Translate this page into:
Presacral tumors: A systematic review of literature

*Corresponding author: Jeffrey Otote, Department of Surgery, Barking Havering and Redbridge Univerisity Hospitals NHS Trust, Romford, United Kingdom. j.otote@nhs.net
-
Received: ,
Accepted: ,
How to cite this article: Otote J, Butnari V, Ravichandran P, Mansuri A, Ahmed M, Pestrin O, et al. Presacral tumors: A systematic review of literature. J Clin Imaging Sci. 2024;14:17. doi: 10.25259/JCIS_27_2024
Abstract
Presacral/Retrorectal tumors (RRT) are rare lesions that comprise a multitude of histological types. Data on surgical management are limited to case reports and small case series. The aim of the study was to provide a comprehensive review of the epidemiology, pathological subtypes, surgical approaches, and clinical outcomes. A PubMed search using terms “retrorectal tumor” and “presacral tumor” was used to identify articles reporting RRT of non-urological, non-gynecologic, and non-metastatic origin. Articles included were between 2015 and 2023. A total of 68 studies were included, comprising 570 patients. About 68.2% of patients were female, and the mean overall age of both sexes was 48.6 years. Based on histopathology, 466 patients (81.8%) had benign lesions, and 104 (18.2%) were malignant. In terms of surgical approach, 191 (33.5%) were treated anteriorly, 240 (42.1%) through a posterior approach, and 66 (11.6%) combined. The mean length of stay was 7.6 days. Patients treated using the posterior approach had a shorter length of stay (5.7 days) compared to the anterior and combined approaches. RRT are rare tumors of congenital nature with prevalence among the female sex. R0 resection is crucial in its management, and minimal access surgery appears to be a safer option in appropriate case selection.
Keywords
Retrorectal tumors
Congenital cystic lesions
Perineal approach
Transabdominal approach
Combined abdominoperineal approach
INTRODUCTION
The anatomical complexity and inherent heterogeneity of retrorectal and presacral space lesions pose significant diagnostic hurdles, rendering their surgical management a subject of ongoing debate within the medical community.[1] The configuration of the retrorectal/presacral space presents significant surgical challenges due to its intricate anatomical architecture. This space is defined by the mesorectum anteriorly, the presacral fascia posteriorly, and the lateral boundaries of the iliac vessels and ureters. While precise epidemiological data regarding these lesions are limited, existing literature suggests an estimated incidence of 1 in 40,000–60,000 hospital admissions. Notably, these neoplasms demonstrate a female predilection and a median age of onset at 45 years.[2,3] The rise in diagnostic accuracy for this pathology can be linked to the combined influence of technological advancements in imaging and the increased use of these technologies in gynecological investigations, where the condition is a common finding. This explains the observed higher prevalence among women in their early reproductive years.[4] Retrorectal tumors (RRT) are characterized by a high prevalence of benignity and asymptomatic presentation. Nevertheless, the significant variability in malignant incidence, ranging from 21% to 50%, necessitates careful clinical assessment and prompt management strategies.[5,6] While the majority of RRT exhibit benign histology, surgical resection is often advised in light of the non-negligible risk of malignant degeneration (up to 8%) and the potential for suppurative complications, estimated at approximately 30%.[4,7] Surgical management of retrorectal tumors necessitates meticulous selection of the approach to ensure adequate exposure, minimize iatrogenic injury, and optimize clinical outcomes while mitigating complications. Recognizing the rarity of these lesions and the paucity of dedicated literature, RRTs pose a significant diagnostic and therapeutic challenge for contemporary surgeons. Therefore, this systematic review offers a valuable contribution to the existing literature. As such, this study aims to deliver a comprehensive analysis of the epidemiology, pathological subtypes, surgical approaches, and clinical outcomes associated with RRTs.
MATERIAL AND METHODS
To comprehensively elucidate the intricacies of RRT, we conducted a systematic literature review encompassing their epidemiology, pathologic subtypes, diverse surgical approaches, and associated clinical outcomes.
Search strategy
The review was conducted in line with the protocol, in accordance with the Cochrane handbook for systematic reviews of interventions and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[8,9] This review was registered in PROSPERO: CRD42024500067.
A PubMed search using the terms “retrorectal tumor,” and “presacral tumor” was used to identify the articles reporting of RRT. The search was undertaken on the September 01, 2023, and looked at all articles between March 2015 and September 2023.
Data selection
Initially records were first screened for relevance based on their title and abstract. Two authors (J.O., V.B.) independently screened records for inclusion and were blinded to each other’s decisions. Disagreements between individual judgments were resolved by consensus, and if no agreement could be made, a third author (A.M.) was consulted.
Articles were included if they were full-text case reports, case series or review articles describing primary RRT. The target population consisted primarily of adults (≥16 years old). Articles reporting metastatic disease, urological or gynecological origin of tumor were excluded from the study. Articles which were descriptive and non-English were also excluded from the present study. A flowchart of the selection process in the format of the PRISMA is presented [Figure 1].[9]
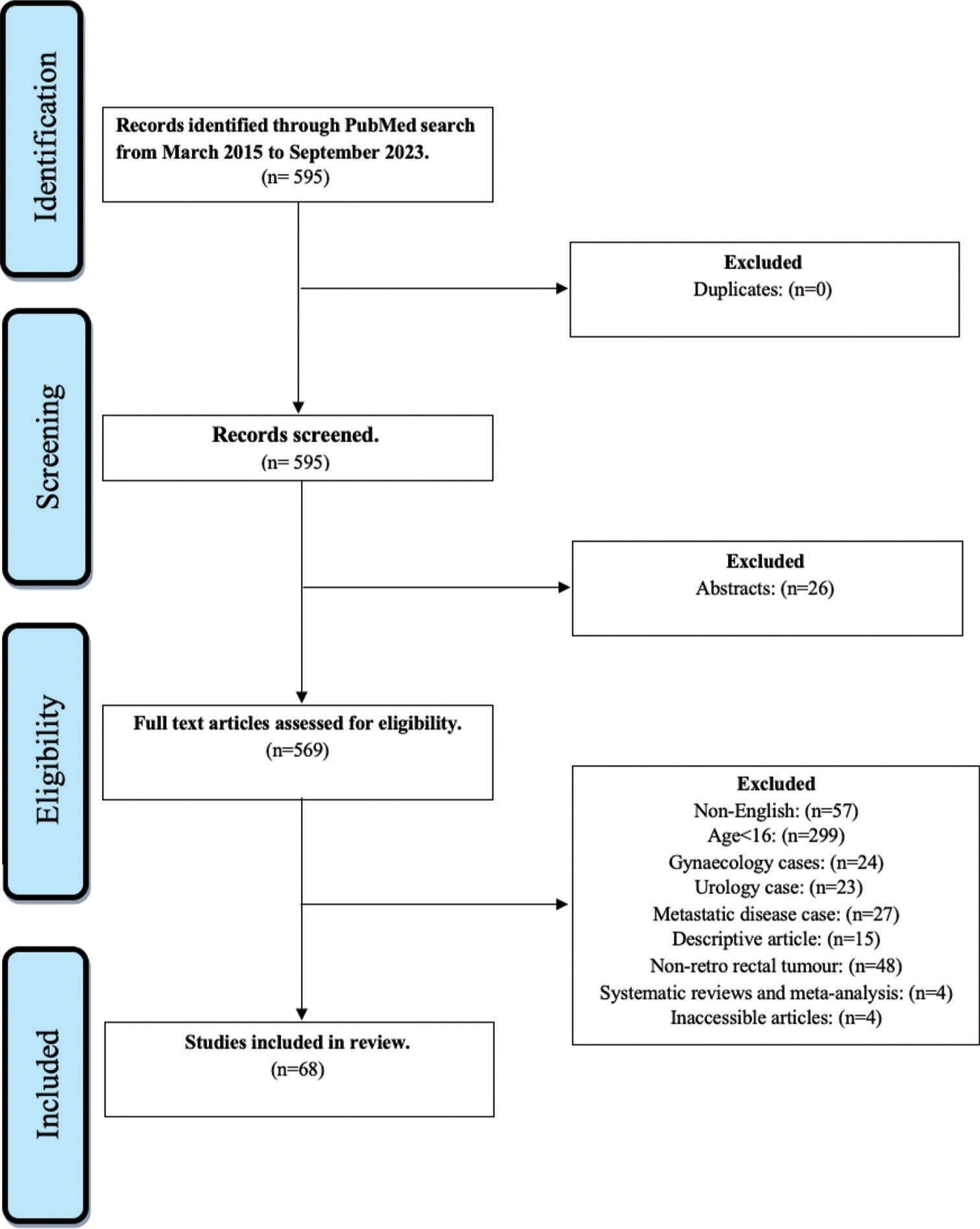
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Data extraction
Two independent reviewers (J.O., V.B.) manually extracted data from full-text articles into a structured database (Microsoft Excel). Extracted fields included primary author, publication year, key study characteristics (sample size, age, gender, and recruitment design), histological type of reported tumors (congenital, neurogenic, osseous, inflammatory, or miscellaneous), surgical approach, operative time, length of stay, resection status for malignancies, overall complication rate, and recurrence status. Disagreements regarding data extraction or categorization were resolved through discussion and consensus. If consensus could not be reached, a senior author (A.M.) adjudicated the case.
Definitions
Tumors were categorized according to established definitions: Congenital – lesions present at birt h (most common); neurogenic – slow-growing tumors arising from peripheral nerves (second most common); osseous – tumors arising from bone, cartilage, or marrow; inflammatory less common, potentially linked to perirectal or abdominal infections; and miscellaneous – encompasses 10–25% of cases, including lipomas, fibromas, hemangiomas, leiomyomas, and liposarcomas.[10]
Data analysis
Given the significant heterogeneity between studies on this topic, a formal narrative synthesis was the most appropriate analytical approach. Statistical analyses primarily relied on descriptive statistics, where findings were presented as rates, means, and ranges. For subgroups defined by histological type (e.g., congenital, neurogenic, osseous, inflammatory, and miscellaneous), survival, malignancy potential, surgical approach, and tumor recurrence rates were expressed as rates and means.
RESULTS
Overview on basic characteristics of included studies
A comprehensive literature search yielded 595 citations encompassing abstracts and full-text articles. Figure 1 illustrates the selection process, ensuring a well-defined and representative sample for subsequent analysis. Rigorous screening eliminated duplicates (26 conference abstracts); irrelevant studies based on selection criteria were excluded (n = 501). Ultimately, 68 studies (570 patients) met the inclusion criteria, focusing on primary RRTs. Table 1 summarizes single case reports, while Table 2 details case series. Females constituted 68.2% of the cohort, with an average age of 48.6 years (range: 18–89).
The primary tumors in this study were classified according to the Uhlig and Johnson classification syste m.[11] This system categorizes tumors into five groups: congenital, neurogenic, osseous, inflammatory, and miscellaneous [Table 3]. The majority of tumors in this study were benign (466, or 81.8%). The most common type of benign tumor was congenital (56.8%), followed by neurogenic (15.8%), miscellaneous (8.9%), inflammatory (7.2%), and osseous (0%).
| Author | Patient Age | Sex | Primary Lesion /Recurrence | Primary Symptoms | Diagnosed Incidentally? | Diagnosis method | Preoperative Histopathology | Mean tumor diameter on cross sectional imaging (cm) | Lesion extension Above/Below S3 | Operative approach (A/P/C) | Operative method (Open/Lap/Robotic/Converted- reason for conversion/TAMIS) | Benign/Malignant | Histopathology | Mean OR (min) | LOS (days) | Complete resection? (Y/N/NR) | Combined resection? (Y/N/NR) | Overall Complication? (Y/N/NR) | Recurrence reported in F/U period? (Y/N/NR) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kumassah PK, et al.[12] | 37 | F | Primary | Coccydynia CIBH | No | MRI | FNA | NR | Above | C | Open | Mal | Cystic hamartoma, with mucinous adenocarcinoma | NR | 6 | Y | Y (en bloc with rectum with coloanal anastomosis) | N | N |
| Iwata E, et al.[13] | 25 | F | Primary | Coccydynia | No | CT | FNA | 3.5 | Below | P (Kraske) | Open | Mal | Tailgut cyst in which a Grade 2 Neuroendocrine Tumor | NR | NR | Y | N | NR | NR |
| Cataneo J, et al.[14] | 34 | F | Primary | CIBH | No | MRI | Not performed | NR | Below | A | Robotic | B | Dermoid cyst | NR | 1 | Y | N | N | N |
| Fang H, et al.[15] | 43 | M | Primary | Asymptomatic | Yes | MRI | FNA | 16.1 | Above | A | Open | B | Tailgut cyst | NR | 5 | Y | N | N | N |
| Mora-Guzmán I, et al.[16] | 56 | F | Primary | Coccydynia, anal fistula | No | MRI | FNA | 4.1 | Below | P (Kraske) | Open | B | Tailgut cyst | NR | 5 | Y | Y (en bloc with the coccyx) | N | N |
| Jun C, et al.[17] | 22 | F | Primary | Asymptomatic | Yes | MRI | FNA | NR | Above | A | Robotic | B | Schwannoma | NR | 3 | Y | N | N | NR |
| Criss CN, et al.[18] | 19 | F | Recurrence | Left buttock and thigh pain | No | MRI | Not performed | 8.2 | Above | A | Robotic | B | Lipoblastoma | 960 | 4 | Y | N | N | NR |
| Yang BL, et al.[19] | 36 | M | Recurrence | Coccydynia | No | MRI | Known from prior resections | 4 | Below | P (Kraske) | Open | Mal | Moderately differentiated adenocarcinoma | NR | NR | Y | N | N | N |
| Cho MH, et al.[20] | 70 | F | Primary | Coccydynia | No | MRI | FNA | 3.7 | NR | NR | NR | B | Myelolipoma | NR | NR | Y | N | N | NR |
| Roy SP, et al.[21] | 29 | F | Primary | Coccydynia | No | USS | Not performed | 4.6 | Above | A | Robotic | B | Tailgut cyst | NR | 2 | Y | N | N | NR |
| Kesavan S, et al.[22] | 51 | F | Primary | Intermittent lower abdominal pain | Yes | USS | Not performed | 4.7 | Above | A | Laparoscopic | B | Epidermoid cyst | NR | 2 | Y | N | N | N |
| Tarchouli M, et al.[23] | 45 | M | Primary | Intermittent lower abdominal pain | No | MRI | Not performed | 7 | Below | P (Kraske) | Open | Mal | Low-grade leiomyosarcoma | NR | 2 | Y | N | N | N |
| Bouzid A, et al.[24] | 22 | F | Primary | Intermittent lower abdominal pain | No | USS | Not performed | 8.5 | Above | A | Laparoscopic converted to open due to suspicion of S1 nerve involvement | B | Ganglioneuroma | NR | 7 | N | N | N | N |
| Gutierrez O, et al.[25] | 37 | F | Primary | Acute pelvic pain | Yes | MRI | Not performed | 2.9 | Below | P (York-Mason) | Open | B | Cystic hamartoma | NR | NR | Y | N | Y | NR |
| Tan GHC, et al.[26] | 76 | M | Primary | Acute pelvic pain | Yes | CT | Not performed | 15 | Above | A | Open | B | Angiomyxoma/liposarcoma | 415 | 43 | Y | N | Y (SSI required surgical drainage) | N |
| Seydafkan S, et al.[27] | 52 | F | Recurrent | Asymptomatic | Yes | USS | Known from prior resections | 5.3 | Below | P (Kraske) | Open | B | Tailgut cyst | NR | NR | Y | N | NR | NR |
| Carchman E, Gorgun E[28] | 76 | M | Primary | Obstructive uropathy | No | CT | FNA | 8 | Above | A | Robotic | B | Low-grade fibromyxoid sarcoma fibrosarcoma | NR | 3 | Y | N | N | NR |
| Alvarez-Sarrado E, et al.[29] | 49 | F | Primary | Asymptomatic | Yes | CT | Not performed | 13.5 | Above | A | Open | B | Epidermoid cyst | NR | 60 | Y | N | Y (Decompensation of epilepsy) | NR |
| Şahin S et al.[30] | 55 | F | Primary | Asymptomatic | Yes | MRI | Not performed | 17.3 | Below | P (Kraske) | Open | Mal | Tailgut cyst (malignant degeneration) | NR | NR | Y | N | NR | NR |
| Pizzuti V et al.[31] | 41 | M | Primary | Coccydynia | No | MRI | Not performed | 4 | Below | P (Modified Kraske) | Open | B | Schwannoma | NR | 4 | Y | N | N | NR |
| Patel A et al.[32] | 54 | F | Recurrence | Coccydynia | No | MRI | Known from prior resections | 9.7 | Below | C | Laparoscopic also Kraske | B | Tailgut cyst | 160 | 4 | Y | NR | N | N |
| Perungo T et al.[33] | 23 | F | Primary | Coccydynia | No | CT | Not performed | 4.6 | Below | P (Kraske) | Open | B | Epidermoid cyst. | NR | NR | Y | N | N | N |
| Inada R et al.[34] | 67 | F | Primary | Asymptomatic | Yes | CT | Not performed | NR | Below | A | Laparoscopic | B | Tailgut cyst | 199 | NR | Y | Y (en block with descending colon) | N | N |
| Huang M, et al.[35] | 34 | M | Primary | Asymptomatic | Yes | USS | FNA | 11.3 | Above | A | Open | Mal | Schwannoma | NR | 7 | Y | N | N | N |
| Kawamura J, et al.[36] | 61 | F | Primary | Asymptomatic | Yes | CT | FNA | NR | Below | P | TAMIS | Mal | Extra-nodal marginal-zone lymphoma of mucosa associated lymphoid tissue (MALT) | 100 | 7 | Y | N | N | NR |
| Zhu XL[37] | 51 | M | Primary | Abdominal pain | No | CT | Not performed | 9.3 | Above | A | Open | Mal | primary alveolar rhabdomyosarcoma | NR | NR | Y | N | N | N |
| Tobias-Machado M[38] | 60 | M | Primary | Abdominal pain | No | CT | Not performed | NR | Above | A | Laparoscopic | B | Schwannoma | 150 | 2 | Y | N | N | NR |
| Alshahri J[39] | 74 | M | Primary | Back pain | No | CT | Not performed | 11.2 | Above | A | Open | Mal | Synchronous Chondroma of sacrum and moderately differentiated adenocarcinoma | NR | NR | Y | Y (en bock with sigmoid colon with end-to-end colorectal anastomosis as well as low sacrectomy with VRAM flap) | Y (SSI requiring IR drainage as well as postoperative PE) | N |
| Kearney D[40] | 58 | F | Primary | Chronic abscess in sacral area | Yes | MRI | FNA | 9.3 | Below | P (Kraske) | Open | B | Tailgut cyst | NR | 5 | Y | N | N | N |
| Mahajan UV[41] | 62 | F | Primary | Back pain radiating to the whole lower limb | No | MRI | FNA | 3.1 | Above | P | Open | B | Perineural | NR | 7 | Y | Y (A sacral laminectomy) | Y | N |
| Li W[42] | 33 | M | Primary | Asymptomatic | Yes | MRI | Not performed | 6.4 | Below | P (Kraske) | Open | B | Tailgut cyst | NR | 10 | Y | Y (en block with coccyx) | N | NR |
| Naf F[43] | 59 | F | Primary | Coccydynia | Yes | MRI | Not performed | 4.7 | Below | A | Laparoscopic | B | Teratoma | NR | 10 | Y | N | NR | NR |
| Singh A, et al.[44] | 63 | M | Primary | CIBH | No | MRI | FNA | 5.3 | Below | NR | NR | Mal | Well-differentiated neuroendocrine tumor (Grade I) | NR | NR | Y | N | NR | N |
| Tokuyama S[45] | 31 | M | Primary | Pain in the thigh | Yes | CT | Not performed | 3.4 | Above | A | Laparoscopic | B | Tailgut cyst | 132 | 6 | Y | N | N | N |
| Borsuk DJ[46] | 31 | F | Recurrence | F/U post previous resection | No | MRI | Not performed | 8 | Below | A | Robotic | B | Epidermoid cyst | NR | 1 | Y | N | N | N |
| Schleinstein HP[47] | 94 | M | Primary | CIBH | No | CT | Not performed | 10 | Below | A | Open | B | Schwannoma | NR | 7 | Y | N | Y (Major bleeding during surgery required 4 units of RBC) | NR |
| Colombo F[48] | 46 | M | Recurrence | Second opinion regarding his recurrent sacral epidermoid tumor |
No | MRI | Not performed | NR | Below | P | Open | Mal | NR | NR | N | N | N | N | Y (local recurrence diagnosed during surveillance at 6 moths) |
| Benjamin B[49] | 29 | F | Primary | Acute lower abdominal pain | Yes | MRI | Not performed | 6 | Below | A | Open | B | Castleman’s | NR | NR | Y | Y (en block with rectosigmoid with end-to-end colorectal anastomosis) | N | N |
| Carvalho BJ[50] | 41 | M | Primary | Acute lower abdominal pain | No | CT | Not performed | 3.9 | Above | A | Laparoscopic | B | Schwannoma | 260 | 1 | Y | N | N | N |
| Turati L[51] | 75 | M | Primary | Coccydynia | No | MRI | Not performed | 14.5 | Below | A | Open | Mal | Leiomyosarcoma | NR | 35 | Y | Y (Total pelvic exenteration) | Y (chylous leak, conservatively treated with an alipidic diet) |
Y (distant disease diagnosed during surveillance at 6 months) |
| Rakici SY, et al.[52] | 55 | F | Primary | Inability to walk that emerged in the last 1 year | No | MRI | Incision biopsy | 1.25 | Above | P | Open | B | Ganglioneuromas | NR | NR | Y | N | Y (unable to urinate post operative day 2) | Y (local disease recurrence diagnosed during surveillance in 1 year) |
| Tsarkov PV[53] | 52 | F | Primary | Acute lower abdominal pain | No | MRI | Not performed | 6 | Above | A | Laparoscopic | B | Tailgut cyst | 120 | 3 | Y | N | N | N |
| Zhao XR[54] | 44 | F | Primary | Coccydynia | No | CT | FNA | 16 | Above | A | Open | Mal | Cystic hamartoma | NR | NR | N (Adherent to surrounding structures, so unresectable) | N | N | NR |
| Maemoto R[55] | 70 | F | Primary | Follow up post rectal cancer surgical treatment | Yes | CT | Not performed | 2.7 | Above | A | Open | B | Epidermoid cyst. | NR | NR | Y | Y (en bloc with colonic involved mesentery preserving vasculature) | N | N |
| Santos AJ[56] | 68 | F | Primary | Lower abdominal pain | Yes | MRI | Not performed | 6.3 | Above | A | Open | B | Schwannoma | NR | 3 | Y | N | N | N |
| Bhadarge PS[57] | 65 | F | Primary | Lower abdominal pain | No | MRI | Not performed | NR | Above | A | Open | Mal | Primitive neuroectodermal tumors | NR | NR | Y | N | N | NR |
| Emohare O[58] | 39 | M | Primary | Chronic low-back pain | Yes | MRI | Not performed | 8 | Above | A | Laparoscopic | B | Schwannoma | 249 | 4 | Y | N | N | N |
| Brackzynski AK[59] | 71 | F | Primary | Severe bacterial meningitis | Yes | MRI | FNA | NR | Above | A | Open | B | Anterior sacral meningocele | NR | NR | Y | N | N | N |
| Rege S[60] | 40 | F | Primary | Multiple miscarriages | Yes | USS | Not performed | 10 | Below | P | Open | B | Fibro collagenous cyst wall lined by stratified squamous epithelium | NR | NR | Y | Y (Sacral laminectomy) | N | N |
| Lorusso D et al[61] | 47 | F | Primary | Anal fissure | Yes | MRI | FNA | 7 | Above | A | Open | Mal | Mucinous adenocarcinoma with osseous metaplasia | NR | NR | Y | N | N | NR |
| Andrade P[62] | 60 | F | Primary | Chronic low-back pain | Yes | MRI | FNA | 4 | Below | P | Open | Mal | Squamous cell carcinoma | NR | NR | Y | Y en block with coccyx | N | N |
| Total | 51 (33F18M) |
45 Primary, 6 Recurrent |
42 symptomatic 9 asymptomatic |
23 Incidental | 30 MRI 15 CT 6 USS |
31 Not performed. 16 FNA 3 Known from prior resections. 1 Incision Biopsy |
26 Above 24 Below 1 NR |
30 A 19 P 2 C 2 NR |
32 Open 9 Laparoscopic 6 Robotic 2 Converted 1 TAMIS 1 NR |
35 Benign 16 Malignancies |
48 complete 3 incompletes |
39 Uncombined 11 Combined 1 NR |
7 Complications 5 NR |
19NR 3 Recurrences |
Key: F – Female, M – Male, NR – Not reported, Y – Yes, N – No, A – Anterior, P – Posterior, C – Combined, CT – Computed Tomography, MRI – Magnetic Resonance Imaging, TAMIS – Transanal Minimally Invasive Surgery, FNA – Fine Needle Aspiration, Mal – Malignant, B – Benign, F/U – Follow Up, USS: Ultrasound scan, CIBH: Change in bowel habit, MALT: Mucosa associated lymphoid tissue, LOS: Length of stay, OR: Operative time, SSI: Surgical site infection, IR: Interventional radiology, PE: Pulmonary embolism
| Author | Number of CS included | Mean Age | F (n) | M (n) | Primary symptoms | Diagnosed incidentally | Asymptomatic | Diagnosis method | Ratio F:M in % | B (n) | Mal (n) | Mean tumor diameter on cross-sectional imaging (cm) | Underwent Surgery | Operative approach (A.P.C) | Operative method (open/lap/robotic/converted/both) | Mean OR (min) | LOS (days) | Complete resection? (Y/N/NR) | Combined resection? (Y/N/NR) | Overall Complication? (Y/N/NR) | Recurrence? (Y/N/NR) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. Hopper, et al.[63] | 69 | 50 | 39 | 30 | Pain (abdominal, buttocks, flank, sacrococcygeal, rectal), bowel, neurological (sciatica, altered sensation) & urinary symptoms, and presence of a mass. | 19 | 3 | CT and MRI performed 28/69 (41%) and 34/69 (49%), respectively | 57:43:00 | 40 | 29 | NR | 27 | 6.15.6 | Open | NR | NR | NR | Y (6/69) | NR | NR |
| Yin J, et al. [64] |
7 | 44 | 6 | 1 | NR | NR | NR | MRI 100% | 86:14:00 | 6 | 1 | NR | All | 5.0.2 | Robotic | 84 | 5.7 | Y (1/7) | Y (2/7) | N | N |
| Leclerc A, et al. [65] |
6 | 52 | 3 | 3 | Constipation, dysuria, radicular or lower back pain | 0 | 0 | All had CT and MRI | 50:50:00 | 6 | 0 | NR | All | A | Open | 240 | 6 | Y (1/6) | NR | N | N |
| Isla A, et al. [66] |
19 | 51 | 11 | 8 | Lower back pain with lower limb radiation, paresthesia, | 6 | 6 | All had CT/MRI, or both |
58:42:00 | 18 | 1 | NR | All | 6.9.4 | Open | NR | NR | Y14/19 | NR | 1 lumbar spinal stenosis, 1 Impairment of external popliteal sciatic nerve | Y 3/19 |
| Kilic A, et al. [67] |
16 | 41 | 10 | 6 | Pelvic, sacral, lower back and perianal pain, discomfort, changes in bowel habits, difficulty in defecation, and tenesmus | 0 | 0 | MRI 100% | 63.5:37.5 | 13 | 3 | NR | 13 | 5.8.1 | NR | NR | NR | NR | NR | Y (2/14) | Y (1/14) |
| Rompen IF, et al. [68] |
5 | 47 | 5 | 0 | NR | 5 | 4 | MRI 100% | 100:0 | 0 | 5 | NR | All | A | Robotic (da Vinci) | 235 | 5.6 | NR | Y (2/5) | NR | NR |
| Manabe T, et al. [69] |
3 | 46 | 1 | 2 | NR | 3 | 3 | CT and MRI were performed | 33:67 | 3 | 0 | NR | All | A | Laparoscopic | 265 | 7.7 | Y | N | N | N |
| Ramalingam K, et al. [70] |
4 | 41 | 4 | 0 | Back pain, constipation, CIBH | 1 | 0 | 3MRI, 1 CT | 100:0 | 4 | 0 | NR | All | P (Kraske) | Open | NR | NR | NR | NR | N | NR |
| Wang B, et al. [71] |
10 | 43 | 9 | 1 | Pain (Abdominal, leg, lower back, perineal, sacral), CIBH, | 0 | 0 | 7CT, 9 MRI, 4 USS | 90:10:00 | 10 | 0 | NR | 6 | 2.4.0 | 5 Open, 1 Laparoscopic | NR | NR | NR | N | Y 3/10 | NR |
| Oguz A, et al. [72] |
17 | 36 | 12 | 5 | Tenesmus, palpable perineal lump, lower urinary tract dysfunction, and rectal hemorrhage | 2 | 0 | 15 MRI, 16 CT | 71:29:00 | 16 | 1 | NR | All | 7.6.4 | NR | NR | 12.4 | NR | NR | Y (4/17) | Y (1/17) |
| Carpelan-Holmström M, et al. [73] |
52 | 43 | 40 | 12 | Lower abdominal pain | 30 | 30 | MRI 100% | 77:23:00 | 48 | 4 | NR | All | 7.44.1 | NR | 118 | 3 | Y (51/52) | N | Y (11/52) | 14/52 (1 mal) |
| Dwarkasing RS, et al. [74] |
28 | NA | 22 | 6 | Nonspecific pelvic pain, obstructed defecation | NR | NR | MRI 100% | 79:21:00 | 23 | 5 | NR | NR | NR | NR | NR | NR | NR | NR | NR | Y (2/28) |
| Maddah G et al. [75] |
50 | 42 | 26 | 24 | CIBH | NR | NR | NR | 52:48:00 | 0 | 50 | NR | All | 22.34.30 | NR | NR | NR | NR | NR | NR | NR |
| Buchs NC, et al. [76] |
62 | 44 | 50 | 12 | Pain, tenesmus, constipation, incontinence | 13 | 13 | 45 MRI, 20 CT, 8 ERUS | 81:19:00 | 49 | 13 | NR | All | 55.7.0 | NR | NR | NR | Y (56/62) | NR | NR | 9 |
| Xu XM [77] |
8 | 34 | 6 | 2 | Sacrococcygeal pain, urinary retention, constipation | 1 | 1 | CT and MRI were performed for all | 75:25:00 | 7 | 1 | NR | All | 1.6.1 | Open | NR | NR | Y | NR | N | Y (1 Mal) |
| Huisman JF [78] |
20 | 64 | 6 | 14 | NR | NR | NR | NR | 30:70 | 20 | 0 | NR | All | P | Laparoscopic | NR | NR | Y | NR | NR | NR |
| Gould LE, et al. [79] |
143 | 46 | 106 | 37 | Pain, CIBH, rectal bleeding | NR | 0 | 76 MRI | 74:26:00 | 125 | 18 | NR | 107 | 24.64.15 | 78 (Open) 10 (Laparoscopic) |
NR | NR | NR | Y (24) | Y (104/143) | Y (5) |
| Total | 519 | 356 | 163 | 80 | 60 | 388 | 131 | 406 28NR |
161.221.64 | 87 Open 6 NR 13 Lap 2 Robotic |
126 complete | 34 reported combined resections | 125 reported complications | 36 reported recurrences |
CS: Case series, F: Female, M: Male, n: Number, Y: Yes, N: No, NR: Not reported, CIBH: Change in bowel habit, CT: Computed Tomography, MRI: Magnetic Resonance Imaging, USS: Ultrasound scan, ERUS: Endoscopic Rectal Ultrasound, B: Benign, Mal: Malignant, A: Anterior, P: Posterior, C: Combined, Lap: Laparoscopic, OR: Operative time, LOS: Length of stay
| Classification | Case | (% from overall total) |
|---|---|---|
| Congenital | 324 | 56.8 |
| Benign | 284 | 49.8 |
| Tailgut Cyst | 145 | 25.4 |
| Teratoma | 33 | 5.8 |
| Epidermoid Cyst | 36 | 6.3 |
| Dermoid Cyst | 25 | 4.4 |
| Developmental Cyst | 1 | |
| Duplication Cyst | 38 | 6.7 |
| Anterior Sacral Meningocele | 4 | |
| Indeterminant Cyst | 2 | |
| Malignant | 40 | 7 |
| Chordoma | 20 | 3.5 |
| Primitive neuroectodermal | 2 | |
| Tailgut cyst (malignant degeneration) | 6 | |
| Malignant Teratoma | 3 | |
| Germ Cell Tumor | 1 | |
| Developmental Cyst (malignant transformation) | 5 | |
| Dermoid Cyst (malignant degeneration) | 2 | |
| Duplication Cyst (malignant degeneration) | 1 | |
| Neurogenic | 103 | 18.1 |
| Benign | 90 | 15.8 |
| Neurofibroma | 5 | |
| Ganglioneuroma | 5 | |
| Paraganglioma | 2 | |
| Schwannoma | 54 | 9.5 |
| Neuroblastoma | 3 | |
| Perineural cyst (Tarlov) | 21 | 3.7 |
| Malignant | 13 | 2.3 |
| Neurofibrosarcoma | 2 | |
| Neuroendocrine Carcinoma | 3 | |
| Neuroblastoma | 1 | |
| Ependymoma | 2 | |
| Ganglioneuroblastoma | 1 | |
| Malignant peripheral nerve sheath tumor | 4 | |
| Osseus | 8 | 1.4 |
| Benign | 0 | 0 |
| Malignant | 8 | 1.4 |
| Ewing Tumor | 2 | |
| Chondrosarcoma | 4 | |
| Synovial Sarcoma | 1 | |
| Malignant Giant Cell Tumor | 1 | |
| Inflammatory | 41 | 7.2 |
| Benign | 41 | 7.2 |
| Abscess | 28 | 4.9 |
| Fibrosis | 3 | |
| Cyst hydatid | 3 | |
| Inflammatory Cyst | 2 | |
| Diverticulitis | 1 | |
| Fistula | 1 | |
| Unknown Cyst | 3 | |
| Malignant | 0 | 0 |
| Miscellaneous | 94 | 16.5 |
| Benign | 51 | 8.9 |
| Leiomyoma | 6 | |
| Fibroma | 4 | |
| Myelomeningocele | 2 | |
| Myelolipoma | 3 | |
| Solitary fibrous tumor (SFT) | 4 | |
| Hemangiopericytoma | 1 | |
| Oleogranuloma | 1 | |
| Lipoma | 1 | |
| Angiomyxoma | 4 | |
| Neuroenteric Cyst | 1 | |
| Angiomyofibroblastoma | 1 | |
| Fibrolipoma | 1 | |
| Peritoneal serous cystadenoma | 1 | |
| Desmoid tumor | 1 | |
| Lymphoid hyperplasia (Castleman's disease) | 1 | |
| Lipoblastoma | 1 | |
| Bronchogenic cyst | 1 | |
| Angiomyolipoma | 1 | |
| Sclerosing Epithelioid Fibrosarcoma | 1 | |
| Hematoma | 1 | |
| Angiofibroma | 1 | |
| Lymphocele | 1 | |
| Retention cyst of anal gland | 4 | |
| Vascular | 1 | |
| Normal tissue | 1 | |
| Unknown | 6 | |
| Malignant | 43 | 7.5 |
| Gastrointestinal stromal tumor | 9 | |
| Lymphoma | 4 | |
| Leiomyosarcoma | 4 | |
| Liposarcoma | 3 | |
| Undifferentiated sarcoma | 7 | |
| Rhabdomyosarcoma | 1 | |
| Adenocarcinoma | 5 | |
| Mucinous Tumor | 1 | |
| Squamous cell carcinoma | 1 | |
| Spindle cell tumor | 1 | |
| Desmoid type fibromatosis | 1 | |
| Plasmacytoma | 2 | |
| Intra-osseus ganglion cyst | 1 | |
| Unknown | 3 | |
| Total | 570 | 100 |
This study identified 324 patients (56.8%) with congenital tumors, of whom 284 (87.7%) harbored benign lesions (primarily tailgut cysts at 44.8%). Malignant tumors were diagnosed in 40 patients (12.3%), exhibiting a statistically significant association with older age (51.4 vs. 43.1 years). Compared to benign counterparts, malignant tumors displayed a markedly higher recurrence rate (24.2% vs. 2%).
Amongst the studied patients, 18.1% (n = 103) presented with neurogenic tumors, characterized by a mean age of 48 years and a striking predominance of benignity (87.4%, n = 90). Schwannomas emerged as the leading benign subtype (9.5%, n = 54), while retention cyst of the anal gland constituted the primary form of malignancy (n = 4). Notably, malignant neurogenic tumors exhibited a significantly higher recurrence rate compared to their benign counterparts (7.1% vs. 3.2%) [Table 4].
| Classification | Case (%) | Mean age (range) | Complication (%) | Recurrence (%) |
|---|---|---|---|---|
| Congenital | 324 (56.8) | 44.8 (21-74) | 2 | 6.5 |
| Benign | 284 (49.8) | 43.1 (21-71) | 2.1 | 2.4 |
| Malignant | 40 (7.0) | 51.4 (25-74) | 1.5 | 24.2 |
| Neurogenic | 103 (18.1) | 48 (22-94) | 6.4 | 3.6 |
| Benign | 90 (15.8) | 48 (22-94) | 7.1 | 3.2 |
| Malignant | 13 (2.3) | 47 | 0 | 7.1 |
| Osseus | 8 (1.4) | 39.8 (16-58) | 25 | NR |
| Benign | 0 | - | - | - |
| Malignant | 8 (1.4) | 39.8 (16-58) | 25 | NR |
| Inflammatory | 41 (7.2) | 47.6 | 2.4 | 7.3 |
| Benign | 41 (7.2) | 47.6 | 2.4 | 7.3 |
| Malignant | 0 | - | - | - |
| Miscellaneous | 94 (16.5) | 51.3 (19-76) | 1.6 | 3.2 |
| Benign | 51 (8.9) | 49.3 (19-76) | 0.7 | 0 |
| Malignant | 43 (7.5) | 53.6 (35-75) | 4.7 | 14 |
| Overall benign | 466 (81.8) | |||
| Overall malignant | 104 (18.2) | |||
| Total | 570 (100) |
Osseous tumors comprised a mere 1.4% (n = 8) of all cases, characterized by a strikingly young mean age of 39.8 years and exclusive malignancy. Interestingly, chondrosarcomas constituted half of these malignant lesions. When compared to other tumor types, osseous tumors emerged as the youngest subgroup within the analyzed population.
This study identified a subset of 41 patients (7.2%) harboring inflammatory tumors, exclusively benign in nature. The spectrum encompassed abscesses (28 cases), diverticulitis (1 case), fibrosis (3 cases), cyst hydatid (3 cases), inflammatory cysts (2 cases), and unclassified cysts (3 cases). Notably, this group exhibited a higher mean age compared to other tumor categories, at 47.6 years.
Among the analyzed tumors, a noteworthy 16.5% (n = 94) were classified as miscellaneous, encompassing a diverse spectrum of lesions. Patients with miscellaneous tumors exhibited a higher mean age compared to other groups, at 51.3 years. A concerning trend emerged within this category: malignant miscellaneous tumors displayed significantly higher recurrence and complication rates compared to their benign counterparts (14% vs. 0% and 4.7% vs. 0.7%, respectively). Malignant composition – gastrointestinal stromal tumors took the lead among malignant miscellaneous tumors, with nine cases identified. Undifferentiated sarcomas followed closely behind, accounting for five cases. Further investigation into the specific subtypes and clinical characteristics of these aggressive miscellaneous tumors is warranted.
The operative approaches are listed in Table 5. The posterior approach was performed in 240 patients (42.1%), anterior approach in 191 patients (33.5%), and a combined approach in 66 patients (11.6%). The mean post-operative hospital stay was 8.4 days after an anterior approach was performed; 5.7 days after a posterior approach; and 6.6 days after a combined approach.
| Approach | Case # | Mean OR (min)* | Mean LOS (days)* | ||||
|---|---|---|---|---|---|---|---|
| Anterior | 191 | 293.2 | 8.4 | ||||
| Posterior | 240 | 100 | 5.7 | ||||
| Combined | 66 | 160 | 6.6 | ||||
| Operation method | Case # | Mean OR (min)* | Mean LOS (days)* | Complete resection (%) | Combined resection (%) | Complication rate (%) | Recurrence rate (%) |
| Open | 119 | 327.5 | 13.1 | 82.8 | 9.5 | 32.1 | 12 |
| Minimally invasive | 31 | 242.8 | 4.2 | 85.1 | 20 | 10 | 0 |
The overall post-operative complication rate was 20.2% and included surgical site infection, neurological complications (lower limb weakness, paresthesia), hematoma, postoperative bleeding, and change in bowel habit. The overall post-operative recurrence rate was 6.9%.
Thirty-one patients underwent a minimally invasive procedure [Table 5], an approach which had lower recurrence and complication rates when compared to open surgery (0 vs. 12 % and 10 vs. 32.1%, respectively) and was associated with shorter length of stay (4.2 vs. 13.1 days). The operative time was also shorter in minimally invasive surgery (242.8 vs. 327.5 min).
DISCUSSION
RRT management presents a unique dilemma due to their rarity and diverse histology. While open surgery dominates existing literature, minimally invasive techniques are gaining traction. However, robust data scarcity, limited to case reports and small series, impedes definitive conclusions on patient selection, perioperative complications, and oncological outcomes for minimally invasive approaches. Our systematic review reveals female predominance, benign etiology, and congenital classification as common features. Malignant lesions, more prevalent in males, exhibit higher complication and recurrence rates post-resection. Posterior approach emerges as the minimally invasive method of choice with minimal morbidity. Laparoscopy and robotic-assisted laparoscopy, though primarily used in benign cases, show promise with shorter hospital stays and potentially lower recurrence rates. Further research with robust data is crucial to solidify the role of minimally invasive techniques in retrorectal tumor management, particularly for malignant cases.
Radiological presentations of RRT range from asymptomatic and incidental findings to symptomatic manifestations, often associated with infectious or malignant processes. Barraqué et al. report 50% asymptomatic cases in a series of 53, highlighting the potential for subclinical presentation.[80] Symptomatic cases typically present with abdominal and lower back pain, possibly indicating underlying infection or malignancy.[81] Other reported symptoms include rectal fullness and sciatic pain.[82]
The diagnosis of RRTs presents a significant challenge due to their complex anatomical location and diverse presentations. A multimodal approach is essential for accurate diagnosis. While physical examination, often aided by proctoscopy, has limited sensitivity, with palpable lesions detectable in only approximately 35% of cases, it can be useful in excluding lower rectal involvement.[82] Proctoscopy and flexible sigmoidoscopy offer restricted visualization but can be employed to detect mucosal involvement. In cases with a draining sinus, fistulography may be utilized.[83] Magnetic resonance imaging has emerged as the gold standard imaging modality due to its exquisite soft-tissue resolution, enabling accurate assessment of tumor size, location, extent, and relationship to adjacent structures, thereby facilitating surgical planning.[84] Computed tomography (CT) scanning serves as a complementary tool, providing valuable information regarding RRT consistency (cystic vs. solid) and the degree of invasion into surrounding tissues.[81] In addition, CT scan features can offer clues to the underlying pathology, with homogeneous lesions suggesting benignity and heterogeneous lesions indicating a higher likelihood of malignancy.[80] In select cases, transrectal ultrasound can be beneficial, particularly in differentiating solid from cystic lesions and evaluating the tumor’s proximity to the rectum.[85]
The definitive role of biopsy in diagnosing RRTs remains a subject of debate. While biopsy is generally avoided for cystic lesions due to their high likelihood of benignity and potential for infection, image-guided tissue acquisition remains crucial for definitive diagnosis and treatment planning.[1,83] This is particularly true for specific tumor types such as Ewing sarcoma, osteogenic sarcoma, neurofibrosarcomas, and desmoid tumors, which benefit from neoadjuvant therapy.[86,87]
Precise surgical planning and meticulous execution are paramount, as highlighted by Balci et al. The extent of tumor invasion dictates resection levels, with abdominoperineal or sacral resection necessary in severe cases.[80] The efficacy of neoadjuvant therapy remains under debate, with conflicting results concerning its impact on surgical difficulty and overall outcome.[88] In specific cases, adjuvant radiotherapy or palliative chemotherapy can be considered.[80]
The limitations of this study were that many of the articles were confined to small case series and case studies which also meant that adequately comparing certain criteria such as surgical outcomes based on approach, for instance, was difficult owing to the lack of this information in many of the reviewed articles.
CONCLUSION
RRTs are rare tumors and most commonly congenital in nature with a predominance among the female sex. It is recommended that surgical management with R0 resection is crucial in the management of these tumors, and the minimal access surgery approach appears to be a safer option in appropriate case selection, having an association with shorter length of stay, lower recurrence rates, and shorter operative time.
Author’s contributions
Jeffrey Otote (J.O.), Valentin Butnari (V.B.), Praveen Ravichandran (P.R.), Ahmer Mansuri (A.M.), Mehnaz Ahmed (M.A.), Olivia Pestrin (O.P.), Nirooshun Rajendran (N.R.), Sandeep Kaul (S.K.). Conceptualization, S.K. and V.B.; methodology, J.O.; software, V.B.; validation, A.M., N.R. and S.K; formal analysis, V.B. and J.O.; investigation, J.O. and P.R.; resources, P.R. , O.P. and M.A. .; data curation, J.O., V.B., and P.R.; writing—original draft preparation, J.O.; writing— review and editing, V.B. , J.O. P.R; visualization, J.O., V.B., P.R, M.A. and O.P.; supervision, A.M. , N.R. and S.K.; project administration, V.B., A.M. , N.R. and S.K. All authors have read and agreed to the pub-lished version of the manuscript.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Presacral tumors: Diagnosis and management. Clin Colon Rectal Surg. 2009;22:84-93.
- [CrossRef] [PubMed] [Google Scholar]
- Presacral masses: Multimodality imaging of a multidisciplinary space. Radiographics. 2013;33:1145-67.
- [CrossRef] [PubMed] [Google Scholar]
- Kraske approach to retrorectal tumors: Surgical technique. J Visc Surg. 2022;159:229-33.
- [CrossRef] [PubMed] [Google Scholar]
- Retrorectal tumors. Mayo Clinic experience, 1960-1979. Dis Col Rectum. 1985;28:644-52.
- [CrossRef] [PubMed] [Google Scholar]
- The management of presacral masses in the adult. Tech Coloproctol. 2002;6:43-9.
- [CrossRef] [PubMed] [Google Scholar]
- Benign pre-sacral teratoma and vestigial retrorectal cysts in the adult. J Chir. 2006;143:310-4.
- [Google Scholar]
- Cochrane handbook for systematic reviews of interventions (2nd ed). Chichester, United Kingdom: John Wiley and Sons; 2019. p. :241-84.
- [CrossRef] [Google Scholar]
- Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264-9.
- [CrossRef] [PubMed] [Google Scholar]
- Retrorectal tumors: A challenge for the surgeons. World J Gastrointest Surg. 2021;13:1327-7.
- [CrossRef] [PubMed] [Google Scholar]
- Presacral tumors and cysts in adults. Dis Colon Rectum. 1975;18:581-9.
- [CrossRef] [PubMed] [Google Scholar]
- Mucinous adenocarcinoma of a tailgut cyst. Ghana Med J. 2022;56:46-50.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroendocrine tumor arising in a tailgut cyst: A rare presacral tumor. Int J Surg Pathol. 2019;27:336-42.
- [CrossRef] [PubMed] [Google Scholar]
- Robotic excision of retrorectal mass. J Gastrointest Surg. 2018;22:1811-3.
- [CrossRef] [PubMed] [Google Scholar]
- Multidisciplinary treatment of giant presacral solitary fibrous tumour: A case report and literature review. J Int Med Res. 2022;50:03000605221135458.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroendocrine tumour arising inside a tailgut cyst. Ann R Coll Surg Engl. 2017;99:e91-3.
- [CrossRef] [PubMed] [Google Scholar]
- Robot-assisted resection of pre-sacral schwannoma. Neurosurg Focus. 2018;45(VideoSuppl1):V1.
- [CrossRef] [PubMed] [Google Scholar]
- Robotic resection of recurrent pediatric lipoblastoma. Asian J Endosc Surg. 2019;12:128-31.
- [CrossRef] [PubMed] [Google Scholar]
- Primary presacral adenocarcinoma: A case report and review of the literature. Acta Chir Belg. 2015;115:155-8.
- [CrossRef] [PubMed] [Google Scholar]
- A case report of symptomatic presacral myelolipoma. Medicine (Baltimore). 2018;97:e0337.
- [CrossRef] [PubMed] [Google Scholar]
- Robotic resection of a multicystic tailgut cyst. BMJ Case Rep. 2019;12:e231286.
- [CrossRef] [PubMed] [Google Scholar]
- Laparoscopic management of epidermoid cyst in an unusual location. BMJ Case Rep. 2019;12:e228043.
- [CrossRef] [PubMed] [Google Scholar]
- Perineal approach for surgical treatment in a patient with retro-rectal tumor: A case report and review of the literature. BMC Res Notes. 2015;8:470.
- [CrossRef] [PubMed] [Google Scholar]
- Unusual retrorectal ganglioneuroma: A case report of laparoscopic assisted approach. Pan Afr Med J. 2021;38:241.
- [CrossRef] [PubMed] [Google Scholar]
- Presacral mass in the setting of an ovarian cyst and abdominal pain. BMJ Case Rep. 2017;2017:bcr2017219803.
- [CrossRef] [PubMed] [Google Scholar]
- A solitary fibrous tumour mimicking an aggressive angiomyxoma/liposarcoma. BMJ Case Rep. 2017;2017:bcr2016218202.
- [CrossRef] [PubMed] [Google Scholar]
- Pathology report: Presacral noncommunicating enteric duplication cyst. Cancer Control. 2016;23:170-4.
- [CrossRef] [PubMed] [Google Scholar]
- Robotic-assisted resection of presacral sclerosing epithelioid fibrosarcoma. Tech Coloproctol. 2015;19:177-80.
- [CrossRef] [PubMed] [Google Scholar]
- Retrorectal tumour simulating vaginal birth: An exceptional case of emergency surgery indication. BMJ Case Rep. 2017;2017:bcr2017219211.
- [CrossRef] [PubMed] [Google Scholar]
- Adenocarcinoma in a tailgut cyst: A rare case report. Turk Patoloji Derg. 2020;36:169-72.
- [CrossRef] [Google Scholar]
- Computed tomography-guided posterolateral transsacral ala approach to presacral L5 schwannoma: Technical note. World Neurosurg. 2019;128:55-61.
- [CrossRef] [PubMed] [Google Scholar]
- Combined laparoscopic and perineal approach for the management of recurrent tailgut cyst. Asian J Endosc Surg. 2019;12:181-4.
- [CrossRef] [PubMed] [Google Scholar]
- An elusive cause of perianal pain in a patient with Hirschsprung's disease. BMJ Case Rep. 2016;2016:bcr2016215173.
- [CrossRef] [PubMed] [Google Scholar]
- Laparoscopic synchronous resection for descending colon cancer and tailgut cyst. Acta Med Okayama. 2021;75:529-32.
- [Google Scholar]
- Giant presacral schwannoma in man: Report of a case with emphasis on imaging findings. World Neurosurg. 2020;133:14-6.
- [CrossRef] [PubMed] [Google Scholar]
- Transanal minimally invasive approach for the resection of retrorectal tumour-a video vignette. Colorectal Dis. 2018;20:646-7.
- [CrossRef] [PubMed] [Google Scholar]
- Primary alveolar rhabdomyosarcoma of retrorectal-presacral space in an adult patient: A case report of an uncommon tumor with rare presentation. Medicine (Baltimore). 2019;98:e13416.
- [CrossRef] [PubMed] [Google Scholar]
- Laparoscopic resection of prescral and obturator fossa schwannoma. Int Braz J Urol. 2017;43:566.
- [CrossRef] [PubMed] [Google Scholar]
- Sacral chordoma with incidental rectal adenocarcinoma: A case report. J Med Case Rep. 2021;15:195.
- [CrossRef] [PubMed] [Google Scholar]
- Excision of a recurrent retrorectal tailgut cyst after 58 years. BMJ Case Rep. 2019;12:e230286.
- [CrossRef] [PubMed] [Google Scholar]
- Pudendal tumor mimicking cauda equina syndrome and acute radiculopathy: Case report. Spinal Cord Ser Cases. 2022;8:71.
- [CrossRef] [Google Scholar]
- Retrorectal adenocarcinoma arising from tailgut cysts: A rare case report. BMC Surg. 2019;19:180.
- [CrossRef] [PubMed] [Google Scholar]
- Atypical case of a painful presacral tumor. Am J Case Rep. 2015;16:760-2.
- [CrossRef] [PubMed] [Google Scholar]
- Deodhar K. A well-differentiated neuroendocrine tumor (Grade I) arising in a tailgut cyst. J Cancer Res Ther. 2019;15:258-60.
- [CrossRef] [PubMed] [Google Scholar]
- A rare case of pelvic bronchogenic cyst treated by laparoscopic surgery. Asian J Endosc Surg. 2020;13:227-30.
- [CrossRef] [PubMed] [Google Scholar]
- Robotic excision of a difficult retrorectal cyst-a video vignette. Colorectal Dis. 2020;22:226-7.
- [CrossRef] [PubMed] [Google Scholar]
- Retrorectal tumor: A case report of a patient with “schwannoma”. Arq Bras Cir Dig. 2015;28:151-2.
- [CrossRef] [PubMed] [Google Scholar]
- Carcinoid transformation of presacral dermoid cyst in patient with currarino syndrome: A case report. Br J Neurosurg. 2019;33:285-6.
- [CrossRef] [PubMed] [Google Scholar]
- Presacral mass discovered during pregnancy followed by myasthenia gravis. Isr Med Assoc J. 2015;17:318-20.
- [Google Scholar]
- Laparoscopically excised retroperitoneal presacral Schwannoma: Atypical pre and postoperative manifestations-case report. BMC Surg. 2019;19:148.
- [CrossRef] [PubMed] [Google Scholar]
- Large retrorectal leiomyosarcoma: Case report and considerations about a rare and challenging disease. Updates Surg. 2016;68:423-4.
- [CrossRef] [PubMed] [Google Scholar]
- An urban legend: Malignant transformation caused by radiotherapy in patients with presacral ganglioneuroma. The necessity and first-time administration of radiotherapy. Case report and literature review. J Cancer Res Ther. 2021;17:248-54.
- [CrossRef] [PubMed] [Google Scholar]
- Laparoscopic dissection of a pre-sacral cyst and transvaginal extraction of the specimen-a video vignette. Colorectal Dis. 2019;21:1101-2.
- [CrossRef] [PubMed] [Google Scholar]
- The malignant transformation of retrorectal cystic hamartomas with blood irregular antibodies positive: A case report. Medicine (Baltimore). 2015;94:e2253. Erratum in: Medicine (Baltimore) 2016;95:e5672. Erratum in: Medicine (Baltimore) 2017;96:e6010
- [CrossRef] [PubMed] [Google Scholar]
- Intra-abdominal desmoid tumor after laparoscopic low anterior resection for rectal cancer: A case report. Asian J Endosc Surg. 2020;13:426-30.
- [CrossRef] [PubMed] [Google Scholar]
- A 68-year-old woman presenting with recurrent abdominal pain and a diagnosis of a presacral retroperitoneal benign schwannoma that mimicked an ovarian tumor on pelvic magnetic resonance imagining. Am J Case Rep. 2022;23:e935985.
- [CrossRef] [Google Scholar]
- Primitive neuroectodermal tumor presenting as a presacral mass: A rare case report with review of literature. Indian J Pathol Microbiol. 2017;60:250-2.
- [CrossRef] [PubMed] [Google Scholar]
- A minimally invasive pericoccygeal approach to resection of a large presacral schwannoma: Case report. J Neurosurg Spine. 2015;23:81-5.
- [CrossRef] [PubMed] [Google Scholar]
- Anterior sacral meningocele infected with Fusobacterium in a patient with recently diagnosed colorectal carcinoma-a case report. BMC Neurol. 2017;17:212.
- [CrossRef] [PubMed] [Google Scholar]
- An unusual presentation as recurrent abortions in a case of giant presacral epidermoid cyst mimicking an anterior sacral meningocele. World Neurosurg. 2019;122:77-80.
- [CrossRef] [PubMed] [Google Scholar]
- A rare case of incidental mucinous adenocarcinoma with osseous metaplasia associated with cysts of the presacral space. Turk J Gastroenterol. 2019;30:495-6.
- [CrossRef] [PubMed] [Google Scholar]
- Squamous cell carcinoma arising from a presacral cyst in a patient with ulcerative colitis under azathioprine and infliximab: First case report. Int J Colorectal Dis. 2016;31:1509-10.
- [CrossRef] [PubMed] [Google Scholar]
- Progress in the management of retrorectal tumours. Colorectal Dis. 2016;18:410-7.
- [CrossRef] [PubMed] [Google Scholar]
- Robot-assisted sacral tumor resection: A preliminary study. BMC Musculoskelet Disord. 2018;19:186.
- [CrossRef] [PubMed] [Google Scholar]
- Management of giant presacral schwannoma. Clinical series and literature review. Clin Neurol Neurosurg. 2021;200:106409.
- [CrossRef] [PubMed] [Google Scholar]
- Surgery of neurogenic tumors of the sacrum. Neurocirugia (Astur: Engl Ed). 2022;33:53-60.
- [CrossRef] [PubMed] [Google Scholar]
- A clinical and surgical challenge: Retrorectal tumors. J Cancer Res Ther. 2019;15:132-7.
- [CrossRef] [PubMed] [Google Scholar]
- Robotic-assisted laparoscopic resection of tailgut cysts. JSLS. 2021;25
- [CrossRef] [PubMed] [Google Scholar]
- Laparoscopic excision of neurogenic retrorectal tumors. Asian J Endosc Surg. 2017;10:223-6.
- [CrossRef] [PubMed] [Google Scholar]
- Retrorectal cyst: Proteus in the backyard-case series and literature review. BMJ Case Rep. 2020;13:e231080.
- [CrossRef] [PubMed] [Google Scholar]
- Presacral tarlov cyst as an unusual cause of abdominal pain: New case and literature review. World Neurosurg. 2018;110:79-84.
- [CrossRef] [PubMed] [Google Scholar]
- Retrorectal tumors in adults: A 10-year retrospective study. Int Surg. 2015;100:1177-84.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical management of 52 consecutive retro-rectal tumours treated at a tertiary referral centre. Colorectal Dis. 2020;22:1279-85.
- [CrossRef] [PubMed] [Google Scholar]
- Primary cystic lesions of the retrorectal space: MRI evaluation and clinical assessment. AJR Am J Roentgenol. 2017;209:790-6.
- [CrossRef] [PubMed] [Google Scholar]
- Problems in diagnosis and treatment of retrorectal tumors: Our experience in 50 patients. Acta Med Iran. 2016;54:644-50.
- [Google Scholar]
- A multicenter experience with perirectal tumors: The risk of local recurrence. Eur J Surg Oncol. 2016;42:817-22.
- [CrossRef] [PubMed] [Google Scholar]
- Adult sacrococcygeal teratoma: A retrospective study over eight years at a single institution. J Zhejiang Univ Sci B. 2019;20:670-8.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of endosponge therapy for the management of presacral abscesses following rectal surgery. Tech Coloproctol. 2019;23:551-7.
- [CrossRef] [PubMed] [Google Scholar]
- Evolution of the management of retrorectal masses: A retrospective cohort study. Colorectal Dis. 2021;23:2988-98.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical management of retro-rectal tumors in the adult. J Visc Surg. 2019;156:229-37.
- [CrossRef] [PubMed] [Google Scholar]
- Retrorectal tumors: A comprehensive literature review. World J Surg. 2016;40:2001-15.
- [CrossRef] [PubMed] [Google Scholar]
- Retrorectal tumors: A diagnostic and therapeutic challenge. Dis Colon Rectum. 2005;48:1581-7.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging and management of rectal cancer. Semin Ultrasound CT MR. 2020;41:183-206.
- [CrossRef] [PubMed] [Google Scholar]
- Transrectal ultrasound of the prostate In: Practical urological ultrasound. New York: Springer; 2013. p. :155-70.
- [CrossRef] [Google Scholar]
- Retrorectal tumor: A single-center 10-years' experience. Ann Surg Treat Res. 2020;99:110-7.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: https://www.sematicscholar.org/reader/ef8736c7ab077505e4348346e9421ca9bf21370f [Last accessed on 2024 Feb 10]







