagery with magnetic resonance angiography examination during 2016–2017 periods.
Results:
After statistical analysis, 43.27% were male while 56.72% female, 39% were younger than 40 years old. Age interval lies from 11 to 84 years old, mean age 46. The most common variations or 9.74% is when communicant anterior artery absence and absence of both posterior communicant arteries (Type G*/E) more rarely is H*/G (0.2%), G*/D (1.75%), G*/G (0.6%), H*/D (0.4%), H*/E (3.39%), H*/H (0.4%), J*/E (0.6%), while combination J*/D, J*/G, J*/H, G*/H not found. The most often combination is absence of anterior communicant artery and absence of both posterior communicant artery (Type G*/E), more in male 10.36% than female 9.6%.
Conclusion:
The CAC is considered to play a critical role in preventing future stroke events in patients with absent of any of the arteries. Knowledge on variations in arteries forming the CAC is with clinical significance, as it is one of the components of CAC which stabilizes cerebral blood flow when principle conduits fail. Knowing the structure of arteries provide clinical knowledge to the surgeons before planning neurovascular surgeries.
Keywords
Circulus arteriosus cerebri
Clinical importance
Variations of circulus arteriosus cerebri
Cerebral artery
Interventional of cerebral artery
INTRODUCTION
The circulus arteriosus cerebri (CAC) distributes oxygenated blood in whole brain and it represents the largest and the only brain anastomosis. Even though it was recognized by many researchers, it was Thomas Willis with his detailed description to recognize CAC function as a compensation mechanism when internal carotid artery (ICA) is stenosed or totally closed. CAC can be found at all people, with different morphological variations. The typical CAC with its components behaves as a closed circle, in which blood can flow from every entrance and still get back to the same one. Extra-cranial arterial wall consists of three tunicas, namely, tunica intimae, tunica media, and tunica adventitia. Tunica intima is comprised a single endothelial cell layer which creates a continual barrier between the arterial wall and flowing blood.
Tunica media is the thickest layer of arterial wall composed of smooth musculature, elastic fibers, and extracellular matrix. External elastic lamina separates tunica media from the adventitia which is made up of adipocytes and connective tissue. Nerves and vessels (vasa vasorum) penetrate the adventitia and can stretch to the media. Intracranial arteries differ morphologically from extra-cranial arteries.
Intracranial arteries do not have external elastic membrane, but there is a subtitle tunica intimae though. Tunica media and adventitia are poor in elastic fibers compared to extra-cranial arteries with same diameter.
ICA and basilar artery (BA) carry blood to the CAC; therefore, they are considered as afferent arteries, while their branches are considered efferent arteries.
According to Terminologia Anatomica (TA-1988), ICA is parted in four segments: (1) The cervical segment, (2) the petrouse segment, (3) the cavernouse segment, and (4) the cerebal segment. Meanwhile, in February 2017, Federative International Programme for Anatomical Terminology proposed the neuroanatomical terminology, thus, based on Terminologia Neuroanatomica, ICA is comprised seven segments: Pars cervicalis (segmentum C1), pars petrosa (segmentum C2), pars lacera (segmentum C3), pars cavernosa (segmentum C4), pars clinoidea (segmentum C5), and pars ophtalmica (segmentum C6) dhe pars communicans (segmentum C7).[1]
While ICA vascularize major parts of the brain, the posterior part of brain hemisphere receives blood supply by vertebrobasilar system, including here the posterior part of lobus occipitalis and lobus temporalis, cerebellum, and truncus cerebri. The forming arteries of circulus arterious cerebri vary in diameter and length, and often, they are not well formed or are absent at all. Sometimes, the pars precommunicalis diameter is smaller than pars post-communicalis diameter. Except the altered diameter, arteries may differ in origin and atypical configuration. Thus, may variations are described in arteries which form the CAC as a consequence of anomalies such as absensce of artery, division, doubling, tripling, hypoplasia, or secondary arteries.
Hereby, CAC variations are divided in anterior part and posterior part variations. Each of ICA supplies 2/5 of the brain, while the vertebrobasilar system only 1/5 of the common brainstem. Regardless of the smaller size, the backbone includes the truncated trunk, very critical median line structure without which consciousness, motion and sensitivity cannot be stored.[2]
Cerebrovascular diseases represent, which are one of the main health issues nowadays. These events cause high mortality rates or to those who survive the cerebrovascular event (transient ischemic attack [TIA], brain ischemic attack, or apoplexia) are accompanied with pyramidal and extrapyramidal system disorder.[3]
Being a very well vascularized organ, brain utilizes 20% of total blood circulation or 25% of oxygen during one cycle of breathing. As a matter, brain blood circulation is 60–100 ml/100 g. of brain tissue per minute. If blood flow drops below 15 ml/100 g/min, oxygen tissue level drops markedly and anaerob metabolism is required to generate energy. This non-efficient metabolism causes lactic acid levels to rise, which is very toxic for brain tissue. Neurons only do aerobe respiration and for this reason, they are dependent to uninterrupted metabolic substrate flow.[4] Therefore, collateral circulation is the main security factor which protects brain tissue from the occlusion or total closure of one or more major arteries. The most common arteries to be affected by occlusion is ICA mainly in cervical segment. After the narrowing or closure, collateral circulation is possible by the opposite carotide system, through anterior communicating artery anterior or from the vertebrobasilar system through ipsilateral posterior communicating artery posterior or from both arteries. A TIA is defined as a reversible episode of neurologic deficit caused by blood vessel insuffiency which can last from 5 to 30 min, but it can persist for 24 h or rarely even for days (the longer lasting attack, higher the possibility of tissue destruction).
Variations in CAC affect blood flow and thus increase susceptibility to vascular disease.[5,6] Special clinical importance variations can be considered: In anterior part absence of anterior communicating artery (AComA) (Type G), absence of anterior cerebral artery-A1 (ACA-A1) segment of one side (Type H), and AComA agenesis; meanwhile, middle cerebral artery (MCA) flows as two branches (Type J). In posterior part, absence of posterior communicating artery (PComA) of one side while the other side is present (Type D), PComA absence of both sides (Type E), PComA absence together with fetal-type of PCA (fPCA) with hypoplastic P1 segment of PCA of the same side (Type G) and fPComA one side, and P1 absence of the same side accompanied by PComA absence of the opposite side or variation absence (Type H) [Figure 1].[7] These variations interrupt communication between anterior and posterior parts and also left and right side. When these variations happen to be combined and appear to the same person, the risk is higher, concretely speaking, in case of ICA or BA occlusion because there is no blood flow in occlusion side and as a consequence no blood flow circulates in that brain territory.
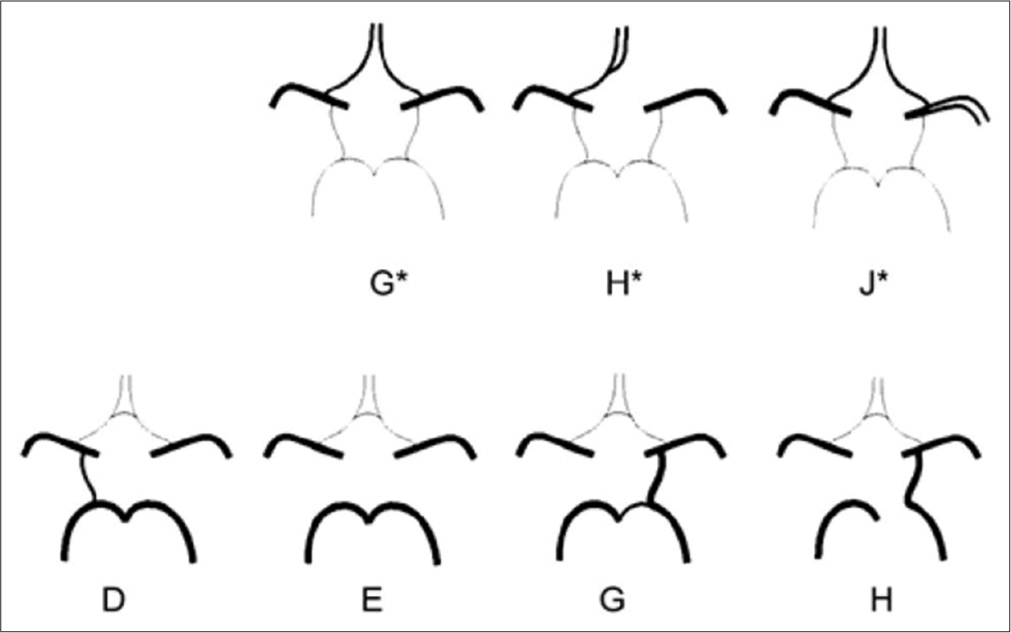
- Schematic view of anatomic variations of anterior and posterior part. (G*) Hypoplasia or absence of an anterior communication. (H*) One pre-communicating segment of an anterior cerebral artery-A1 (ACA) is hypoplastic or absent, the other pre-communicating segment gives rise to both post-communicating segments of the ACAs. (J*) Hypoplasia or absence of an anterior communication. (D) Unilateral PComA present. (E) Hypoplasia or absence of both posterior communicating artery (PComA) and isolation of the anterior and posterior parts of the circle at this level. (G) Unilateral fetal-type posterior cerebral artery and hypoplasia or absence of the contralateral PComA. (H) Unilateral fetal-type posterior cerebral artery and hypoplasia or absence of both pre-communicating segments of the posterior cerebral artery and the PComA.
Clinical usage of transcranial doppler (TCD), magnetic resonance angiography (MRA), and computerized tomography angiography (CTA) provide detailed information of blood circulation on CAC vivo. In the recent years, TCD and MRA have developed greatly hence, which have proven to be the two most valuable non-invasive techniques on intracranial hemodynamic blood circulation evaluation.[8-13]
Furthermore, there is a possible connection between CAC anomalies and patients with psychiatric and cerebrovascular disorders.[14,15] Likewise, recognizing anatomic variations have impact on diagnosing and managing subarachnoid hemorrhage.
MATERIAL AND METHODS
In this study, 513 patients without clinical manifestation in regard to cerebrovascular diseases, who are considered healthy on CAC anatomy, are included in the study. Patients who were instructed by clinicians for head imagery with MR underwent three-dimensional time of flight (3D-TOF) and MRA examination during 2016–2017 periods. This cross-sectional study was realized using analytic and observatory techniques. MR scanner 1.5T Siemens Symphony at the Neuro-Radiologic Department at Clinic of Radiology, in University Clinical Centre of Kosovo was used, with the parameters which are shown in Table 1. Even though angiography with MR cannot discover any artery which is invisible with conventional angiography, it offers 89.2% sensitivity for AComA and 81.3% for PComA, while 100% for ACA-A1, PCA-P1, and MCA. Based on great attributes of MRA and taking into consideration patient’s category that were included on my thesis dissertation, this method was ideal for studying variations of CAC in Republic of Kosovo. Arteries diameter measurement was done 5 mm from the artery origin.
| 3D-TOF | |
| TR | 30–40 m/sc |
| TE | 6–10 m/sc |
| FA | 20–25 |
| Thickness | 0.8 mm |
| Number | 96 |
| Voxel (mm) | 0.8×0.8×0.8–1.0 |
3D-TOF: Three-dimensional time of flight
At first sight MRA offers similar morphologic information as does conventional angiography, but the principles of getting images are totally different. Due to non-invasive nature, MRA made it possible to study CAC in vivo in healthy individuals, as well as in patients; thus, avoiding risks which are linked to conventional angiography as is thromboembolism which results from intra-arterial catheter maneuvering, pseudo aneurisms, allergic reactions against contrast used, artery dissection, and hematoma formation at wound place. CTA uses X-ray to produce images and intravenous contrast in large amount should be used to visualize the arteries, which means only patients with vessel pathological changes would be examined, which is not our aim of study.
3D-TOF technique creates the image based on blood flow inside the blood vessel differing it from static structure of organism, in our case presenting brain as hyper-signal, and post-processive procedures 3D shapes of blood vessels are created.
TOF technique makes the difference between stationary tissue and blood flow while maneuvering magnetizing levels.
RESULTS
After statistical analysis, 43.27% (n = 222) patients were male, while 56.72% (n = 291) were female [Table 2].
| Age-group | Female nr. (%) | Male nr. (%) | Total (%) |
|---|---|---|---|
| under 20 years | 22 (7.5 ) | 15 (6.7) | 37 (7.1) |
| 21–30 | 45 (15.5) | 29 (13) | 74 (14.4) |
| 31–40 | 51 (17.5) | 44 (19.8) | 95 (18.5) |
| 41–50 | 62 (21.3) | 35 (15.8) | 97 (18.9) |
| 51–60 | 46 (15.8) | 39 (17.6) | 85 (16.6) |
| 61–70 | 41 (14.1) | 40 (18) | 81 (15.8) |
| above 70 years | 24 (8.3) | 20 (9) | 44 (8.6) |
| Total | 291 (100) | 222 (100) | 513 (100) |
Meanwhile, distribution of patients in regard of age groups and gender is shown in Table 3. As it is described in introduction, hypoplasia or absence of pars pre-communicating of (ACA-A1), Type H and AComA, Type G, and J interrupt blood flow circulation between left and right side. Furthermore, absence of PComA (type D, E, G and H) interrupt the circulation between anterior and posterior parts of CAC.
| G* (%) | H* (%) | J* (%) | |
|---|---|---|---|
| D | 4 (1.37) | 1 (0.34) | - |
| E | 28 (9.6) | 8 (2.75) | - |
| G | 2 (0.68) | 1 (0.34) | - |
| H | 1 (.34) | 2 (0.7) | - |
If combination of these appears to the same person, and it come ICA or vertebra-basilar system occlusion, CAC compensation ability will have clinical damage consequences, including here TIA or brain ischemic attack.
In Table 4, it can be seen that 90 patients of the total number (n = 513) coincide with these combinations described above.
| G* (%) | H* (%) | J* (%) | |
|---|---|---|---|
| D | 9 (1.75) | 2 (0.4) | - |
| E | 50 (9.74) | 20 (3.39) | 3 (0.6) |
| G | 3 (0.6) | 1 (0.2) | - |
| H | - | 2 (0.4) | - |
In Table 3, it can be seen the variations type of anterior and posterior with high clinical importance in females.
Table 5 shows variations type of anterior and posterior with high clinical importance in males, Type G*/E is common than others variations.
| G* (%) | H* (%) | J* (%) | |
|---|---|---|---|
| D | 5 (2.25) | 1 (0.45) | - |
| E | 23 (10.36) | 11 (4.95) | 3 (1.35) |
| G | - | - | - |
| H | - | - | - |
The most common variations or 9.74% is when communicant anterior artery absence and absence of both posterior communicant arteries (Type G*/E), while the more rarely is H*/G while not found J*/D, J*/G, J*/H, G*/H [Figures 2-6].

- Type G* – (a and b) show absence of anterior communicating artery of anterior part of circulus arteriosus cerebri (blue arrows).

- Type H* – (a-d) show absence of anterior cerebral artery-A1 of anterior part of circlus arteriosus cerebri (blue arrows).

- Type D – absence of posterior communicating artery of one side while the other side of posterior part of circulus arteriosus cerebri (blue arrows).

- Type E – (a-c) show absence of both sides posterior communicating artery of posterior part of circulus arteriosus cerebri (blue arrows).

- Type H – (a-d) show P1 absence of the same side accompanied by posterior communicating artery absence of the opposite side or variation absence of posterior part of circulus arteriosus cerebri (blue arrows).
After analyzing Tables 3 and 5, we can see that these variations occur more often in female, 9.74% versus 9.35% to occur in males. The most often combination is absence of AComA and absence of both PComA (Type G*/E), more in male 10.36% than female 9.6%.
DISCUSSION
The cerebral arteries are derived from the internal carotid and vertebral arteries. The three trunks which together supply each cerebral hemisphere arise from the CAC. From anterior part of CAC proceed the two anterior cerebral arteries, from antero-lateral parts the middle cerebral arteries and from posterior part the posterior cerebral arteries.[16,17] These principal arteries give origin to two different systems of secondary vessels. They are ganglionic system of vessels to supply the thalami and corpus striatum and the cortical system of vessels which are ramify in the pia mater and supply the cortex and subjacent brain substance.[18] These two systems do not communicate at any point of their peripheral distribution, but are entirely independent of each other. The parts supplied by the two systems are borderland of diminished nutritive activity, where softening is especially liable to occur in the brains of old people.[19,20]
The AComA is the artery which completes the CAC anteriorly, by connecting two internal carotid arterial system. Although PComA is a smaller branch of the ICA, it gives the main contribution in the formation of CAC by communicating with the internal carotid arterial system and the vertebrobasilar arterial system. AComA is supposed to be a short bridging vessel between 2 ACAs of equal diameter. It is grouped as simple and complex type on the basis of its number and morphology. The AComAs were absent in a small proportion of people, in such cases, ACAs from two sides fused to avoid the necessity of an AComA.[21] Marinković et al. reported that A1 of 2 sides fused to replace the AComA in 4.5%.[22] In normal circumstances, occipital lobes of cerebrum enjoy nutrition both from vertebrobasilar and internal carotid system of arteries. In different morphological variations, the situation changes. If aplasia of PComA or P1 segment, blood supply to the occipital pole retains from a single source. In such cases, collateral circulation lacks. Blood supply may get hampered due to hemodynamic factors affecting vessel wall to change in the development of aneurysm or occlusion.[23] Some workers considered the circle to be complete, even when a normal AComA was absent and when the anterior cerebral arteries were joined together. The variation in absent AComA and PComA in the present study was 9.74%, more in male 10.36% than female 9.6%. However, some evidences suggest that PComA hypoplasia per se predisposes to thalamic lacunar stroke due to the critical role of PComA in the collateral supply of proximal PCA territory.[24]
The future development of collateral circulation followed by the progression of intracranial atherosclerotic stenosis appears to play a critical role in determining patient outcomes. If intracranial atherosclerotic stenosis progresses continuously, there are two ways to compensate for the reduced blood flow. One involves direct collateral flows through the CAC (antegrade collaterals) and the other involves leptomeningeal collaterals (retrograde collaterals). Leptomeningeal collaterals to offset the large arterial territory may require flow augmentation, which can be achieved by systemic hypertension or with additional flows through the CAC from other arterial territories. In other words, the CAC can be a route for antegrade collaterals and a route for blood flow augmentation as supplied by retrograde collaterals, which is why we designed CAC scoring system.[25] Hypertension may have two roles in intracranial atherosclerotic stenosis: One is an atherosclerotic risk factor and the other is a physiologic response to maintain cerebral perfusion. If a patient has good CAC integrity, hypertension may be an atherosclerotic risk factor rather than a physiologic response. In such a situation, hypertension needs to be controlled. However, if a patient has poor CAC integrity, hypertension may be a physiologic response to augment blood flow through leptomeningeal collaterals, and maintaining a high blood pressure can be a reasonable treatment.[26,27]
CONCLUSION
From the present study, we can conclude that large numbers of variations were seen in hypoplasia or absent of AComA and PComA (Type G*/H). The CAC is considered to play a critical role in preventing future stroke events in patients with absent of any of the arteries in CAC. Anatomical variations of CAC and its constituent vessels are important in clinical and surgical approaches. Magnetic resonance imaging angiography is useful in diagnosis of cerebrovascular diseases.[28] Preliminary anatomical knowledge and incidences of vascular variations of brain help us as future prospective in understanding vascular pattern. Thus, knowing the structure of arteries provide clinical knowledge to the surgeons before planning neurovascular surgeries.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Terminologia anatomica: International anatomical terminology and terminologia histologica: International terms for human cytology and histology. J Anat. 2009;215:221.
- [CrossRef] [Google Scholar]
- Posterior Circulation Cerebrovascular Syndromes-UpToDate. 1996. United Kingdom: Blackwell; Available from: https://www.uptodate.com/contents/posterior-circulation-cerebrovascular-syndromes [Last accessed on 2018 Mar 02]
- [Google Scholar]
- Angiography analysis of variations of the posterior segment of the circle of willis. Bosn J Basic Med Sci. 2005;5:30-4.
- [CrossRef] [PubMed] [Google Scholar]
- Shape optimization in steady blood flow: A numerical study of non-Newtonian effects. Comput Methods Biomech Biomed Engin. 2005;8:127-37.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between variations in the circle of Willis and flow rates in internal carotid and basilar arteries determined by means of magnetic resonance imaging with semiautomated lumen segmentation: Reference data from 125 healthy volunteers. AJNR Am J Neuroradiol. 2006;27:1770-5.
- [Google Scholar]
- Hemodynamics of cerebral aneurysms. Annu Rev Fluid Mech. 2009;41:91-107.
- [CrossRef] [PubMed] [Google Scholar]
- Arterial variations in man In: Classification and Frequency. Munich, Germany: Bergmann, Springer; 1985.
- [CrossRef] [Google Scholar]
- Intracranial stenoses and occlusions, and circle of willis collaterals. Front Neurol Neurosci. 2006;21:117-26.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of the collateral function of the circle of Willis: Three-dimensional time-of-flight MR angiography compared with transcranial color-coded duplex sonography. Am J Neuroradiol. 2003;24:456-62.
- [Google Scholar]
- MR imaging of the human brain at 1.5 T: Regional variations in transverse relaxation rates in the cerebral cortex. AJNR Am J Neuroradiol. 2001;22:1732-7.
- [Google Scholar]
- Incompleteness of the circle of Willis is related to EEG-based shunting during carotid endarterectomy. Eur J Vasc Endovasc Surg. 2013;46:631-7.
- [CrossRef] [PubMed] [Google Scholar]
- The anatomy of circulus arteriosus cerebri (circle of willis): A study in Turkish population. Turk Neurosurg. 2016;26:54-61.
- [CrossRef] [Google Scholar]
- Normal variants of the cerebral circulation at multidetector CT angiography. Radiographics. 2009;29:1027-43.
- [CrossRef] [PubMed] [Google Scholar]
- Observations on the length and diameter of vessels forming the circle of Willis. J Anat. 1981;133:419-23.
- [Google Scholar]
- Anomalies of the encephalic arteries among the insane. A study of the arteries at the base of the encephallon in two hundred and twenty consecutive cases of mental disease, with special reference to anomalies of the circle of Willis. J Comp Neurol Psychol. 1907;17:493-517.
- [CrossRef] [Google Scholar]
- Cortical blood vessels of the human brain. Brain Res Bull. 1981;7:519-79.
- [CrossRef] [Google Scholar]
- On the mode of entry of blood vessels into the cerebral cortex. J Anat. 1970;106:507-20.
- [Google Scholar]
- A functional perspective on the embryology and anatomy of the cerebral blood supply. J Stroke. 2015;17:144-58.
- [CrossRef] [PubMed] [Google Scholar]
- Anterior cerebral artery angioplasty for intracranial atherosclerosis. J Vasc Interv Neurol. 2008;1:16-8.
- [Google Scholar]
- Absent anterior communicating artery and varied distribution of anterior cerebral artery. Neurosciences (Riyadh). 2008;13:441-4.
- [Google Scholar]
- Branches of the anterior communicating artery, Microsurgical anatomy. Acta Neurochir (Wien). 1990;106:78-85.
- [CrossRef] [PubMed] [Google Scholar]
- Variations of anterior cerebral artery in human cadavers. Neurol Asia. 2013;18:249-59.
- [Google Scholar]
- Posterior communicating artery hypoplasia as a risk factor for acute ischemic stroke in the absence of carotid artery occlusion. J Clin Neurosci. 2008;15:1376-81.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical significance of the circle of Willis in intracranial atherosclerotic stenosis. J Neurointerv Surg. 2016;8:251-5.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation. 2007;115:2969-75.
- [CrossRef] [PubMed] [Google Scholar]
- Circle of Willis: Morphologic variation on three-dimensional time-of-flight MR angiograms. Radiology. 1998;207:103-11.
- [CrossRef] [PubMed] [Google Scholar]
- Review on anatomy of cerebral arterial system-clinical importance. J Clin Biomed Sci. 2014;4:305-8.
- [Google Scholar]







