Accuracy of Routine Clinical Ultrasound for Staging of Liver Fibrosis
Address for correspondence: Dr. Sudhakar K. Venkatesh, Department of Diagnostic Radiology, National University Hospital, 5 Lower Kent Ridge Road, Singapore 119074, Singapore. E-mail: venkatesh.sudhakar@mayo.edu
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
To determine the diagnostic accuracy of routine clinical ultrasound in the staging of liver fibrosis in chronic viral hepatitis.
Materials and Methods:
A retrospective evaluation of the ultrasound images of 156 patients with chronic viral hepatitis who underwent liver biopsy was performed. Two radiologists in consensus, blind to the biopsy results and clinical details, evaluated the ultrasound images for liver fibrosis. The readers specifically assessed three features — surface nodularity, liver edge, and parenchymal echotexture — with scores of 0 to 3 (0 = normal, 1 = mild, 2 = moderate, 3 = severe). Accuracies of each sonographic feature for the detection of mild fibrosis and above (≥F1), significant fibrosis (≥F2), severe fibrosis (≥F3), and cirrhosis (F4) were determined with histopathology as the reference standard.
Results:
Fibrosis was present in 99 patients (F1=34, F2=20, F3=22, and F4=23) and absent in 57 patients. The sensitivities for the detection of significant fibrosis with surface nodularity, liver edge, and parenchymal echotexture were 57%, 15%, and 41%, respectively. The accuracies for the detection of ≥F1, ≥F2, ≥F3, and F4 stages were 50.5%, 59%, 59%, and 65% for liver surface, 51%, 53%, 54%, and 55% for liver edge, and 58%, 59%, 63%, and 63% for parenchyma echotexture, respectively. The combined scores from all three features had accuracies of 56%, 59%, 62%, and 66% for the detection of ≥F1, ≥F2, ≥F3, and F4, respectively.
Conclusion:
Routine clinical ultrasound is a not a sensitive predictor of early fibrosis in chronic viral hepatitis. Surface nodularity is the most sensitive sonographic feature for the detection of significant fibrosis and routine clinical ultrasound is the most useful for the detection of cirrhosis.
Keywords
Accuracy
fibrosis
liver
ultrasound
INTRODUCTION

Chronic viral hepatitis, especially chronic hepatitis B is the most common cause of chronic liver disease in SE Asia. Although most chronic hepatitis B carriers do not develop complications, approximately 15 to 40% develop complications that include the development of liver fibrosis and eventually cirrhosis, with a predisposition to hepatocellular carcinoma (HCC).[12] The risk of HCC increases with the degree of fibrosis and is highest when associated with chronic hepatitis C, which has a 13 to 30% five-year cumulative incidence of HCC.[34]
Antiviral treatment in patients with chronic hepatitis B or C is indicated in severe fibrosis.[56] Many studies have demonstrated that hepatic fibrosis or even cirrhosis is potentially reversible with treatment, especially when treatment is started in the early stages of liver fibrosis.[7–10] The risk of HCC may also be reduced.[1112] Therefore the staging of fibrosis is an important determinant of prognosis and management.
Liver biopsy is the gold standard to determine the stage of fibrosis. However, it is invasive and carries the risk of bleeding, in particular when patients with liver disease may have abnormal clotting times and low platelet counts. The procedure often invokes patient anxiety, and may also be non-diagnostic due to suboptimal sampling.[1314] Liver biopsy is further limited by intra-observer and inter-observer variability even among experienced pathologists.[1516] Therefore, there is a need for reliable noninvasive tests that can accurately reflect the spectrum of hepatic fibrosis, not only for prognostic purposes, but also to detect the progression of fibrosis and to monitor treatment response.
Ultrasound is easily accessible in most health-care centers, making it the most commonly used imaging technique to evaluate chronic liver disease. Previous studies have demonstrated that ultrasound can predict liver cirrhosis or significant fibrosis.[1718] Given that ultrasound is a routine clinical investigation at our institute for patients with chronic liver disease, we sought to determine the diagnostic accuracy of routine ultrasound in predicting the stages of hepatic fibrosis.
MATERIALS AND METHODS
Subjects
This retrospective analysis study was approved by the Institution Review board with a waiver of informed consent from the patients. A search of the database between October 2004 and November 2009 for patients with a history of chronic viral hepatitis who underwent liver biopsy and ultrasound was performed. The search yielded 156 patients who had liver biopsies performed within six months of the ultrasound examination and before any antiviral treatments were initiated. We limited the time interval between the biopsy and ultrasound to six months to minimize the effect of progression of chronic liver disease and its correlation with ultrasound features.
Ultrasound examination and interpretation
Using standard department protocol, ultrasound studies were performed by trained sonographers using both linear and curvilinear transducers on the available five different ultrasound scanners in the department: Toshiba Aplio Mx and Toshiba Aplio XG1 (Toshiba Medical Systems, Tokyo, Japan) using both 3.5 MHz and 7.5 MHz transducers, Phillips ATL and iU22 (Phillips Ultrasound, Bothell, WA) using both C5-1 and L12-5 transducers, and GE LOGIQ9 (GE Healthcare., Milwaukee, WI) with both M12 and 4C transducers. The frequency settings ranged from 1.9 to 6 MHz when using curvilinear probes and from 6.2 to 12 MHz for linear high-resolution probes. Overall, the livers were studied with low frequency around 3.5 MHz for general evaluation and >7 MHz for evaluation of the surface and the parenchymal echotexture. The ultrasound study protocol consisted of obtaining a series of images of both lobes of the liver for evaluation of the parenchymal echotexture, focal lesions, volumetric changes, and edge evaluation. High-resolution images of the surface of the right and left lobes of the liver were obtained with high-frequency probes for evaluation of the surface of the liver. Additional images were obtained if required. Two experienced radiologists, in consensus, retrospectively reviewed the ultrasound images on picture archiving and communication system (PACS). Both readers were blinded to the clinical findings and histopathological results. For the study purpose, three features reported – surface nodularity, liver edge, and parenchymal echotexture – were evaluated. We assessed the sensitivity of these features with a scoring system modified from a previously published scoring system.[19] A single score was assigned to each of the three features: (1) Liver surface where 0 = smooth, 1 = mildly nodular, 2 = moderately nodular, 3 = severely nodular; (2) liver edge where 0 = sharp, 1 = mildly blunted, 2 = moderately blunt, 3 = rounded; (3) parenchymal echotexture where 0 = normal, 1 = mildly coarse, 2 = moderately coarse, 3 = severely coarse [Figure 1]. A normal liver with no fibrosis was expected to receive a score 0 for all three features. A liver with mild fibrosis would be expected to have either surface nodularity or altered echotexture. Moderate liver fibrosis was expected to show some surface nodularity and mild coarsening of echotexture with or without blunting of the liver edge. In severe fibrosis, the liver was expected to appear more nodular and show coarse echotexture changes with blunting of liver edge. Finally, cirrhotic livers were expected to demonstrate all the above features and receive higher scores.
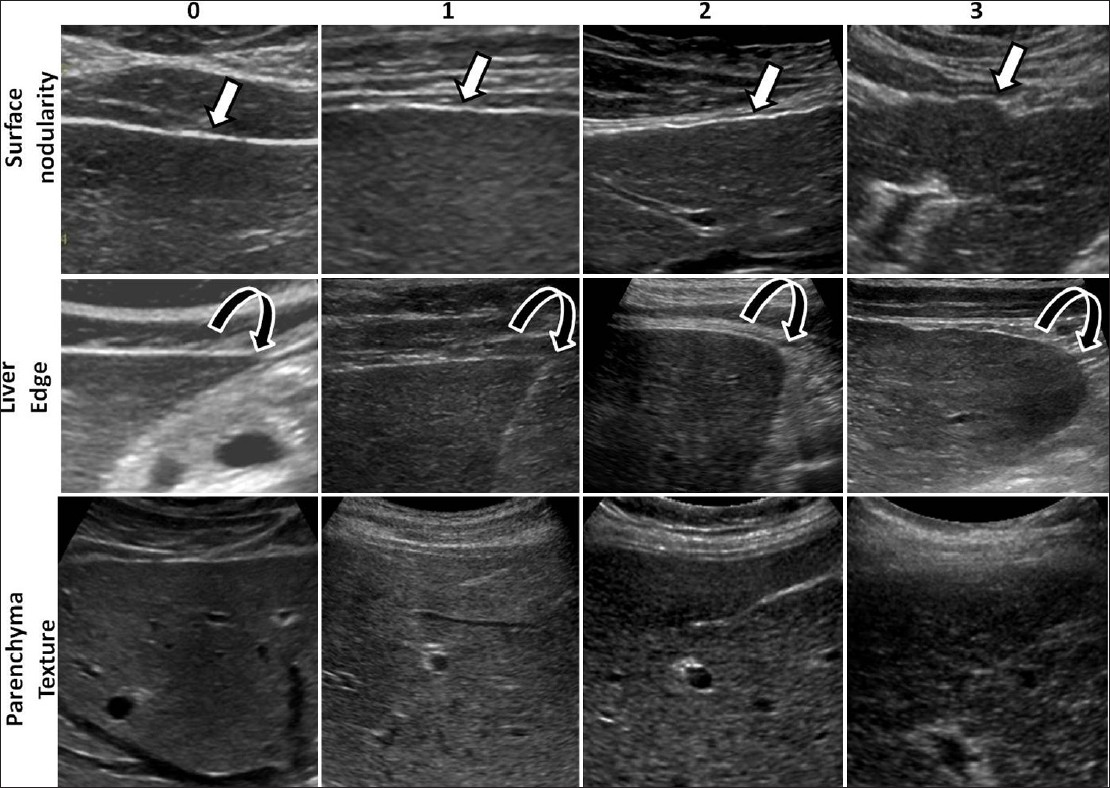
- Examples of ultrasound images from different patients illustrating the ultrasound features evaluated and scores assigned to the features. In the top row are images showing the liver surface demonstrating smooth to severe nodularity (arrows) and in the middle row, images showing the liver edges from sharp to rounded edge (curved arrows). In the bottom row, note the change in fine granular echoes in normal liver to severely coarse echoes in cirrhotic liver.
Histopathological findings
The staging of hepatic fibrosis was performed by an expert histopathologist who was blinded to the scores and the laboratory reports available from the hospital electronic data system. The staging of fibrosis was assigned using the METAVIR scoring system as follows: F0 = no fibrosis, F1 = portal fibrosis without septa, F2 = portal fibrosis with few septa, F3 = bridging fibrosis without cirrhosis, F4 = cirrhosis.[20]
Statistical analysis
Correlation between scores of ultrasound features and severity of fibrosis was performed with Spearman's rank correlation test. Receiver operator curve analysis was performed for determining the optimal cutoff score for each ultrasound feature, their combinations (sum of the scores for individual features), and total scores for the detection of fibrosis. Sensitivity, specificity, positive predictive value, negative predictive value, and accuracies for the detection of all degrees of fibrosis (≥F1), significant fibrosis (≥F2), severe fibrosis (≥F3), and cirrhosis (F4) were also determined. Comparison of receiver operating characteristic (ROC) curves was performed for each feature, combinations, and total scores for predicting different stages, and only significant differences were reported. A P value of <0.05 was considered significant. All statistical analyses were performed with Med Calc version 12.1.4.0 (Med Calc Software, Mariakerke, Belgium).
RESULTS
The study population was predominantly male (72%) with a mean age of 46 (range 20–84 years). The etiology for chronic liver disease was hepatitis B in 136 patients (87%) and hepatitis C in 20 patients (13%).
Histological findings
Histological findings showed that 99 patients (63.5%) had fibrosis. Among them, 34 patients (34.3%) had mild fibrosis (F1), 20 patients (20.2%) had moderate fibrosis (F2), 22 patients (22.2%) had severe fibrosis (F3), and 23 patients (23.2%) had cirrhosis (F4). There was no fibrosis in 57 patients. Mild inflammation was present in 56 patients (36.5%), moderate inflammation in 30 (19.2%), and severe inflammation in nine (5.8%), and there was no inflammation in 51 (32.6%). Fatty change was present in 46 patients (ranging from 1 to 20% of the biopsy sample) (29.5%).
Correlation of ultrasound scores with staging of fibrosis
There was some correlation between scores of ultrasound features and increasing severity of fibrosis [Table 1]. The Spearman's rank correlation coefficient for the surface nodularity, liver edge, and parenchymal texture with all stages of fibrosis were 0.16 (P=0.03), 0.08 (P=0.33), and 0.21(P=0.009), respectively. The cumulative scores also had a significant but low correlation of 0.22 (P=0.005) with all stages of fibrosis.
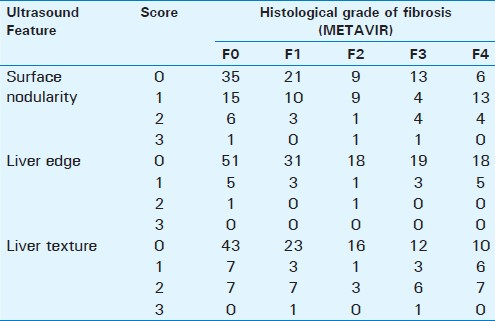
Detection of liver fibrosis
Fibrosis was present in 99 patients with a disease prevalence of 63.5% [Table 1] in the study population. Among the individual features, surface nodularity had high sensitivity, whereas liver edge had the highest specificity, and parenchymal echotexture had the best positive predictive value; however, these differences were not statistically significant. The best sensitivity (53%) was with combined scores of surface + liver edge, whereas, the best specificity (94%) and positive predictive value (87%) was with combined scores of surface + texture [Table 2]. Negative predictive values were low with all features. There were no significant differences between the accuracies among the individual features and combined scores.
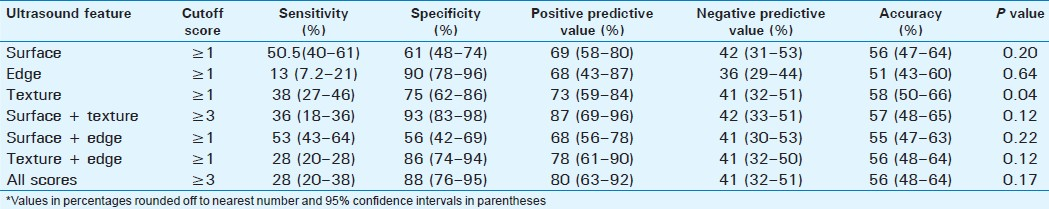
Detection of significant fibrosis
The prevalence of significant fibrosis was 41.7% [Table 3]. Surface nodularity was the most sensitive single feature detected in 57% of the cases with an accuracy of 59%. Combined scores of surface + texture and texture + edge had better accuracies of 61% and 60%, respectively, but the overall accuracy was similar to surface nodularity, and these differences were not statistically significant. The combined scores of surface + edge, surface + texture, and total scores had significantly better accuracy than liver edge score alone (P=0.025, P=0.04, P=0.03, respectively).
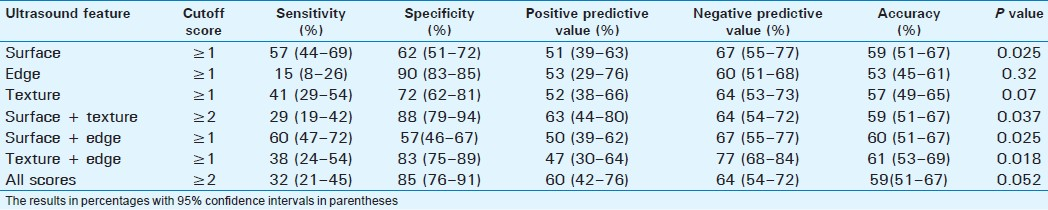
Detection of severe fibrosis
Severe or advanced fibrosis was present in 45 patients (28.8%). Surface nodularity was the most sensitive single feature (58%) with an overall accuracy of 59% [Table 4]. The best accuracy (63%) was with combined scores of surface + texture. The liver edge score had a high specificity of 91%, but a low sensitivity of 18% and an accuracy of 54%, and this was significantly lower compared to scores for liver texture (63%, P=0.003), edge + texture (62%, P=0.04), surface + texture (64%, P=0.023), and all scores (63%, P=0.026).
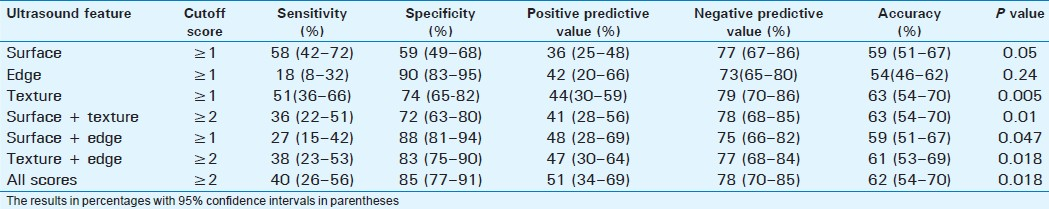
Detection of cirrhosis
Cirrhosis was present in 23 patients (14.7%). Surface nodularity had the best sensitivity of 74% with an accuracy of 65%. However, the score for surface + texture combination had the best accuracy (67%) for detection of cirrhosis [Table 5]. Combined score had the best positive predictive value of only 31%. Surface nodularity had the highest negative predictive value of 93%.
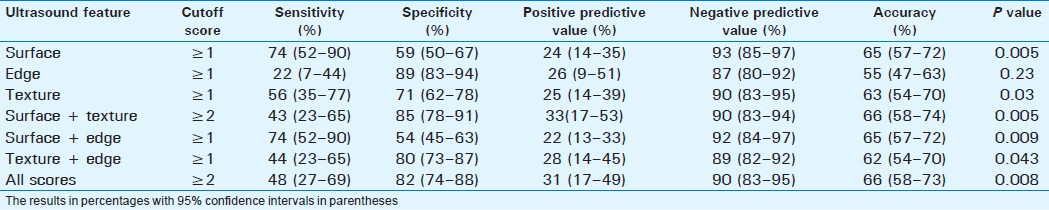
DISCUSSION
Our study results show that the ultrasound features of surface nodularity, coarsened echotexture, and blunting of liver edge have low sensitivity and specificity for the detection of early stages of liver fibrosis. Although the specificities are moderately high, the negative predictive values are less than 50%, suggesting that routine clinical ultrasound does not have sufficient accuracy for detecting and ruling out early fibrosis. Routine clinical ultrasound is also not accurate for the detection of significant fibrosis, an important stage for management decisions. However, ultrasound has a high negative predictive value for cirrhosis and this may be useful for ruling out cirrhosis or stage F4 liver fibrosis in patients who are usually on surveillance for the detection of complications, such as HCC and portal hypertension.
The role of ultrasound in predicting cirrhosis has been a subject of interest for decades, with liver surface nodularity being the most commonly used ultrasound feature for detection. Surface nodularity was the most sensitive of the three features in our study as well. There are a few studies describing the role of ultrasound in detecting early fibrosis. Nishiura et al., found that ultrasound evaluation of the three liver features (surface, edge, and echotexture) were reliable predictors of the full spectrum of liver fibrosis.[19] By utilizing a scoring system, they reported that both higher individual and total ultrasound scores were strongly predictive of an increasing stage of fibrosis. Our study did not demonstrate higher accuracies but showed a similar trend of increasing sensitivity and accuracy with the stage of fibrosis. Reasons for these differences could be due to the use of dedicated high-resolution scans in their study and differences in the populations studied.
In our retrospective study, there was no significant correlation between ultrasound scores and the stage of fibrosis. However, we confirmed that amongst the three features, surface nodularity is the best predictor of fibrosis in predicting significant fibrosis. Our study results reinforce that surface nodularity should be looked for carefully when interpreting the ultrasound studies. Coarsened echotexture and blunting of the liver edge are not sensitive in detecting significant fibrosis. However, the presence of either feature is highly specific in predicting significant fibrosis. Blunting of the liver edge is the least sensitive but it has a high specificity, suggesting a potential use of this feature for ruling out cirrhosis and significant fibrosis.
The reasons for the low sensitivity and accuracy of ultrasound may be due to many factors. The pattern of fibrosis affects the extent of nodularity and echogenicity, and may account for the differences in the diagnostic performance seen between hepatitis B- and hepatitis C-related cirrhosis on ultrasound.[19] However, the complex pattern of changes in chronic liver disease that is reflected in histopathology includes mixed features of steatosis, necrosis, and inflammation. These may affect the morphological appearance of the liver on ultrasound, rather than the presence of fibrous tissue alone.[21–23] Joseph et al., found that only two out of three cirrhotic livers had abnormal bright echoes on ultrasound and, in particular, the majority with piecemeal necrosis or macronodular cirrhosis had normal echopatterns, even in the presence of extensive fibrous tissue on histology.[23] Increased echoes in chronic liver disease may not only reflect fibrosis, but also fatty infiltration, portal tract fibrosis, and hepatitis. However, in our study, only a fraction of the patients had fatty change and the degree of fatty change was mild to moderate. Although the presence of steatosis and inflammation may affect the evaluation of the parenchymal texture, the evaluation of surface nodularity and edge would not be significantly altered.
Other studies have demonstrated the diagnostic performance of multiple ultrasound parameters including liver morphology and hemodynamics for secondary signs of portal hypertension.[2425] Gaiani el al., studied seven ultrasound variables and found that liver surface nodularity and portal flow velocity were the best independent features in predicting fibrosis.[24] When these two features were combined, 82% of cirrhotic livers were accurately identified (82% sensitivity and 79% specificity). In another study by Aube et al., where 11 ultrasound variables were reviewed, stepwise analysis showed 73% accuracy in predicting significant fibrosis, according to liver length followed by portal velocity and liver surface, respectively.[25] However, spleen length, followed by ascites and liver length, respectively, were better predictors of severe fibrosis with an accuracy of 84%. Although combining scores from three ultrasound features increased sensitivity marginally, it did not increase accuracy significantly. Combined features of surface nodularity and texture produced the best accuracy for the detection of various stages of fibrosis, in particular for cirrhosis. Surface nodularity was the most sensitive feature in our study, and other features had many false positives, and therefore, combining the scores did not improve accuracy.
Many of the previous studies examined the utility of ultrasound for the detection of cirrhosis.[25–34] The most commonly used ultrasound feature in the studies for detecting cirrhosis is liver surface nodularity. The diagnostic performances of these studies in detecting severe fibrosis or cirrhosis were widely variable, with sensitivities ranging from 12.5 to 91%. In comparison, our study showed a moderately high sensitivity but slightly lower specificity and therefore, accuracy. Many investigators on the noninvasive monitoring of chronic liver disease report a variety of algorithms and scoring systems that may be useful to improve the discrimination of fibrosis, including combinations of biochemical, radiological, and laparoscopic studies. Some studies allow the identification of patients with significant fibrosis. However, major limitations include a low predictive accuracy when patients fall into the intermediate zone of scoring indices, and also low detection of patients with mild fibrosis (F1) who are at a risk of progression.[2627]
Our study has several limitations. First, this was a retrospective study. The study was performed to produce a database to be used as a reference for a prospective study in the future. Second, the scans were performed by sonographers and on five different scanners which may induce operator bias and nonuniformity of the quality of ultrasound. Although all the ultrasound studies are considered to be of satisfactory to high quality for interpretation, the differences between machines and operator dependency may affect image characteristics. Our department protocol uses the frequency of 2.5–4 MHz, which is standard. However, the use of higher resolution probes with a frequency of 5–9 MHz may be useful for the evaluation of surface and texture features. Using high-resolution probes for screening the whole liver is not routine in our clinical practice except for obtaining selected images of the liver surface. This may have reduced the sensitivity of the study. Third, we did not perform inter-observer variability which would have made the study more interesting. However, it is common knowledge that ultrasound interpretation is variable among readers. We chose to combine the experience of two readers to improve the accuracy of routine clinical ultrasound studies. We plan to perform inter-observer variability in a prospective study wherein the readers can be trained appropriately for uniform interpretation of the findings. Fourth, sampling error of the liver biopsy may also affect our results. An experienced pathologist reported the liver biopsies and scored the fibrosis and inflammation. We did not test for inter-observer variability among pathologists. Although this is a retrospective study with relatively small numbers, it was performed under conditions similar to clinical practice and therefore confirms that routine clinical ultrasound is not a reliable diagnostic test especially for staging early or mild fibrosis.
The wide range of ultrasound parameters and variable recommended algorithms reflect the limitations of ultrasound, including operator dependency and limited accuracy in the staging of fibrosis. Currently, transient elastography (Fibroscan) and magnetic resonance elastography (MRE) provide the most reliable results in predicting fibrosis. However there is a need for larger longitudinal studies to define standardized diagnostic criteria for staging fibrosis with reproducible results before a noninvasive imaging technique can replace liver biopsy.[17]
CONCLUSION
In summary, our study demonstrates that routine clinical ultrasound is a not an accurate predictor of early and significant liver fibrosis in chronic hepatitis. Therefore, routine clinical ultrasound is not reliable for the staging of liver fibrosis and for making therapeutic decisions and for the assessment of prognosis in chronic liver disease secondary to chronic hepatitis. However, ultrasound is still useful in detecting cirrhosis.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2012/2/1/58/101000
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- How big is the financial burden of hepatitis B to society? A cost-of-illness study of hepatitis B infection in Singapore. J Viral Hepat. 2009;16:53-63.
- [Google Scholar]
- Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35-50.
- [Google Scholar]
- Hepatitis C virus related cirrhosis: Time to occurrence of hepatocellular carcinoma and death. Gut. 2000;47:131-6.
- [Google Scholar]
- AASLD Practice Guidelines: Chronic hepatitis B: Update 2009. Hepatology. 2009;50:661-2.
- [Google Scholar]
- Diagnosis, management, and treatment of hepatitis C: An update. Hepatology. 2009;49:1335-74.
- [Google Scholar]
- Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: A meta-analysis of individual patient data. Hepatology. 2004;39:333-42.
- [Google Scholar]
- Direct and indirect evidence for the reversibility of cirrhosis. Hum pathol. 2006;37:1519-26.
- [Google Scholar]
- Regression of hepatic fibrosis in hepatitis C with long term interferon treatment. Dig Dis Sci. 1998;43:2573-76.
- [Google Scholar]
- Reversibility of hepatic fibrosis in autoimmune hepatitis. Ann Intern Med. 1997;127:981-5.
- [Google Scholar]
- Interferon therapy reduces the risk for hepatocellular carcinoma: National surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT study group. inhibition of hepatocarcinogenesis by interferon therapy. Ann Intern Med. 1999;131:174-81.
- [Google Scholar]
- Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051-5.
- [Google Scholar]
- Sampling error and intraobserver varation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-8.
- [Google Scholar]
- Second opinion pathology in liver biopsy interpretation. Am J Gastroenterol. 2001;96:3158-64.
- [Google Scholar]
- The value of second opinion in gastrointestinal and liver pathology. Arch Pathol Lab Med. 2001;125:736-9.
- [Google Scholar]
- Can imaging modalities diagnose and stage hepatic fibrosis and cirrhosis accurately? J Hepatol. 2009;50:17-35.
- [Google Scholar]
- Noninvasive monitoring of patients with chronic hepatitis C. Hepatology. 2002;36(5 Suppl 1):S57-64.
- [Google Scholar]
- Ultrasound evaluation of the fibrosis stage in chronic liver disease by the simultaneous use of low and high frequency probes. Br J Radiol. 2005;78:189-97.
- [Google Scholar]
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20(1 Pt 1):15-20.
- [Google Scholar]
- Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig Liver Dis. 2002;34:516-22.
- [Google Scholar]
- Quantitative US attenuation in normal liver and in patients with diffuse liver disease: Importance of fat. Radiology. 1986;160:65-71.
- [Google Scholar]
- Ultrasound in the detection of chronic liver disease (the “bright liver”) Br J Radiol. 1979;52:184-8.
- [Google Scholar]
- What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? A prospective study comparing ultrasonography and percutaneous liver biopsy. J Hepatol. 1997;27:979-85.
- [Google Scholar]
- Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol. 1999;30:472-8.
- [Google Scholar]
- A non-invasive algorithm accurately predicts advanced fibrosis in hepatitis C: A comparison using histology with internal-external validation. J Hepatol. 2008;49:564-71.
- [Google Scholar]
- The diagnosis of cirrhosis by high resolution ultrasound of the liver surface. Br J Radiol. 1999;72:29-34.
- [Google Scholar]
- Severe liver fibrosis or cirrhosis: Accuracy of US for detection – Analysis of 300 cases. Radiology. 2003;227:89-94.
- [Google Scholar]
- Cirrhosis: Diagnosis with sonographic study of the liver surface. Radiology. 1989;172:389-92.
- [Google Scholar]
- Limitation of liver surface US in the diagnosis of cirrhosis. Radiology. 1992;185:21-3.
- [Google Scholar]
- Prediction of compensated liver cirrhosis by ultrasonography and routine blood tests in patients with chronic viral hepatitis. Korean J Hepatol. 2010;16:369-75.
- [Google Scholar]
- Ultrasonographic and biochemical parameters in the non-invasive evaluation of liver fibrosis in hepatitis C virus chronic hepatitis. Aliment Pharmacol Ther. 2005;22:769-74.
- [Google Scholar]
- Correlation between ultrasonographic and pathologic diagnosis of hepatitis B and C virus-related cirrhosis. J Gastroenterol. 2003;38:153-7.
- [Google Scholar]






