Translate this page into:
A Rare Case of Primary Malignant Pericardial Mesothelioma
Address for correspondence: Dr. Rajoo Ramachandran, Department of Radiology, Sri Ramachandra University, Porur, Chennai - 600 116, Tamilnadu, India. E-mail: drrajoor@gmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Primary malignant pericardial mesothelioma (PMPM) is a rare tumor of the pericardium. The cause of this tumor is unknown and it has a very poor prognosis. Exposure to asbestos is correlated with the onset of pleural and peritoneal mesothelioma; however, the role of asbestos in pericardial mesothelioma is unclear. Here we highlight the radiological features of this rare tumor and its correlative pathological confirmation with the help of new immunohistochemical (IHC) markers.
Keywords
Immunohistochemical markers
pericardial effusion
pericardial mesothelioma
pericardial thickening
INTRODUCTION

Primary malignant pericardial mesothelioma (PMPM) is a rare tumor of the pericardium. It accounts for approximately 2-3% of all cardiac and primary pericardial tumors. It is the third most encountered tumor after angiosarcoma and rhabdomyosarcoma.[1] However, the most common malignant tumor involving the pericardium is metastasis. Here we report a case of PMPM with emphasis on its radiological imaging findings and clinicopathological correlation.
A 55-year-old woman presented to the emergency department with complaints of breathing difficulty that had persisted for 2 days. The patient is a home maker with no history of exposure to asbestos. On examination, the patient was in sinus rhythm with a blood pressure of 110/80 mmHg and ECG showed sinus tachycardia. Arterial blood gas analysis revealed respiratory alkalosis. Ultrasound identified bilateral mild to moderate pleural effusion. The patient was then shifted to the intensive care unit because of severe breathing difficulty. Echocardiogram showed mild pericardial effusion with an ejection fraction of 65%. Based on the clinical assessment, ultrasonography (USG), and echocardiogram (ECHO) findings, the patient was evaluated with computed tomography (CT).
RADIOLOGICAL FEATURES
On the basis of clinical assessment and ECHO findings, the patient was referred for cross-sectional imaging. She underwent contrast-enhanced computed tomography (CECT) scan of the thorax on a 64-slice CT scanner (General Electric Light Speed, Milwaukee, WI, USA). CECT was performed by injecting 60 ml of intravenous contrast Iohexol (Omnipaque; General Electric Healthcare, Milwaukee, WI, USA) through an 20-gauge needle in the antecubital vein at a rate of 3.5 ml/s. Scanning parameters used were tube current of 300 mA and peak tube voltage of 120 kVp. Acquisition was done at a slice thickness of 5 mm. In addition to a baseline non-enhanced scan, image acquisition was done during venous phase (50 s). The 5-mm-thick axial images were reformatted into thinner sections in three orthogonal planes (0.625 mm thick), which demonstrated enhancing irregular lobular thickening of the pericardium (maximum thickness of 15 mm) and fluid collections within the pericardial sleeves [Figure 1a and b]. Multiple enlarged lymph nodes were found in the right upper and lower paratracheal, prevascular, aortopulmonary regions and in the pericardial fat pads, with the largest measuring 11.5 mm [Figure 1c and d]. Bilateral moderate pleural effusions with no enhancing pleural nodules were noted. No mass lesions were seen in either of the lung fields. In view of the absent primary lesions elsewhere and enlarged lymph nodes in the pericardial fat pad, pericardial mesothelioma was the probable diagnosis. Therapeutic and diagnostic pericardial and pleural tapping was done and sent for cytological examination, which showed presence of malignant cells in the pericardial aspirate and absence of the same in the pleural aspirate. An F-18 fluorodeoxyglucose (FDG-18) positron emission tomography/computed tomography (FDG-PET/CT) showed metabolically active uptake in the pericardial thickening (SUV 5 g/ml) and in mediastinal lymph nodes (SUV 4.6 g/ml) [Figure 2a and b]. No metabolically active lesions were found elsewhere. Following radiological suspicion, CT-guided biopsy of the pericardial thickening was performed for further histopathological analysis.
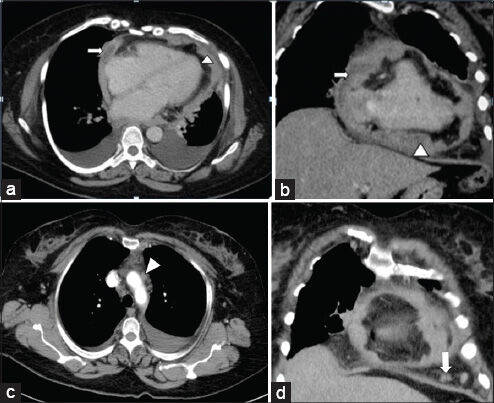
- 55-year-old woman with breathing difficulty diagnosed with primary malignant pericardial mesothelioma. (a and b) Contrast-enhanced axial reformatted CT images of the thorax show lobulated thickening of the pericardium (arrow head) and fluid collections within the pericardial sleeves (arrow). (c and d) Contrast-enhanced coronal reformatted CT images of the thorax show enlarged prevascular (arrow head) and pericardial lymph nodes (arrow).
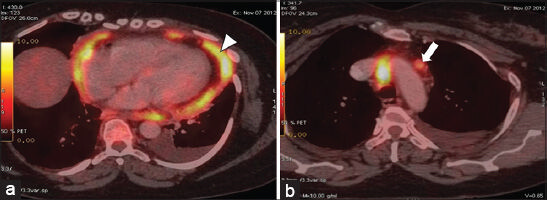
- 55-year-old woman with breathing difficulty diagnosed with primary malignant pericardial mesothelioma. (a and b) FDG-PET/CT axial images show metabolically active uptake in the pericardial thickening (arrow head) and in the mediastinal lymph node (arrow).
PATHOLOGICAL FEATURES
On routine histopathologic examination, the tumor cell morphology of the mesothelioma and other metastatic tumors, especially adenocarcinoma of the lung, overlap a lot. Gland formation can be seen in both. Presence of papillary processes and biphasic tumors cells (epithelial and spindle cells) when present, favors a diagnosis of mesothelioma. In our case, gland formation was noted with occasional papillary process [Figure 3a]. In this core biopsy, it was very difficult to definitely diagnose this as a case of mesothelioma, especially with only hematoxylin and eosin stained slides, and hence immunohistochemistry was advised.
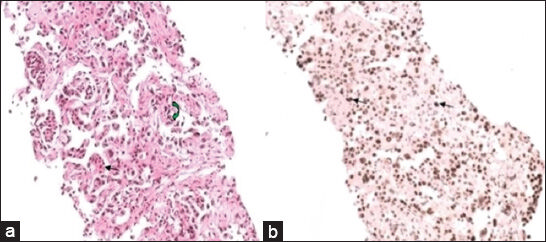
- 55-year-old woman with breathing difficulty diagnosed with primary malignant pericardial mesothelioma. a) Photomicrograph of hematoxylin and eosin (×200) stained biopsy sample of the pericardium shows tumor cells forming glands (black arrow) and occasional papillary process (green arrow). b) Immunohistochemistry analysis (×200) of the biopsy tissue shows tumor cells with nuclear positivity for Wilm's Tumor 1 (WT1) marker (black arrow).
Immunohistochemistry in the diagnosis of mesothelioma
Previously, electron microscopy was the Gold Standard in the differential diagnosis of adenocarcinoma of lung and mesothelioma. Adenocarcinomas show short microvilli, but the mesotheliomas show long, slender microvilli. Currently, specific immunohistochemical (IHC) markers like Wilm's Tumor 1 (WT1) and Thyroid Transcription factor 1 (TTF1) have almost eliminated the use of electron microscopy. While the former is highly specific for mesothelial cells, the latter is a prudent marker for primary carcinoma of the lung. In our case, the tumor cells were positive for WT1 [Figure 3b] and p63 (another marker for mesothelial cells) and negative for TTF1. These staining patterns, in addition to its pathognomic radiological features, confirmed the diagnosis of primary malignant mesothelioma of the pericardium.
DISCUSSION
Primary tumors involving the pericardium are rare and can be benign (teratoma, fibroma, lipoma) or malignant (mesothelioma, sarcoma). In contrast, secondary tumors (i.e. metastasis from the lung, breast, melanomas, lymphoma, or leukemia) are more common.[2] The incidence of PMPM was found to be 0.0022%[3] and can occur at any age with a male to female ratio of 3:1.[4]
Mesothelioma is a malignancy derived from serous epithelial cells of the mesothelium. It occurs most commonly in the pleura (88.8%), peritoneum (9.6%), both pleura and peritoneum (0.6%), and the tunica vaginalis of testis (0.2%).[56]
The cause of this rare tumor is unknown. Exposure to asbestos is correlated with the onset of pleural and peritoneal mesothelioma; however, the role of asbestos in pericardial mesothelioma is unclear.
Ante-mortem diagnosis is extremely difficult because the clinical presentation is nonspecific. The clinical presentation is that of constrictive pericarditis or as pericardial effusion with or without tamponade.[7] The majority of physical findings are nonspecific. Tachycardia is the usual presentation. Heart sounds may be attenuated if pericardial fluid is present. Clinically cardiac tamponade produces visible juglar venous pulsations, hypotension, or in rare cases shock.
Pericardial mesothelioma may present as a localized tumor associated with pericardial effusion[8] or as a mass encasing the pericardium.[9] Local invasion into the cardiac chambers occurs occasionally.[10] Distant metastasis to the pericardial mesothelioma is very rare.
CT-guided percutaneous biopsy is very useful in case of mediastinal, parenchymal, and pleural pathologies. CT-guided pericardial biopsy is minimally invasive with less mortality and morbidity as compared to conventional open pericardiectomy for tissue diagnosis; hence, it is a preferred mode for collection of biopsy tissue.
The treatment options for this rare tumor are surgery, radiotherapy, and chemotherapy. But the results are modest and provide no significant difference in prognosis, with a median survival time of about 6 months from diagnosis.
CONCLUSION
Overall, the prognosis of PMPM is very poor due to its late presentation, inability to completely eradicate the tumor by surgery, and its poor response to radiotherapy and chemotherapy. Imaging studies can be useful in aiding the surgeon to decide if the tumor is localized and amenable to resection or if it is encapsulating, in which case resection is inappropriate. The new IHC markers help to differentiate this tumor from lung primary and metastasis from other organs. Careful clinical, radiological, and pathological correlation is extremely necessary for the diagnosis of this very rare tumor.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2014/4/1/47/139737
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Primary pericardial mesothelioma presenting as constrictive pericarditis. Arc Oncol. 2005;13:150-2.
- [Google Scholar]
- Malignant pericardial diseases: Diagnosis and treatment. Am Heart J. 1987;113:785-90.
- [Google Scholar]
- Primary malignant pericardial mesothelioma-a rare cause of pericardial effusion and consecutive constrictive pericarditis: A case report. J Med Case Rep. 2009;3:9256.
- [Google Scholar]
- Primary malignant pericardial mesothelioma presenting as pericardial constriction. Ann Thorac Cardiovasc Surg. 2008;14:396-2007.
- [Google Scholar]
- Malignant Mesothelioma 1982: Review of 4710 published cases. Br J Dis Chest. 1983;77:321-43.
- [Google Scholar]
- Cardiac constriction due to malignant disease of the pericardium. Ir J Med Sci. 1983;152:454-5.
- [Google Scholar]
- Magnetic resonance imaging findings in a patient with pericardial mesothelioma. Am Heart J. 1988;115:1321-2.
- [Google Scholar]
- Primary pericardial mesothelioma demonstrated by magnetic resonance imaging. Jpn Circ J. 1996;60:898-900.
- [Google Scholar]
- Primary malignant pericardial mesothelioma mimicking left atrial myxoma. Scand J Thorac Cardiovasc Surg. 1987;21:273-5.
- [Google Scholar]






