Translate this page into:
Partially calcified giant hemorrhagic syringomyelia and hematomyelia

*Corresponding author: Kreshnike Dedushi Hoti, Department of Radiology, Faculty of Medicine, University of Prishtina “Hasan Prishtina”, Kosovo, Albania. kreshnike.dedushi@uni-pr.edu
-
Received: ,
Accepted: ,
How to cite this article: Duriqi A, Dedushi Hoti K, Gocaj K, Hyseni F. Partially calcified giant hemorrhagic syringomyelia and hematomyelia. J Clin Imaging Sci. 2025;15:22. doi: 10.25259/JCIS_138_2024
Abstract
Syringomyelia is a rare condition characterized by the formation of a fluid-filled cyst within the spinal cord, leading to myelopathy. In addition, the pathological enlargement of the central canal is referred to as hydromyelia or cleft-like syrinx. We present a case of idiopathic syringomyelia and hematomyelia in a 50-year-old female patient with a 5-year follow-up on her disease progression. Magnetic resonance imaging (MRI) images revealed low-signal intensity on T1 and high-signal intensity on T2, with elevated hemorrhagic signal intensity on T1 and low peripheral signal intensity on T2. A fluid-filled lesion measuring 12 × 36 mm was observed between the C7 and Th3 vertebrae, with separation from some of the detailed components. No contrast enhancement was noted following IV contrast administration. Based on the MRI findings, a diagnosis consistent with giant hemorrhagic syringomyelia was established. Subsequently, a neurosurgical intervention was performed, resulting in a reduction in the size of the syringomyelia and a moderate improvement in the patient’s symptom profile.
Keywords
Giant hemorrhagic syringomyelia
Hematomyelia
Magnetic resonance imaging
Syringomyelia
INTRODUCTION
Spinal epidural hematoma is an uncommon condition typically characterized by sudden, intense pain at the hemorrhage site, which can radiate to the limbs. It may quickly progress to significant neurological impairments. The underlying mechanisms are often unclear, but in the lumbar region, it is commonly attributed to the rupture of Batson’s vertebral venous plexus.[1] Syringomyelia, on the other hand, is a distinct disorder caused by disruptions in normal cerebrospinal fluid (CSF) flow. A thorough imaging assessment using advanced three-dimensional constructive interference in steady-state (CISS) and four-dimensional contrast imaging techniques is essential for identifying the underlying lesion, as this will guide the appropriate surgical approach.[2]
Syringomyelia’s causes include factors that disrupt CSF circulation. Typically, it results from obstructions in the spinal subarachnoid space. The potential causes include:
Syrinx with no identifiable cause
Syringomyelia with obstructions at the foramen magnum (developmental):
Chiari Type 1 Malformation (CM1): Most commonly associated[3]
Basilar invagination.
Syringomyelia with other spinal cord diseases (acquired):
Post-inflammatory:
Postinfectious: Granulomatous infections (e.g., tuberculosis and fungal), postoperative meningitis
Chemical/sterile inflammation: Following subarachnoid hemorrhage or myelography (metrizamide).
Post-traumatic
Spinal Cord Tumors: Especially intramedullary tumors such as hemangioblastoma
Secondary myelomalacia: Including cord compression (due to herniated discs, spondylosis, tumors), infarction, and hematomyelia.[4]
Epidemiological information on syringomyelia is sparse. Studies suggest its prevalence ranges from 8.4/100,000 to 0.9/10,000, with variations across different ethnic and geographic groups.[5]
Magnetic resonance imaging (MRI), with or without contrast, is the preferred diagnostic tool. It provides detailed anatomical information and allows for accurate visualization of the syrinx in both sagittal and axial planes. MRI effectively shows the location, size, and extent of the syrinx cavity and the degree of cerebellar tonsillar ectopia. In patients with CM1, MRI typically shows compression of retro-cerebellar CSF spaces. It also helps exclude cystic lesions or spinal tumors. Leptomeningeal enhancement may indicate infection, while MRI can reveal arachnoid scarring. In addition, MRI can monitor syrinx progression over time, documenting the natural history of syringomyelia. This imaging method also enables non-invasive analysis of CSF hydrodynamics, including disturbances in CSF velocity/flow at the foramen magnum (particularly in patients with less than 5 mm tonsillar ectopia), spinal cord wall motion, and syrinx fluid motion during cardiac systole and diastole.[6]
The natural history of syringomyelia remains poorly understood; its unpredictable and variable nature complicates prognosis. Factors such as etiology, the extent of neurological deficits, the size and location of the syrinx cavity, a syrinx diameter exceeding 5 mm, and associated edema can predict rapid deterioration. The rarity of the condition, its variable progression, and short follow-up periods challenge the assessment of treatment outcomes.[7]
Myelopathy, a major complication of syringomyelia, can lead to severe conditions such as spasticity, which may progress to paraplegia or quadriplegia, along with complications like decubitus ulcers, recurrent pneumonia, and bowel and bladder dysfunction.[8]
CASE REPORT
A 50-year-old female with a history of hypertension and low back pain, attributed to L4-L5 and L5-S1 lumbar spondylolisthesis, presented with progressively worsening central spinal cord syndrome over the past year. Initially diagnosed in 2018 with spondylolisthesis and pain in both legs, particularly the left leg, she was treated with analgesics and corticosteroids, but her symptoms persisted. The pain eventually spread to the thoracic region and chest.
MRI of the cervical spine revealed an extensive T1-T2 intramedullary lesion with an intramedullary hematoma extending into the ependymal canal, accompanied by hemato/syringomyelia. The lesion measured 12 × 36 mm and was located between the C7 and Th3 vertebral levels, with a clearly visible fluid-fluid level [Figure 1]. Neurological examination revealed increased spasticity in the central spine, with lower extremity strength graded 2/5 on the right and 2.5/5 on the left, a sensory level at T2–3, and minimal involvement of the upper C8 roots. Marked osteotendinous reflexes were noted (3+ on both sides), along with clonus pedis and urinary retention.
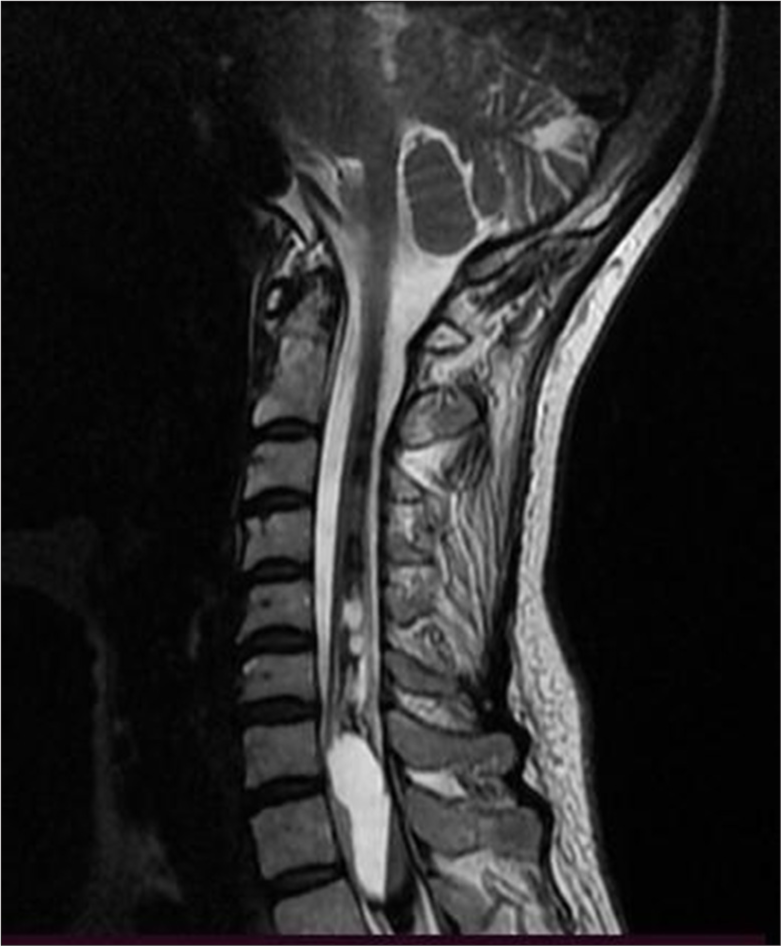
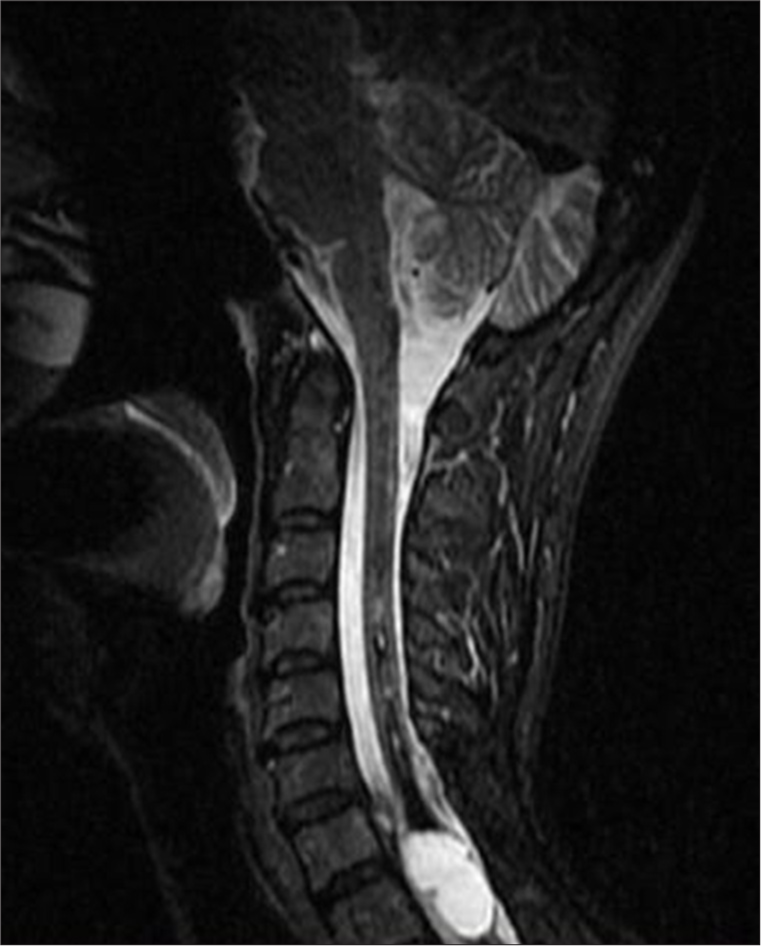
- A 50-year-old female presenting with symptoms of syringomyelia, subsequently diagnosed with a cervicothoracic intramedullary lesion. Sagittal T2-weighted magnetic resonance imaging of the cervical and upper thoracic spine demonstrates an elongated lesion extending from the distal medulla to the upper thoracic spinal cord, measuring 12 × 36 mm, with a distinct fluid-fluid level visible between the C7 and Th3 vertebral levels.
In 2018, an MRI showed an elongated lesion in the distal cervical medulla and upper dorsal spinal cord, exhibiting high-signal intensity on T2-weighted imaging [Figure 2] and heterogeneous hypointensity on T1-weighted images (T1WIs). These findings were consistent with hemato/syringomyelia. A more detailed short tau inversion recovery (STIR) sequence confirmed the fluid-fluid level between C7 and Th3 [Figure 3]. Despite inconclusive digital subtraction angiography, suspicion of a vascular anomaly remained.
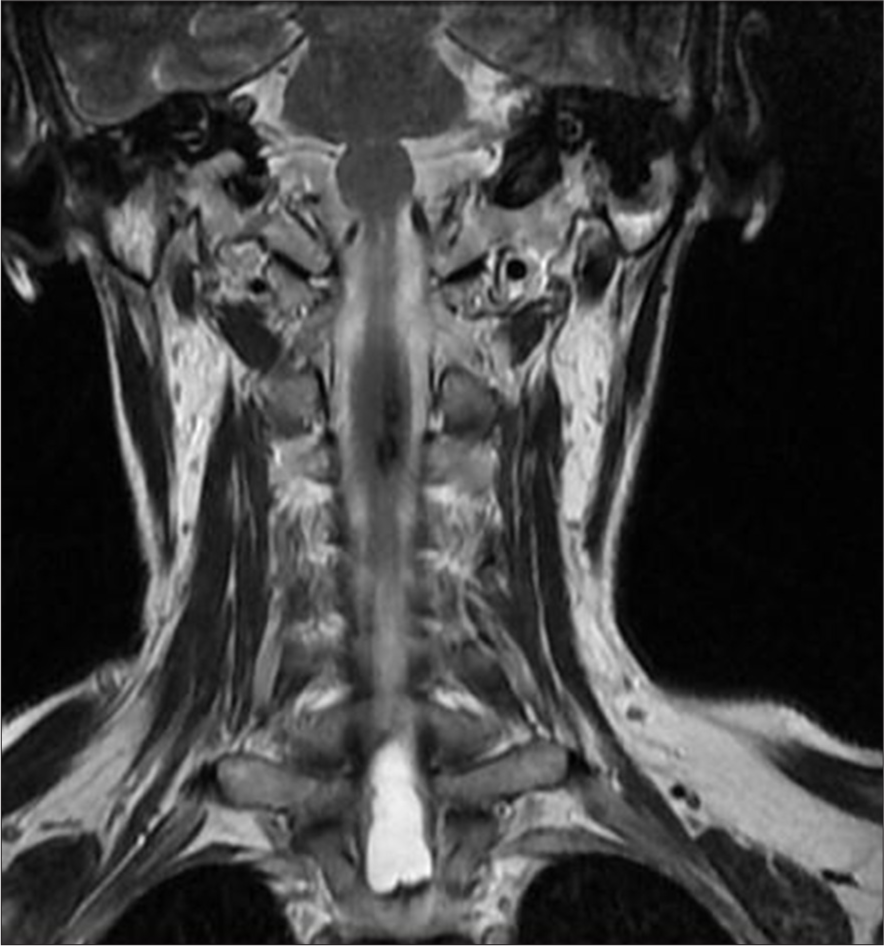
- A 50-year-old female with syringomyelia. Coronal T2-weighted magnetic resonance imaging of the cervicothoracic spine reveals an elongated intramedullary lesion involving the distal medulla, cervical, and upper thoracic spinal cord. The lesion exhibits high-signal intensity and demonstrates fluid-fluid levels.
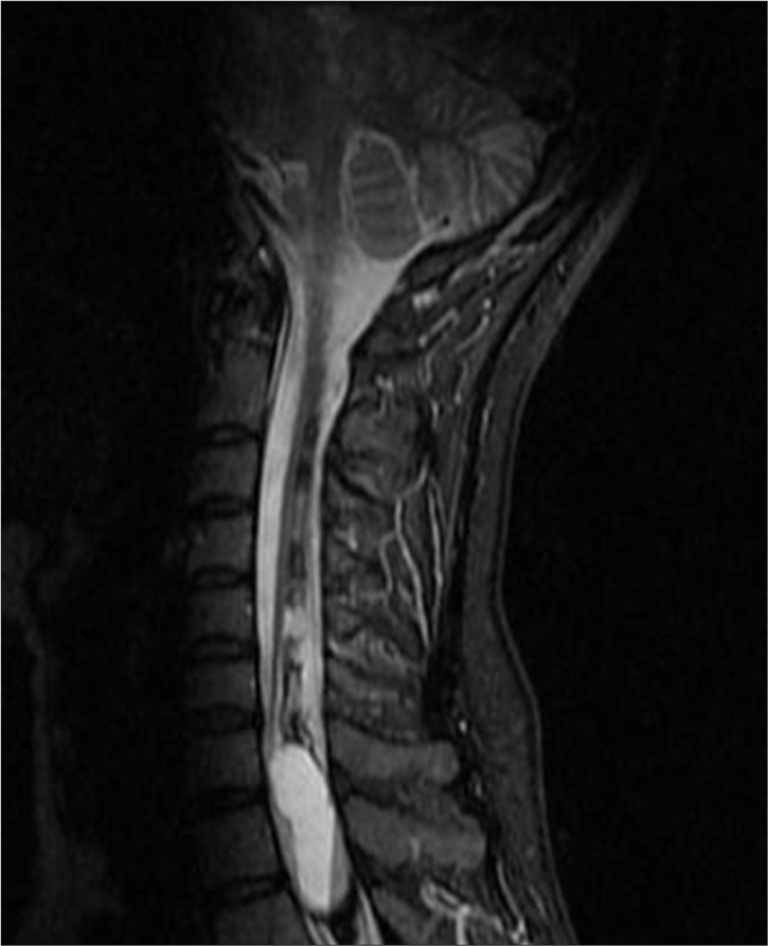
- A 50-year-old female with syringomyelia. Sagittal short tau inversion recovery magnetic resonance imaging of the cervicothoracic spine demonstrates an expansile intramedullary lesion measuring 12 × 36 mm. The lesion contains fluid-fluid levels separated by a thin septum, consistent with giant hemorrhagic syringomyelia and hematomyelia.
Surgical intervention was recommended, and in 2019, the patient underwent a C7-T2 laminectomy and minimal midline T1 myelotomy. The intramedullary fluid was yellow-red, with clotted blood and hemosiderin deposits. A partially calcified lesion was identified in the proximal part of the cavity, and a gross total resection (GTR) was successfully achieved. Postsurgery, MRI imaging showed a reduction in the size of the syrinx and a noticeable improvement in the patient’s symptoms [Figure 4].
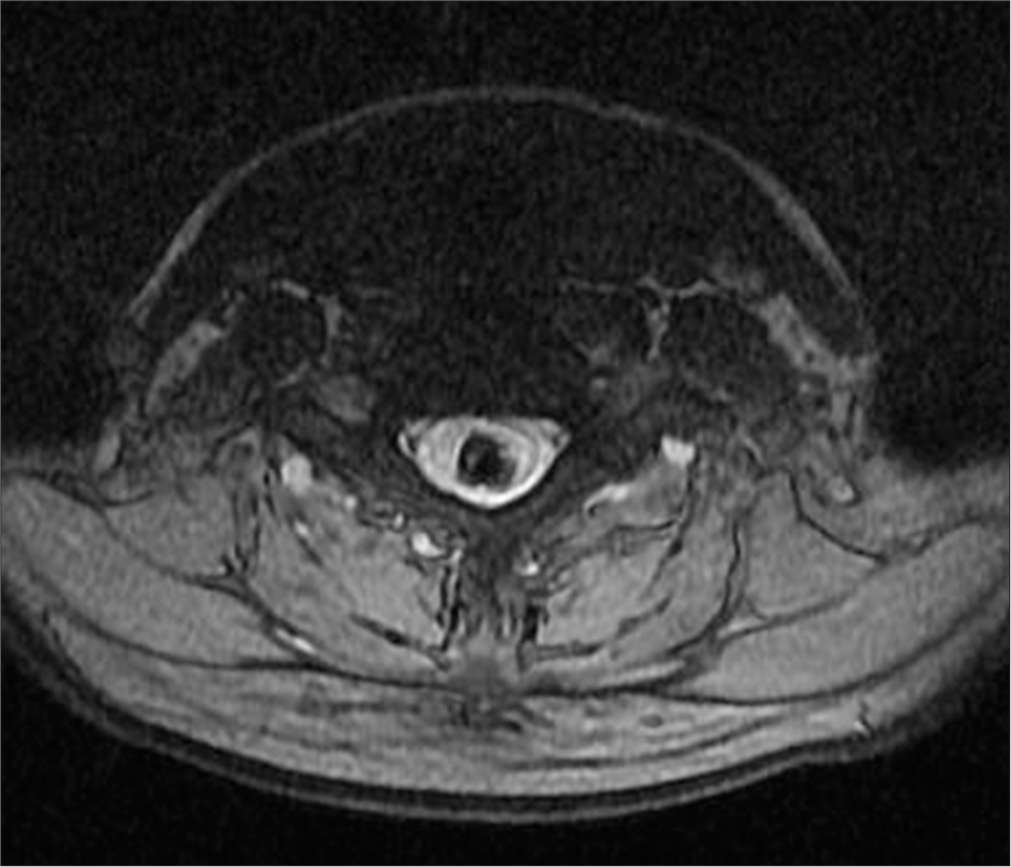
- A 50-year-old female with syringomyelia. Axial T2-weighted magnetic resonance imaging of the cervicothoracic spine shows an elongated intramedullary lesion with low-signal intensity and hemorrhagic characteristics. A thin septum divides the lesion, further supporting the diagnosis of hemorrhagic syringomyelia.
Post-operative MRI revealed minimal syringomyelia in the cervical spinal cord, with decreased T2 signal intensity and thickness of the cervical spinal cord [Figure 5]. A multilobulated expansile intramedullary lesion was still noted between the Th1-Th2 vertebrae, measuring 14 × 26 mm, with peripheral contrast enhancement indicating a vascular malformation [Figures 6 and 7]. Follow-up imaging showed further reduction in the spinal cord thickness and changes consistent with postoperative effects [Figures 8 and 9].
- A 50-year-old female with syringomyelia. Axial T1-weighted magnetic resonance imaging of the cervicothoracic spine demonstrates low-signal intensity within the intramedullary lesion, exhibiting hemorrhagic characteristics and fluid-fluid levels between the C7 and Th3 vertebral levels.
- A 50-year-old female with syringomyelia. Post-contrast sagittal T1-weighted magnetic resonance imaging reveals an expansile intramedullary lesion between the C7 and Th3 vertebrae, demonstrating hemorrhagic signal characteristics without contrast enhancement.
- A 50-year-old female with syringomyelia. Post-operative T2-weighted coronal plane magnetic resonance imaging shows a multilobulated, expansile, hyperintense intramedullary lesion between the Th1 and Th2 vertebrae, measuring 20x13 mm, indicating a residual syringomyelia.
- A 50-year-old female with syringomyelia. Post-operative short tau inversion recovery sagittal plane magnetic resonance imaging demonstrates decreased signal intensity in the cervical spinal cord, indicating a reduction in the syringomyelia following surgery.
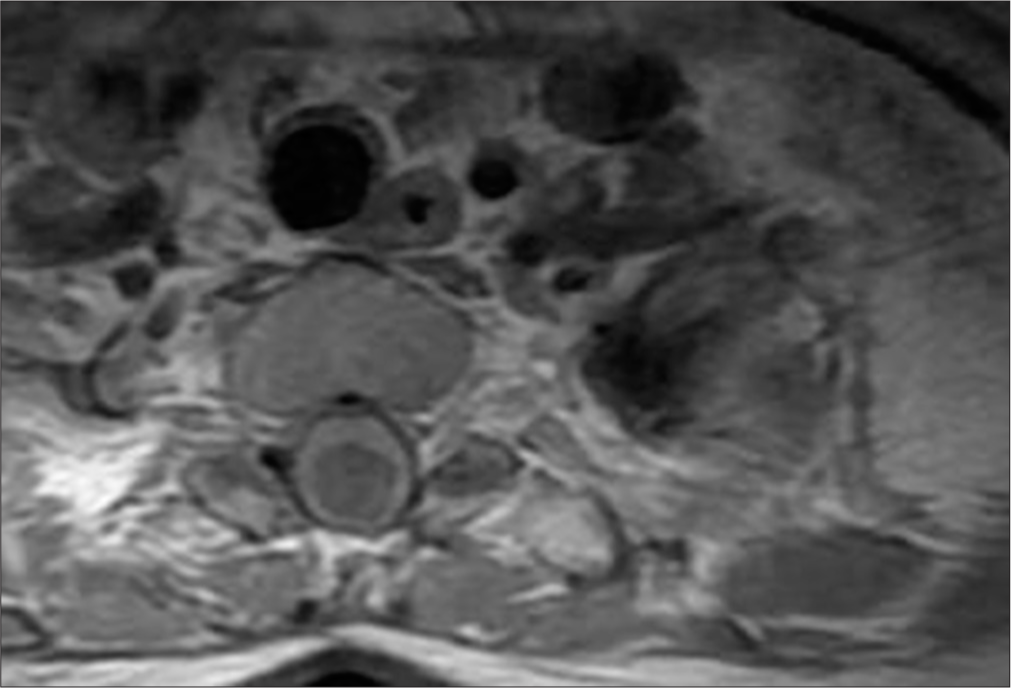
- A 50-year-old female with syringomyelia. T1-weighted post-contrast axial magnetic resonance imaging reveals peripheral contrast enhancement around the lesion between Th1 and Th2, suggesting the presence of a vascular malformation.
The patient’s neurological condition improved moderately after surgery, with partial recovery of motor function and reduction of sensory deficits. A follow-up MRI showed that the remaining expansile hyperintense lesion had stabilized; though some residual syringomyelia was still present [Figure 10].
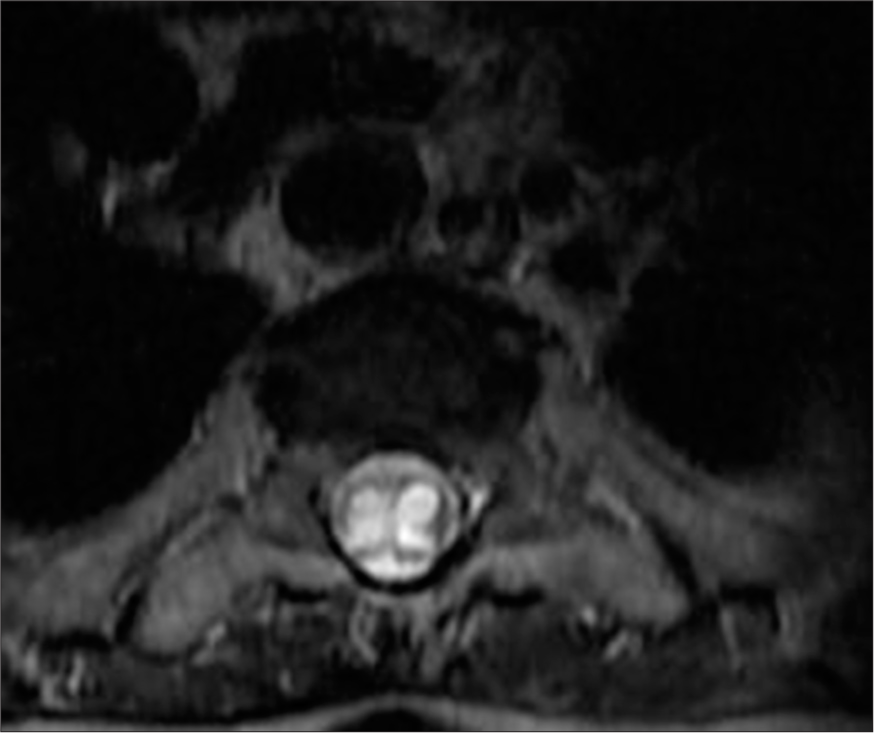
- A 50-year-old female with syringomyelia. T2-weighted axial post-op magnetic resonance imaging shows reduced spinal cord thickness and a remaining expansile hyperintense lesion between Th1 and Th2, consistent with residual syringomyelia.
DISCUSSION
This case involved a complex presentation of syringomyelia combined with hematomyelia, which led to notable neurological symptoms such as spastic paresis, clonus pedis, and urinary retention. MRI findings were consistent with giant hemorrhagic syringomyelia with extensive longitudinal involvement of the spinal cord and the large diameter of the cavity, displaying characteristic low-signal intensity on T1WI, high-signal intensity on T2-weighted images, and hemorrhagic signal characteristics.
Management of this intricate condition required a neurosurgical approach, including C7-T2 laminectomy and minimal midline T1 myelotomy. The intramedullary fluid was yellow-red, containing clotted blood and hemosiderin deposits. A partially calcified lesion was identified in the proximal part of the syrinx cavity, and a gross total resection (GTR) was successfully performed. Post-surgery, there was a noticeable improvement in the patient’s symptoms, underscoring the importance of prompt and appropriate intervention in managing syringomyelia and hematomyelia effectively.[9,10]
Intrasyringeal hemorrhage, where bleeding occurs within a pre-existing syrinx cavity, was first described by Gowers in 1904 and later termed “Gowers’ syringeal hemorrhage” by Wilson in 1955. Such hemorrhages lead to increased pressure within the syrinx cavity, resulting in potentially severe and complex clinical presentations.[11-13]
Although syringomyelia itself is relatively rare, its association with hemorrhage, as illustrated in this case, is even less common. The management of giant hemorrhagic syringomyelia presents distinct diagnostic and therapeutic challenges that necessitate a multidisciplinary approach. Differential diagnoses for syringomyelia include Chiari malformation, spinal cord tumors, and post-traumatic causes.[12]
Hematomyelia, or intramedullary spinal cord hemorrhage, is an uncommon cause of myelopathy and can manifest in various forms, including acute, subacute, stepwise, or chronic presentations. Typically, hematomyelia presents acutely with rapid neurological decline and can result in severe spinal cord syndrome.[14] Prognosis following spinal cord compression is closely related to the extent of neurological impairment and the duration between the onset of symptoms and surgical decompression.[9,10,15]
Early diagnosis and intervention are crucial, as hematomyelia can lead to permanent disability if not addressed promptly. MRI is preferred over conventional myelography or computed tomography (CT) for diagnosing such conditions.[16] Steady-state free precession MRI sequences, as fast imaging employing steady-state acquisition or CISS offer superior contrast resolution between CSF and neural tissue, making them especially valuable for delineating syrinx cavities, detecting subtle septations, and identifying associated arachnoid adhesions or spinal cord tethering. These sequences are highly sensitive for visualizing fluid-filled structures and their relationship with surrounding anatomy. Moreover, dynamic MRI, specifically cine phase-contrast cerebrospinal fluid (CSF) flow studies are particularly valuable for assessing the presence and degree of obstructive CSF flow dynamics associated with syringomyelia. By visualizing and quantifying CSF pulsations in real time, cine MRI can help identify flow disturbances at the foramen magnum or along the spinal canal, which may contribute to syrinx formation or progression. Such information is crucial for determining the need for surgical intervention and tailoring treatment strategies. Moreover, the fluid-fluid level seen on MRI in our case could represent a chronic hemorrhagic process or layering of cellular content, which may complicate further the diagnosis of syringomyelia.
In addition, CT myelography remains an important adjunctive modality, particularly in cases where MRI is inconclusive or contraindicated. It provides high-resolution imaging of the subarachnoid space and is especially useful in identifying CSF flow obstruction or adhesions that may contribute to syrinx formation. Incorporating these advanced imaging modalities into the diagnostic workflow allows for a more comprehensive evaluation and can guide therapeutic decision-making with greater precision. Our study has a limitation, due to not performing a spinal angiogram to clarify the possibility of an underlying vascular lesion such as an arteriovenous malformation, AVF, or cavernoma, which cannot be definitively excluded based on the imaging and clinical data available.
Further, clinical cases presenting with progressive neurological deficits and hemorrhagic cavity formation warrant urgent surgical intervention. Thus, neurosurgical intervention remains the primary treatment strategy to address the bleeding source and to restore neurological function. Timely surgical intervention significantly improves disease outcomes, reducing both mortality and disability.[15,17]
This case emphasizes the critical importance of early diagnosis, a multidisciplinary approach, and long-term follow-up in managing giant hemorrhagic syringomyelia and hematomyelia. Continued research is necessary to enhance understanding of these complex neurological conditions and to explore new treatment strategies.
In conclusion, this case underscores the significance of recognizing and managing giant hemorrhagic syringomyelia and hematomyelia, highlighting the benefits of early intervention and comprehensive care in optimizing patient outcomes.
CONCLUSION
Syringomyelia and giant hemorrhagic hematomyelia are intricate neurological conditions requiring meticulous management and long-term care. Advances in diagnostic imaging, particularly MRI, have proven crucial in the evaluation and treatment of these disorders. Early and precise diagnosis through MRI, coupled with timely surgical intervention, significantly enhances patient outcomes. The sophistication of modern imaging techniques enables detailed assessment of these conditions, facilitating early detection and optimal treatment planning. Thus, the judicious use of MRI and thorough interpretation are vital for achieving favorable results and improving patient prognosis in cases of syringomyelia and giant hemorrhagic hematomyelia.
Ethical approval:
The Instituional Review Board approval is not required.
Declaration of Patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirms that they have used artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript the images are original and not AI generated.
Financial support and sponsorship: Nil.
References
- Spinal epidural hematoma. J Am Acad Orthop Surg. 2010;18:494-502.
- [CrossRef] [PubMed] [Google Scholar]
- Chiari I malformation redefined: Clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44:1005-17.
- [CrossRef] [PubMed] [Google Scholar]
- Non-communicating syringomyelia: A feature of spinal cord involvement in multiple sclerosis. Brain. 2008;131:1776-82.
- [CrossRef] [PubMed] [Google Scholar]
- Syringomyelia fluid dynamics and cord motion revealed by serendipitous null point artifacts during cine MRI. AJNR Am J Neuroradiol. 2017;38:1845-7.
- [CrossRef] [PubMed] [Google Scholar]
- Syringomyelia and its surgical treatment--an analysis of 75 patients. J Neurol Neurosurg Psychiatry. 1981;44:273-84.
- [CrossRef] [PubMed] [Google Scholar]
- Disordered swallowing due to a syrinx: Correction by hunting. Neurology. 1984;34:1497-8.
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic spontaneous intraspinal intramedullary hemorrhage: A report of two cases and literature review. Clin Neurol Neurosurg. 2013;115:1134-6.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous intramedullary hematoma initially mimicking myocardial infarction. Am J Emerg Med. 2014;32:1294.e3-4.
- [CrossRef] [PubMed] [Google Scholar]
- Hematomyelia as a complication of syringomyelia: Gowers' syringal hemorrhage. Case report. J Neurosurg. 1966;25:447-51.
- [CrossRef] [PubMed] [Google Scholar]
- Intrasyringal hemorrhage of the cervical cord associated with Chiari type I malformation-case report. Neurol Med Chir (Tokyo). 1995;35:243-6.
- [CrossRef] [PubMed] [Google Scholar]
- Gowers intrasyringal hemorrhage. Case report and review of the literature. J Neurosurg Spine. 2005;3:477-81.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic progressive hematomyelia: Case reports and review of the literature. Surg Neurol. 1999;51:559-63.
- [CrossRef] [PubMed] [Google Scholar]
- Non-traumatic Hematomyelia: A rare finding in clinical practice. Eur J Case Rep Intern Med. 2018;5:000961.
- [CrossRef] [PubMed] [Google Scholar]
- Spinal cord dysfunction caused by non-traumatic hematomyelia. Spinal Cord. 1996;34:268-71.
- [CrossRef] [PubMed] [Google Scholar]
- Hematomyelia as a consequence of arteriovenous malformation disruption. Russ Neurol J. 2021;26:40-5.
- [CrossRef] [Google Scholar]






