Translate this page into:
Abnormal Findings on “T1WI or DWI or MRCP:” An Effective Boolean Interpretation Model in Discriminating Small Pancreatic Ductal Adenocarcinoma from Control Group

*Corresponding author: Naoko Mori, Department of Diagnostic Radiology, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan. naokomori7127@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ren H, Mori N, Hirasawa M, Hamada S, Mugikura S, Masamune A, et al. Abnormal Findings on “T1WI or DWI or MRCP:” An Effective Boolean Interpretation Model in Discriminating Small Pancreatic Ductal Adenocarcinoma from Control Group. J Clin Imaging Sci 2021;11:54.
Abstract
Objectives:
The objectives of the study was to evaluate the diagnostic performance of findings on T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and magnetic resonance cholangiopancreatography (MRCP) separately and to identify an optimal Boolean interpretation model for discriminating patients with small pancreatic ductal adenocarcinoma (PDAC) from control groups in clinical practice.
Material and Methods:
We retrospectively enrolled 30 patients with surgery confirmed small PDAC (≤20 mm) and 302 patients without pancreatic abnormality between April 2008 and February 2020. The presence of masses was evaluated by T1WI, T2WI, and DWI. Abnormality of the main pancreatic duct (MPD) was evaluated by T2WI and MRCP. Multivariate logistic regression analysis was performed to select significant sequences for discriminating the small PDAC and control groups. Boolean operators “OR” or “AND” were used to construct sequence combinations. Diagnostic performances of these sequences and combinations were evaluated by X2 tests.
Results:
The sensitivity of T2WI was lowest (20%) for detecting masses. For evaluating MPD abnormality, sensitivity was higher for MRCP than for T2WI (86.7% vs. 53.3%). Multivariate logistic regression analysis showed that T1WI and DWI for detecting the presence of masses and MRCP for evaluating MPD abnormality were significantly associated with differentiation between the two groups (P = 0.0002, P = 0.0484, and P < 0.0001, respectively). Seven combinations were constructed with T1WI, DWI, and MRCP. The combination of findings on “T1WI or DWI or MRCP” achieved the highest sensitivity of 96.7% and negative predictive value of 99.6%.
Conclusion:
The combination of findings on “T1WI or DWI or MRCP” might be an optimal interpretation model for discriminating small PDAC from control groups in clinical practice.
Keywords
Non-contrast magnetic resonance imaging
Pancreatic ductal adenocarcinoma
Early diagnosis
Screening
Boolean operators
INTRODUCTION
The prognosis of pancreatic ductal adenocarcinoma (PDAC) is extremely poor because most patients are diagnosed at advanced stages and unresectable. Early stage PDAC (small PDAC) is generally defined as a solid pancreatic lesion originating from the intraductal epithelium with the largest diameter ≤20 mm without lymph nodes or distant metastases.[1] The resectability rate and postoperative cumulative 5-year survival rate are higher for small PDAC than for PDAC (>20 mm).[2,3]
Several non-genetic risk factors such as age, smoking, alcohol, diabetes, and chronic pancreatitis are associated with the development of PDAC.[4,5] Furthermore, approximately 10% of PDACs are related to an underlying familial history or genetic mutation.[6,7] Therefore, the goal for PDAC screening should include the early detection of small PDACs that are amenable to receive surgical resection and the follow-up for identified high-risk individuals.[6]
In this regard, several studies have evaluated the diagnostic performance of endoscopic ultrasound sonography (EUS), US, computed tomography (CT), and magnetic resonance imaging (MRI).[3,4,8-13] MRI has an advantage over EUS, US, and CT in its higher soft-tissue contrast.[8,14,15] T1- weighted imaging (T1WI), T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), magnetic resonance cholangiopancreatography (MRCP), and dynamic contrast-enhanced (DCE) MRI are generally used for diagnosing PDAC.[16] Park et al. reported the equivalent diagnostic performance of non-contrast T1WI, T2WI, and DWI set and DCE-MRI with MRCP set for detecting small PDAC.[3] Furthermore, Kulkarni et al. also described that DCE-MRI might not be required for the detection of PDAC or for follow-up surveillance of cystic pancreatic lesions.[6]
In clinical practice, we occasionally perform non-contrast MRI for screening or follow-up of high-risk individuals of PDAC. The presence of masses was evaluated on T1WI, T2WI, and DWI. The abnormality of main pancreatic duct (MPD) was evaluated on T2WI and MRCP.[17] These findings are intuitively evaluated; however, no studies have provided an interpretation model for detecting small PDAC by applying “logical sum, OR” or “logical product, AND” Boolean operators between the findings to our knowledge.[18] When a radiologist makes a clinical judgment, it is important to know whether to treat the patient as suspected small PDAC if positive findings are found only in one sequence or in two or more sequences.
The purpose of this study was to evaluate the diagnostic performance of findings on T1WI, T2WI, DWI, and MRCP separately and to identify an optimal Boolean interpretation model for discriminating patients with small PDAC from control groups in clinical practice.
MATERIAL AND METHODS
Patients
Our institutional review board approved this retrospective study and waived the requirement for informed consent. We reviewed the surgical database during April 2008 and February 2020 at our institution. A total of 43 patients underwent surgery without any prior treatment and were histologically diagnosed as PDAC. The initial MRI evaluation was performed for all the 43 patients. Among them, patients with the longest diameter of >20 mm on portal phase of DCE-MRI were excluded (n = 13). Thus, 30 patients were enrolled as the small PDAC group. The median time interval between MRI examinations and surgery was 13 days (range, 0–61 days). For the control group, consecutive patients who underwent the abdominal MRI examination were reviewed during the same period. Initially, a total of 735 patients without any solid or cystic tumors in the pancreas were selected. Because observers might be affected by prominent positive findings in other abdominal organs, the following patients were excluded: (a) Patients with percutaneous transhepatic cholangiodrainage treatment (n = 33); (b) patients with severe hepatobiliary dilatation or jaundice (n = 306); (c) patients with apparent intrahepatic cholangiocarcinoma (n = 80); (d) patients with liver metastasis (n = 5); (e) patient with follicular lymphoma in the retro-pancreatic space (n = 1); and (f) patients with poor image quality of MRCP (n = 8). The control group showed no abnormal findings in the pancreas on MRI and persistently negative findings on follow-up CT or MRI for at least 2 years. Consequently, 302 patients (161 men, 141 women; age range, 14–91 years; mean age, 59 years) were enrolled as the control group.
MRI protocols
All patients underwent MR examination of the abdomen using a 3.0-T system (MAGNETOM Trio, A Tim System, Siemens Healthcare GmbH, Erlangen Germany). Patients were imaged in the supine position with 12 coil elements. The MR imaging protocols included the following sequences: (1) A T1WI 2D dual-gradient-recalled echo sequence (in-phase and out-of-phase) (189/2.46 [repetition time in milliseconds/ echo time in milliseconds]; flip angle, 80°; section thickness, 5 mm; acquisition matrix, 182 × 320; bandwidth, 400 Hz/ pixel; field of view (FOV), 340 mm × 340 mm; acquisition time, 17 s); number of excitations (NEX), 2; (2) a breath- hold multi-shot T2WI sequence (2600/102 [repetition time in milliseconds/echo time in milliseconds]; flip angle, 120°; section thickness, 5 mm; acquisition matrix, 182 × 320; bandwidth, 300 Hz/ pixel; FOV, 340 mm × 340 mm; acquisition time, 18 s); NEX, 2; and (3) a navigator- triggered axial DWI sequence with single-shot echo planar imaging with motion-probing gradients in three directions (1000/64 [repetition time in milliseconds/ echo time in milliseconds], flip angle, 90°; section thickness, 5 mm; acquisition matrix, 90 × 128; bandwidth, 2298 Hz/ pixel; FOV, 380 mm × 380 mm; acquisition time, 18 s); NEX, 1; application of motion-probing gradient pulse along the x, y, and z directions. Apparent diffusion coefficient (ADC) maps were automatically created with b-values of 0 s/mm2 and 800 s/mm2; (4) a navigator-triggered three- dimensional MRCP turbo spin-echo sequence (3D MRCP) with 1000/604 [repetition time in milliseconds/echo time in milliseconds]; flip angle, 180°; acquisition matrix, 369 × 384; section thickness, 0.9 mm; bandwidth, 449 Hz/pixel; FOV, 250 mm × 250 mm; acquisition time, 180–240 s. Maximum intensity projection (MIP) in an orientation analogous to that of the navigator-triggered 3D MRCP turbo spin-echo sequences was obtained. To measure the lesion size in the small PDAC group, the portal phase of DCE-MRI was used. DCE-MRI scans were performed with a 3D fat-suppressed T1WI 3D turbo field-echo sequence to obtain arterial phase (30– 35 s), portal phase (65–70 s), delayed phase (3 min), and hepatobiliary phase (15 min) of gadoxetic acid disodium after the injection of contrast agent (Primovist, Bayer Schering; 0.025 mmol/kg). The contrast agent was injected as a rapid bolus and immediately followed by a 30–35 mL saline flush through a power injector at a rate of 2 mL/s.
Image analysis
Two observers (observer 1 and 2 with 2 and 12 years of experiences in abdominal imaging, respectively) independently reviewed all MR images. They were only aware that the study was performed to detect small PDAC but blinded to all other information, such as patients’ clinical data, other imaging (CT or US), and histological findings. To perform the reading tests, MRI DICOM data of small PDAC group (n = 30) and control group (n = 302) were transferred from a commercially available workstation (HMC Viewer Ver. V1.0.0, Hitachi, Japan) to a personal computer and presented by software (RadiAnt DICOM Viewer, version 2020.1; https://www.radiantviewer.com/). The reading tests contained four sessions of T1WI, T2WI, DWI, and MRCP, separately. Each session contained one single sequence. Images of patients were randomly allocated without repetition under anonymized conditions. The time interval between each session was at least 2 weeks to reduce learning effects. The presence of a mass was evaluated on T1WI, T2WI, and DWI sequences [Figures 1a-c, respectively]. The signal intensity (SI) of the lesion was compared to the surrounding pancreatic parenchyma, and a mass with low SI was recorded as positive on T1WI, a mass with iso- or high SI was recorded as positive on T2WI, and a mass with high SI was recorded as positive on DWI. In case when the presence of a mass was evaluated as positive on T1WI or T2WI, we placed oval or round regions of interest (ROIs) as large as possible within the lesion and surrounding pancreatic parenchyma by consensus. The ratio of SI (SIratio) between the lesion and surrounding pancreatic parenchyma was calculated as follows;
SIratio=SIlesion/SIparenchyma
For DWI, we placed an oval or round ROI as large as possible within the lesions by consensus in case when the presence of a mass was evaluated as positive on DWI. The mean ADC values of the ROIs were recorded.
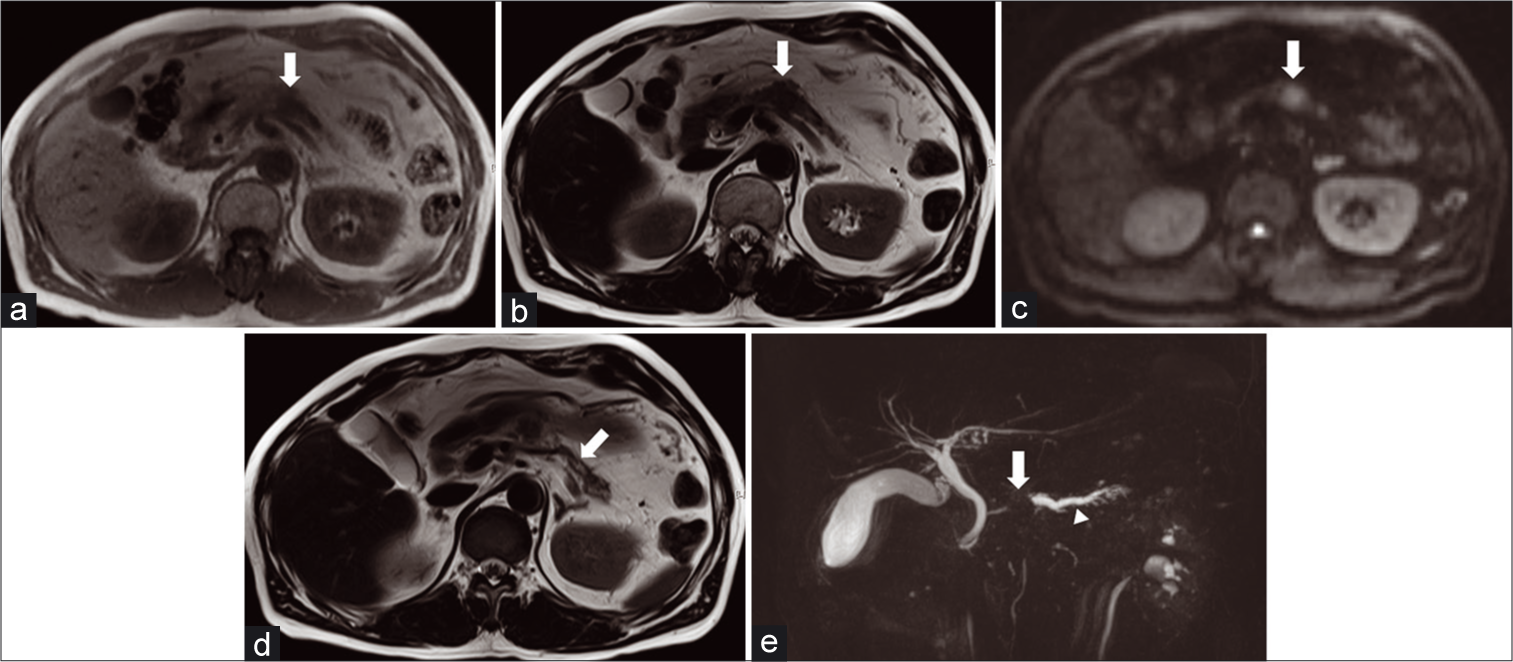
- A 63-year-old man diagnosed with pancreatic ductal carcinoma by ultrasound-guided fine-needle aspiration. T1-weighted imaging shows a mass with low signal intensity in the pancreatic body (arrow) (a). T2-weighted imaging shows a mass in the pancreatic body (arrow) (b). Diffusion-weighted imaging (b value 800 s/mm2) shows a mass with high signal intensity in the pancreatic body (arrow) (c). Mild dilatation of the main pancreatic duct in the pancreatic tail (arrow) (d). The maximum intensity projection image of magnetic resonance cholangiopancreatography shows abrupt cutoff of the main pancreatic duct (arrow) with dilatation of the upstream main pancreatic duct and side branches (e).
The MPD abnormality was evaluated on T2WI and MRCP [Figures 1d and e, respectively]. Dilatation of the MPD on T2WI and dilatation (maximal diameter of a MPD >3 mm) or narrowing of the MPD on MRCP was detected as positive.[3,19] The presence of a mass on T2WI was recorded as T2-mass, and MPD abnormality on T2WI was recorded as T2-MPD. Lesion size (maximum diameter in the axial plane) and location (head, uncinate, body, and tail) were evaluated based on the portal phase of DCE-MR images, because the portal phase provides good contrast of lesion to surrounding pancreatic parenchyma and anatomical information. Our purpose was to evaluate the diagnostic performance of combination of findings on non-contrast MRI sequences, and portal phase was not used for the Boolean combination. Other patients’ clinical data, such as gender and age, were also recorded.
Statistical analysis
The patients’ gender was compared between the two groups using Fisher’s exact test. The patients’ age was compared between the small PDAC and control groups using Mann– Whitney U test. Reading results (T1WI, T2- mass, DWI, T2-MPD, and MRCP) were treated as binomial variables (negative: 0, positive: 1), and the diagnostic performances of T1WI, T2-mass, DWI, T2-MPD, and MRCP were evaluated using X2 tests, and sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. To select the findings of imaging sequences significantly associated with differentiation between the small PDAC and control groups, multivariate logistic regression analysis was performed. From the selected sequences for separately evaluating mass and MPD abnormality, combinations were subsequently constructed by applying Boolean operators “logical sum, OR” or “logical product, AND.”[18] When the operator “OR” was applied, a combination was recorded as “positive” if there was at least one positive finding from the selected sequences. None of them was recorded as “negative.” When the operator “AND” was applied, a combination was recorded as “positive” if positive findings were found in every involved sequence, otherwise a combination was recorded as “negative.” Diagnostic performances of the combinations were evaluated using X2 tests, and sensitivity, specificity, PPV, and NPV were calculated. The interobserver agreement between the two observers was calculated using κ statistics. A κ value of 1.0 indicated perfect agreement; 0.81–0.99, almost perfect agreement; 0.61–0.80, substantial agreement; 0.41–0.60, moderate agreement; 0.21–0.40, fair agreement; and ≤0.20, slight agreement, respectively.[20] The SIratio on T1WI and T2WI, and the mean ADC value of a lesion were recorded.
Statistical analyses were performed on JMP Pro 15 (SAS Institute, Cary, NC). P < 0.05 was considered as statistically significant.
RESULTS
No significant difference was found in gender between the small PDAC group and control group (P = 0.99). Age was significantly higher in the small PDAC group than in the control group (P = 0.0083). The lesion size of the small PDAC group ranged from 8 to 20 mm (median, 17 mm). Seventeen lesions located in the pancreatic head, four in the uncinate, eight in the body, and one in the tail [Table 1]. In detecting the presence of mass, the sensitivity of T2-mass was lowest [20%, Table 2]. In detecting the presence of MPD abnormality, the sensitivity was higher for MRCP than for T2-MPD (86.7% vs. 53.3%, respectively). Multivariate logistic regression analysis showed that T1WI and DWI for detecting the presence of mass were significantly associated with the differentiation between small PDAC and control groups (P = 0.0002 and P = 0.0484, respectively). The SIratio on T1WI was 0.74 ± 0.10, indicating small PDACs presented lower SI compared to surrounding pancreatic parenchyma on T1WI. The SIratio on T2WI was 1.22 ± 0.39, indicating small PDACs presented slightly higher SI compared to surrounding pancreatic parenchyma on T2WI. The mean ADC of lesion was 1.49 ± 0.34 × 10−3 mm2/s. MRCP for detecting MPD abnormality was also significantly associated with the differentiation between the two groups (P < 0.0001), whereas T2-mass and T2-MPD were not (P = 0.6680 and 0.5904, respectively). The κ value for T1WI and DWI was 0.67 and 0.62, indicating substantial interobserver agreement. The κ value for MRCP was 0.82, indicating almost perfect interobserver agreement [Table 2]. The selected sequences were T1WI, DWI, and MRCP. The seven combinations were constructed as follows: T1WI or DWI or MRCP, (T1WI or DWI) and MRCP, (T1WI or MRCP) and DWI, T1WI and DWI and MRCP, (T1WI and DWI) or MRCP, (T1WI and MRCP) or DWI, and (DWI or MRCP) and T1WI. Among these combinations, the combination “T1WI or DWI or MRCP” showed the highest sensitivity in detecting small PDAC (96.7%). The specificity of “T1WI or DWI or MRCP” was 93.4%, lower than the other combinations. The NPV of “T1WI or DWI or MRCP” was 99.6%, higher than the other combinations [Table 3]. The κ value for the combination “T1WI or DWI or MRCP” was 1.0, indicating perfect interobserver agreement.
| Variables | Small PDAC group (n=30) | Control group (n=302) | P-value |
|---|---|---|---|
| Gender (Male/Female) (n (%)) | 16 (53)/14 (47) | 161 (53)/141 (47) | 0.99 |
| Age (median [range]), (years) | 64.5 (54−84) | 60.5 (14−91) | 0.0083a |
| Lesion Size (median [range]), (mm) | 17 (8−20) | N/A | N/A |
| Lesion location (n (%)) | |||
| Head | 17 (57) | N/A | N/A |
| Uncinate | 4 (13) | N/A | N/A |
| Body | 8 (27) | N/A | N/A |
| Tail | 1 (3) | N/A | N/A |
aStatistically significant. N/A: Not applicable
| Sequences | Sensitivity | Specificity | PPV | NPV | P-value (univariate) | P-value (multivariate) | κ | |
|---|---|---|---|---|---|---|---|---|
| Mass | T1WI | 80 | 98.3 | 82.8 | 98 | <0.0001a | 0.0002a | 0.67 |
| T2mass | 20 | 98.7 | 60 | 92.5 | <0.0001a | 0.6680 | 0.34 | |
| DWI | 66.7 | 96 | 62.5 | 96.7 | <0.0001a | 0.0484a | 0.62 | |
| MPD | T2MPD | 53.3 | 98.3 | 76.2 | 95.5 | <0.0001a | 0.5904 | 0.76 |
| MRCP | 86.7 | 99 | 89.7 | 98.7 | <0.0001a | <0.0001a | 0.82 |
| Combinations | Sensitivity | Specificity | PPV | NPV | P-value | κ |
|---|---|---|---|---|---|---|
| T1WI or DWI or MRCP | 96.7 | 93.4 | 59.2 | 99.6 | <0.0001a | 1.00 |
| (T1WI or DWI) and MRCP | 83.3 | 100 | 100 | 98.4 | <0.0001a | 0.81 |
| (T1WI or MRCP) and DWI | 66.7 | 100 | 100 | 96.8 | <0.0001a | 0.74 |
| T1WI and DWI and MRCP | 46.7 | 100 | 100 | 95.0 | <0.0001a | 0.73 |
| (T1WI and DWI) or MRCP | 93.3 | 99 | 90.3 | 99.3 | <0.0001a | 0.81 |
| (T1WI and MRCP) or DWI | 90 | 96 | 69.2 | 99.0 | <0.0001a | 0.68 |
| (DWI or MRCP) and T1WI | 76.7 | 100 | 100 | 97.7 | <0.0001a | 0.80 |
DISCUSSION
In this study, we separately evaluated the diagnostic performance of findings on non-contrast T1WI, T2WI, DWI, and MRCP sequences in detecting small PDAC by evaluating the presence of mass and the MPD abnormality. The combination “T1WI or DWI or MRCP” showed higher sensitivity (96.7%) and NPV (99.6%) than the other combinations. The presence of at least one positive finding from the three sequences suggests the presence of a small PDAC and further clinical examination should be considered.
Independent evaluation of each sequence is not generally performed in clinical practice; however, separate reading tests for evaluating findings on each sequence could help radiologists recognize the roles and relationships of these non-contrast sequences. A few studies have reported the diagnostic performance of non-contrast MRI sequences for detecting small PDAC. Park et al. demonstrated that non-contrast MRI sequences comprising T1WI, T2WI, and DWI showed comparable diagnostic performance to that of DCE-MRI with MRCP.[3] Some studies suggest that gadolinium contrast may not be required for the detection of PDAC.[6] Another study by Kawakami et al. suggested that DWI combined with MRCP was effective for detecting PDAC.[21] Therefore, we enrolled only non-contrast sequences for diagnostic performance evaluation.
We used the Boolean operators, “logical sum, OR” and “logical product, AND” to construct sequence combinations.[18] In this study, “logical sum, OR” means that if there is at least one positive finding in these sequences, the combination is recorded as positive; and “logical product, AND” means that only if all sequences have a positive result, the combination is recorded as positive. Our results showed that T2-mass and T2-MPD were not significantly associated with differentiation between the small PDAC and control groups; therefore, T2WI was excluded from the construction of combinations. Harrington et al. reported that small PDAC could be detected as a mass on T1WI or an MPD abnormality on MRCP.[22] Ichikawa et al. demonstrated the high sensitivity and specificity of DWI for detecting PDAC.[23] Our results were consistent with these previous reports showing the usefulness of T1WI, DWI, and MRCP. We validated the visual reading tests by quantifying the SIratio on T1WI or T2WI. When the presence of a mass was evaluated as positive on T1WI, low SIratio (0.74 ± 0.10) supported the lower SI of lesions compared to surrounding pancreatic parenchyma on T1WI. When the presence of a mass was evaluated as positive on T2WI, the SIratio (1.22 ± 0.39) indicated the slightly higher SI of lesions compared to surrounding pancreatic parenchyma on T2WI. The mean ADC of lesion was 1.49 ± 0.34 × 10−3mm2/s, which was almost similar to the results in a previous study (1.46 ± 0.18).[24]
As for the combination, “T1WI or DWI or MRCP” was constructed by “logical sum, OR” and showed higher sensitivity (96.7%) and NPV (99.6%) but lower PPV (59.2%) than the others. These results suggest that this combination can effectively detect patients with small PDACs. When a patient was judged as negative using the “T1WI or DWI or MRCP” combination, it highly suggested that the case was normal. False positives may be due to the low PPV. To improve PPV, other biomarkers need to be incorporated into non-contrast MRI in the screening of high-risk individuals.
The diagnostic performance of the combination “T1WI or DWI or MRCP” for detecting small PDAC might be addressed from the following mechanisms and drawbacks. On T1WI, the normal pancreas generally has a moderate- to-high SI due to the influence of high protein-containing water in the acinar cells, whereas SI may be lower for lesions with damaged acinar cells than for a normal pancreas. In patients with chronic pancreatitis or atrophy, the entire pancreas might present with low SI on T1WI, making it difficult to detect small PDAC.[25,26] DWI exploits the random motion of water molecules in biological tissues. PDAC with reduced diffusion of water molecules typically shows a high SI on DWI.[27,28] However, one study reported that PDACs could not be detected as a mass with a SI higher than that of a normal pancreas because PDACs are occasionally accompanied by a variable degree of obstructive pancreatitis showing high SI on DWI.[29] Furthermore, the low spatial resolution of DWI might be another drawback. MRCP acquires heavily T2-weighted images and captures abnormalities of the pancreatic duct associated with the presence of PDAC.[17] Especially the MIP, which is reconstructed from three-dimensional MRCP, can identify the three-dimensional anatomy of pancreatic ducts with high resolution.[22] An abrupt cutoff of the MPD strongly suggests the presence of mass.[30,31] However, an abrupt cutoff of the MPD is a secondary finding, and it is difficult to detect small PDAC in patients in which the MPD is unaffected or small lesions in the tail of the pancreas. In this way, the combination “T1WI or DWI or MRCP” could compensate for the drawbacks of each sequence and increase the sensitivity to detect small PDAC.
This study had several limitations. First, this retrospective study had an uneven sample sizes and unavoidable selection bias between the small PDAC and control groups. Although the lesion location of small PDAC varied in this study, sub- group analysis according to the lesion location could not be performed due to the small sample size. Furthermore, the percentages of sensitivity and NPV in this study might be affected by the sample size of small PDAC and control groups. However, the superiority of combination “T1WI or DWI or MRCP” in diagnostic performance compared to other combinations was identified. Multi-center studies with larger sample sizes are needed. Second, patients with other histological types, such as pancreatic endocrine neoplasm, intraductal papillary mucinous neoplasm, and pancreatic cyst were excluded in this study. The clinical management is different between small PDAC and such histological types, and further research to determine how to discriminate these histological types from control groups will be needed. Third, we selected consecutive patients who underwent the abdominal MR examination without apparent pancreatic abnormalities as the control group. PDAC screening might not be performed for the general population because of low disease frequency, and stratification of high-risk individuals with background pancreatic disease, including chronic pancreatitis, diabetes, and pancreatic cystic disease, is necessary for PDAC screening. These high-risk individuals are the differential diagnosis to small PDACs. Our control group without high-risk individuals or differential diagnosis might artificially improve the diagnostic performances of sequence combinations. Further studies to differentiate patients with small PDAC from high-risk individuals should be conducted in the future.
In conclusion, the combination of findings on “T1WI or DWI or MRCP” might be an optimal Boolean interpretation model for discriminating small PDAC from control groups in clinical practice.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Japan Society for the Promotion of Science 18K07742.
Conflicts of interest
There are no conflicts of interest.
References
- Collective review of small carcinomas of the pancreas. Ann Surg. 1986;203:77-81.
- [CrossRef] [PubMed] [Google Scholar]
- Value of unenhanced MRI with diffusion-weighted imaging for detection of primary small (=20 mm) solid pancreatic tumours and prediction of pancreatic ductal adenocarcinoma. Clin Radiol. 2017;72:1076-84.
- [CrossRef] [PubMed] [Google Scholar]
- international cancer of the pancreas screening (CAPS) consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339-47.
- [CrossRef] [PubMed] [Google Scholar]
- A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut. 2016;65:1505-13.
- [CrossRef] [PubMed] [Google Scholar]
- White paper on pancreatic ductal adenocarcinoma from society of abdominal radiology's disease-focused panel for pancreatic ductal adenocarcinoma: Part II, update on imaging techniques and screening of pancreatic cancer in high-risk individuals. Abdom Radiol (NY). 2020;45:729-42.
- [CrossRef] [PubMed] [Google Scholar]
- Is screening for pancreatic cancer in high-risk groups cost-effective?-Experience from a Danish national screening program. Pancreatology. 2016;16:584-92.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic resonance imaging surveillance detects early-stage pancreatic cancer in carriers of a p16-leiden mutation. Gastroenterology. 2011;140:850-6.
- [CrossRef] [PubMed] [Google Scholar]
- Interobserver agreement for EUS findings in familial pancreatic-cancer kindreds. Gastrointest Endosc. 2007;66:62-7.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of small (=2 cm) pancreatic adenocarcinoma and surrounding parenchyma: Correlations between enhancement patterns at triphasic MDCT and histologic features. BMC Gastroenterol. 2014;14:16.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic strategies for early pancreatic cancer. J Gastroenterol. 2015;50:147-54.
- [CrossRef] [PubMed] [Google Scholar]
- Early detection of sporadic pancreatic cancer. Pancreas. 2015;44:7.
- [CrossRef] [PubMed] [Google Scholar]
- Focal parenchymal atrophy and fat replacement are clues for early diagnosis of pancreatic cancer with abnormalities of the main pancreatic duct. Tohoku J Exp Med. 2020;252:63-71.
- [CrossRef] [PubMed] [Google Scholar]
- Added value of diffusion-weighted imaging to MR cholangiopancreatography with unenhanced mr imaging for predicting malignancy or invasiveness of intraductal papillary mucinous neoplasm of the pancreas: DWI of IPMNs. J Magn Reson Imaging. 2013;38:555-63.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of helical CT and MR imaging in detecting and staging small pancreatic adenocarcinoma. Abdom Imaging. 1997;22:429-33.
- [CrossRef] [PubMed] [Google Scholar]
- High signal intensity on diffusion-weighted magnetic resonance images is a useful finding for detecting early-stage pancreatic cancer. Abdom Radiol (NY). 2021;46:4817-27.
- [CrossRef] [PubMed] [Google Scholar]
- MR Imaging techniques for pancreas. Radiol Clin North Am. 2012;50:379-93.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging features of small (=3 cm) pancreatic solid tumors on gadoxetic-acid-enhanced MR imaging and diffusion-weighted imaging: An initial experience. Magn Reson Imaging. 2012;30:916-25.
- [CrossRef] [PubMed] [Google Scholar]
- The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-74.
- [CrossRef] [Google Scholar]
- Diffusion-weighted image improves detectability of magnetic resonance cholangiopancreatography for pancreatic ductal adenocarcinoma concomitant with intraductal papillary mucinous neoplasm. Medicine (Baltimore). 2019;98:e18039.
- [CrossRef] [PubMed] [Google Scholar]
- High-b value diffusion-weighted MRI for detecting pancreatic adenocarcinoma: Preliminary results. Am J Roentgenol. 2007;188:409-14.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic diffusion-weighted imaging (DWI): Comparison between mass-forming focal pancreatitis (FP), pancreatic cancer (PC), and normal pancreas. J Magn Reson Imaging. 2009;29:350-6.
- [CrossRef] [PubMed] [Google Scholar]
- Mass-forming Type 1 autoimmune pancreatitis mimicking pancreatic cancer: Mass-forming autoimmune pancreatitis. J Dig Dis. 2016;17:202-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effective apparent diffusion coefficient parameters for differentiation between mass-forming autoimmune pancreatitis and pancreatic ductal adenocarcinoma. Abdom Radiol (NY). 2020;46:1640-7.
- [CrossRef] [PubMed] [Google Scholar]
- Diffusion-weighted MR imaging of the pancreas: Current status and recommendations. Radiology. 2015;274:45-63.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative detection of small pancreatic carcinoma: Value of adding diffusion-weighted imaging to conventional MR imaging for improving confidence level. Radiology. 2014;273:433-43.
- [CrossRef] [PubMed] [Google Scholar]
- Missed pancreatic ductal adenocarcinoma: Assessment of early imaging findings on prediagnostic magnetic resonance imaging. Eur J Radiol. 2015;84:1473-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic cancer surveillance: Who, when, and how. Curr Treat Options Gastroenterol. 2019;17:681-91.
- [CrossRef] [PubMed] [Google Scholar]







