Translate this page into:
Computed tomography and magnetic resonance imaging findings of intracranial and extracranial meningioma with perineural spread through the foramen rotundum: A case report

*Corresponding author: Hiroki Kato, Department of Radiology, Gifu University, Gifu, Japan. kato.hiroki.w4@f.gifu-u.ac.jp
-
Received: ,
Accepted: ,
How to cite this article: Suto T, Kato H, Ogawa T, Matsuo M. Computed tomography and magnetic resonance imaging findings of intracranial and extracranial meningioma with perineural spread through the foramen rotundum: A case report. J Clin Imaging Sci. 2024;14:36. doi: 10.25259/JCIS_97_2024
Abstract
We report a case of a 73-year-old woman presenting with intracranial and extracranial meningioma with perineural spread crossing the foramen rotundum and pterygopalatine fossa, primarily along the maxillary nerve. She visited our hospital with the chief complaint of the left cheek swelling. Subsequent magnetic resonance imaging revealed a well-demarcated mass containing intracranial (cavernous sinus, Meckel’s cave, and middle cranial fossa) and extracranial components (pterygopalatine fossa, orbit, nasal cavity, ethmoid and sphenoid sinuses, infratemporal fossa, and buccal space) through the foramen rotundum. A biopsy specimen was obtained from the cheek mass, and the histopathological diagnosis was meningothelial meningioma.
Keywords
Meningioma
Perineural spread
Foramen rotundum
Pterygopalatine fossa
Magnetic resonance imaging
INTRODUCTION
Meningiomas are the most common intracranial extraparenchymal tumors and account for approximately 15–20% of all primary intracranial tumors. The majority of extracranial meningiomas are caused by secondary extensions of intracranial tumors that spread to the calvarial bones, perineural spaces, or vascular channels. Extracranial meningiomas that are continuous with intracranial components through the perineural spread (PNS) are extremely rare.
PNS is a form of local invasion in which primary tumor cells spread along the endoneurium or perineurium. In the head and neck region, the most common primary tumors of PNS are squamous cell carcinoma; however, adenoid cystic carcinoma has the highest relative incidence of PNS.[1] Other malignancies that can cause PNS include salivary gland duct carcinoma, mucoepidermoid carcinoma, desmoplastic melanoma, malignant lymphoma, and rhabdomyosarcoma.[2] Although PNS in benign tumors is a relatively rare condition, it can be caused by several benign skin and breast tumors and parotid gland adenomas.[3] To date, only five cases of meningiomas with PNS have been reported.[4-8] As a result, this case report presents a 73-year-old female who had intracranial and extracranial meningioma with PNS crossing the foramen rotundum and pterygopalatine fossa, primarily along the maxillary branch of the trigeminal nerve (V2).
CASE REPORT
A 73-year-old female had been aware of the left cheek swelling for approximately 10 years and visited a nearby clinic when nasal congestion appeared 7 years ago. The clinician identified left nasal polyps with bony protrusion of the left palate, and the nasal polyps were resected. Last year, she went to the nearby clinic for the left serous otitis media, and polypoid lesions were noted in the left oropharynx. Despite being treated with prednisolone, the polypoid lesions did not ameliorate. Her previous medical conditions included mediastinal thyroid goiter, hypertension, diabetes, and insomnia.
Magnetic resonance imaging (MRI) images indicated a well-demarcated mass with intracranial and extracranial components through the left foramen rotundum, primarily along the maxillary nerve. T2- and diffusion-weighted images revealed that the majority of the mass was isointense relative to the cerebral cortex [Figure 1a]. Contrast-enhanced T1-weighted images showed relatively homogeneous contrast enhancement [Figure 1b-d]. In terms of intracranial components, the mass spreads into the left cavernous sinus, Meckel’s cave, and middle cranial fossa [Figure 1b]. Contrast-enhanced T1-weighted images revealed dural thickening in the left middle cranial fossa adjacent to the mass [Figure 1b]. In terms of extracranial components, the mass marked the pterygopalatine fossa as the starting point, which extended in all directions [Figure 1c and d]. The mass spread superiorly into the orbit through the inferior orbital fissure; medially into the nasal cavity, ethmoid sinus, and sphenoid sinus through the sphenopalatine foramen; and laterally into the infratemporal fossa and buccal space through the pterygomaxillary fissure [Figure 1c and d]. Computed tomography (CT) images revealed hyperostosis adjacent to the mass in the sphenoid, ethmoid, and maxillary bones and orbital medial and inferior walls [Figure 2]. The buccal space tumor was subjected to an excisional biopsy through the canine fossa using a sublabial approach. Histopathologically, tumor cells had round nuclei proliferating in whorl formation [Figure 3]. Eosinophilic cytoplasmic invaginations, also known as intranuclear pseudoinclusions, were detected. Few mitotic figures and necrosis were noted [Figure 3]. Immunohistochemical staining revealed positive results for epithelial membrane antigen and progesterone receptor, but negative results were found for AE1/AE3, leukocyte common antigen, calponin, p63, synaptophysin, CD56, chromogranin A, and β-catenin. The Molecular Immunology Borstel 1 index was expressed in <5% of tumor cells. Based on these pathological findings, a final diagnosis of meningothelial meningioma (the World Health Organization [WHO] Grade I) was made.
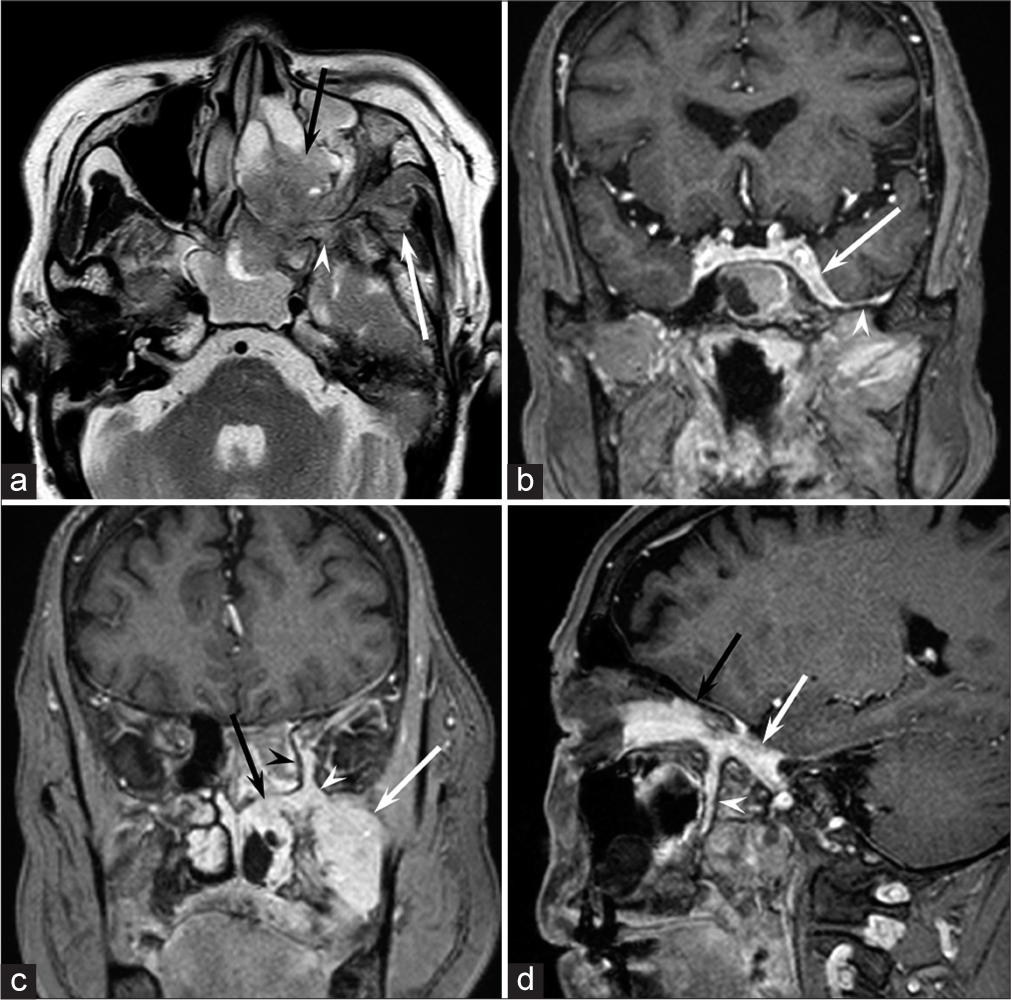
- A 73-year-old woman with meningioma who presented with the left cheek swelling. (a) Axial T2-weighted image shows an isointense mass relative to the cerebral cortex in the left pterygopalatine fossa (white arrowhead) spreading medially into the nasal cavity (black arrow) through the sphenopalatine foramen and laterally into the infratemporal fossa (white arrow) through the pterygomaxillary fissure. (b) Coronal fat-suppressed contrast-enhanced T1-weighted image shows a homogeneously enhanced mass spreading into the left cavernous sinus (white arrow) with dural thickening of the middle cranial fossa (white arrowhead). (c) Coronal fat-suppressed contrast-enhanced T1-weighted image shows a homogeneously enhanced mass in the left pterygopalatine fossa (white arrowhead) spreading superiorly into the orbit (black arrowhead) through the inferior orbital fissure, medially into the nasal cavity (black arrow) through the sphenopalatine foramen, and laterally into the infratemporal fossa (white arrow) through the pterygomaxillary fissure. (d) Sagittal fat-suppressed contrast-enhanced T1-weighted image shows a homogeneously enhanced mass in the left pterygopalatine fossa (white arrowhead) spreading superiorly into the orbit (black arrow) through the inferior orbital fissure and posteriorly into the foramen rotundum (white arrow).
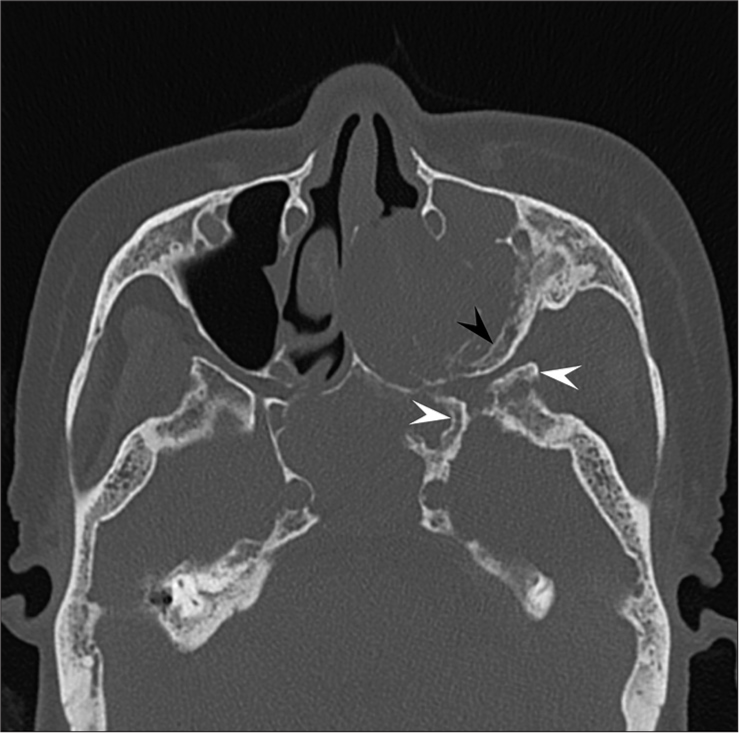
- A 73-year-old woman with meningioma who presented with the left cheek swelling. Axial computed tomography image shows hyperostosis in the left sphenoid (white arrowheads) and maxillary (black arrowhead) bones.
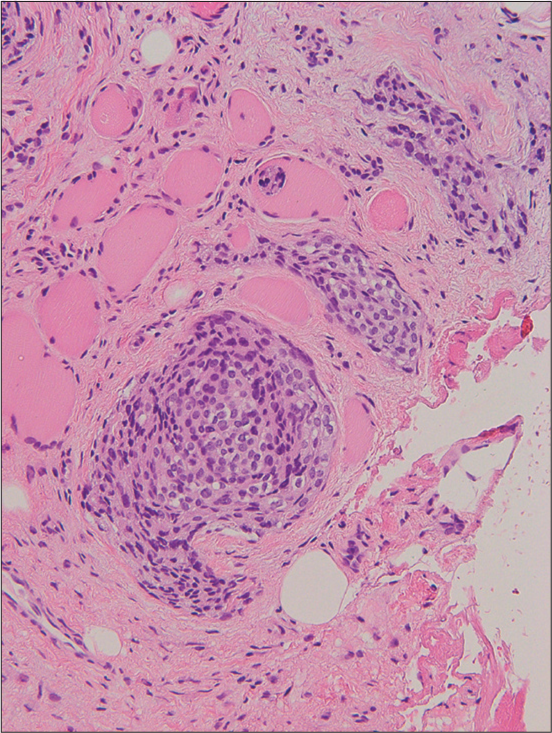
- A 73-year-old woman with meningioma who presented with the left cheek swelling. Hematoxylin and eosin stained image shows tumor cells with round nuclei proliferating in whorl formation. Eosinophilic cytoplasmic invaginations, also known as intranuclear pseudoinclusions, were detected. Few mitotic figures and necrosis w ere noted. Hematoxylin and eosin stained image (×100 magnification)
DISCUSSION
To date, only five cases of meningiomas with PNS have been reported.[4-8] Four meningiomas formed in the middle cranial fossa and spread into the cavernous sinus.[4-7] These meningiomas spread along the mandibular branch of the trigeminal nerve (V3) through the foramen ovale and extended into the infratemporal fossa.[4-7] Three of these four meningiomas spread along the maxillary branch of the trigeminal nerve (V2), passing through the foramen rotundum and extending into the pterygopalatine fossa.[5-7] The remaining meningioma developed in the posterior cranial fossa and extended into the parapharyngeal space through the hypoglossal canal.[8] Among the four meningiomas with PNS in which histological WHO Grade was determined, two were Grade II (atypical meningiomas)[5,6] and two were Grade I (meningothelial and transitional meningiomas).[7,8]
The pathogenesis of PNS is poorly known, and it was initially considered to progress along perineural lymphatics. However, it is currently believed that various signaling pathways exist that cause PNS[3] and are associated with the production of cell molecules with a high affinity for nerves.[9] The signaling pathways involved in the induction of PNS include the phosphoinositide 3-kinase/Akt pathway, which regulates vital cellular functions; mitogen-activated protein kinase pathway, which is crucial for cell proliferation and differentiation; and Janus kinase/signal transducer and activator of transcription pathway, which is known for immune regulation.[3] Nerve growth factor (NGF) in pancreatic and prostate cancers, brain-derived neurotrophic factor (BDNF) in pancreatic and adenoid cystic carcinoma, glial cell line-derived neurotrophic factor in pancreatic cancer, and neurotrophin-3 in pancreatic cancer are all potential causes of PNS. The expression of BDNF is associated with aggressiveness and unfavorable survival in oral squamous cell carcinoma,[10] proliferation and invasion in lung squamous cell carcinoma,[11] and pelvic metastasis in uterine cervical squamous cell carcinoma.[12] NGF and BDNF expressions have been detected in meningiomas and adjacent meninges,[13] indicating that comparable factors may be overexpressed in meningiomas and surrounding tissues, making them more susceptible to PNS. In meningiomas, BDNF is strongly expressed in the vorticoid areas, and this finding suggests that they play a direct role in the development and progression of the meningiomatous cells.[13] One of the characteristic histological features of meningiomas is the vorticoid area, also referred to as whorl formation, which is usually observed in the WHO Grade I meningioma. Therefore, PNS may occur in the WHO Grade I meningioma. Meanwhile, anaplastic (WHO Grade III) meningioma is extremely rare and accounts for 1–3% of all meningiomas. Therefore, PNS may not have been reported in the WHO Grade III meningioma.
The most sensitive imaging approach for identifying PNS is contrast-enhanced T1-weighted images. Fat-suppressed contrast-enhanced T1-weighted images are recommended because the contrast enhancement is more clearly visible and distinguishable from the surrounding fat-suppressed tissue. Although 18F-fluorodeoxyglucose-positron emission tomography (PET)/CT is occasionally used to evaluate PNS, it is inferior to MRI for PNS diagnosis due to the small volume of the tumor and limited spatial resolution of PET. Various diseases that may cause PNS in the head and neck region should be considered when making a differential diagnosis of meningioma. CT attenuation, MRI signal intensity, and degree of contrast enhancement of meningiomas are all non-specific, making it challenging to diagnose meningiomas with PNS. According to previous case reports and our experience, CT images show hyperostosis of the skull base and contrast-enhanced T1-weighted images show dural thickening of cranial fossa, which may indicate meningiomas with PNS.
Among the four meningiomas with PNS in which their treatments were described, two were treated with partial resection and post-operative adjuvant radiotherapy, one was treated with partial resection alone, and one was followed without treatment. In these cases, complete resection could not be achieved due to extensive PNS. Different radiotherapy approaches, such as external beam radiotherapy and single-fraction stereotactic radiosurgery, can be used for meningiomas in the adjuvant setting after surgical resection or to treat disease recurrence.[14] Adjuvant radiotherapy can be proposed for incomplete resection of the WHO Grade I meningioma, for example in high-risk areas such as the cavernous sinus, or if subsequent salvage total resection is not possible, with a dose of approximately 50 Gy.[14] The role of adjuvant radiotherapy is still controversial for the WHO Grade II meningioma, but it can be considered in cases of incomplete resection.[14] In cases of meningioma with PNS, complete resection is considerably difficult; therefore, combined partial resection and post-operative adjuvant radiotherapy may be a reasonable treatment strategy.
CONCLUSION
We have reported a rare case of intracranial and extracranial meningioma with PNS crossing the foramen rotundum and pterygopalatine fossa, primarily along the maxillary branch of the trigeminal nerve (V2). Because meningioma with PNS is uncommon, it is difficult to differentiate it from other head and neck tumors. However, radiologists should be aware that meningioma can cause PNS, particularly if CT images show hyperostosis of the skull base and contrast-enhanced T1-weighted images show dural thickening of the cranial fossa.
Acknowledgment
The authors would like to thank Chiemi Saigo (Department of Pathology, Gifu University) for providing histopathological diagnosis and images.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Mimics of perineural tumor spread in the head and neck. Br J Radiol. 2021;94:20210099.
- [CrossRef] [Google Scholar]
- Perineural invasion and perineural tumor spread in head and neck cancer. Int J Radiat Oncol Biol Phys. 2019;103:1109-24.
- [CrossRef] [Google Scholar]
- Unraveling the mysteries of perineural invasion in benign and malignant conditions. Curr Oncol. 2023;30:8948-72.
- [CrossRef] [Google Scholar]
- Perineural spread of head and neck tumors: How accurate is MR imaging? AJNR Am J Neuroradiol. 1998;19:701-6.
- [Google Scholar]
- Childhood atypical meningioma with perineural spread: MR findings. Pediatr Radiol. 2005;35:895-8.
- [CrossRef] [Google Scholar]
- A rare case of atypical skull base meningioma with perineural spread. J Radiol Case Rep. 2015;9:1-14.
- [CrossRef] [Google Scholar]
- An unusual case of a grade I meningioma with perineural spread. Br J Neurosurg. 2023;37:637-40.
- [CrossRef] [Google Scholar]
- Atypical meningioma with perineural spread along hypoglossal nerve. Indian J Radiol Imaging. 2024;34:566-8.
- [CrossRef] [Google Scholar]
- Perineural invasion in cancer: A review of the literature. Cancer. 2009;115:3379-91.
- [CrossRef] [Google Scholar]
- Activation of BDNF/TrkB/Akt pathway is associated with aggressiveness and unfavorable survival in oral squamous cell carcinoma. Oral Dis. 2019;25:1925-36.
- [CrossRef] [Google Scholar]
- More expression of BDNF associates with lung squamous cell carcinoma and is critical to the proliferation and invasion of lung cancer cells. BMC Cancer. 2016;16:171.
- [CrossRef] [Google Scholar]
- Expression of BDNF, TrkB, VEGF and CD105 is associated with pelvic lymph node metastasis and prognosis in IB2-stage squamous cell carcinoma. Exp Ther Med. 2019;18:4221-30.
- [CrossRef] [Google Scholar]
- Immunohistochemical profile of neurotrophins in human cranial dura mater and meningiomas. Oncol Rep. 2009;21:1373-80.
- [CrossRef] [Google Scholar]
- Meningioma: Not always a benign tumor. A review of advances in the treatment of meningiomas. CNS Oncol. 2021;10:CNS72.
- [CrossRef] [Google Scholar]







