Translate this page into:
Renal functional outcomes after robot-assisted partial nephrectomy and percutaneous cryoablation of clinical T1 renal cell carcinoma – A prospective study

*Corresponding author: Louise Aarup Duus, Department of Radiology, Odense University Hospital, Odense, Denmark. louise.duus@rsyd.dk
-
Received: ,
Accepted: ,
How to cite this article: Duus L, Junker T, Rasmussen B, Vilstrup M, Lund L, Pedersen M, et al. Renal functional outcomes after robot-assisted partial nephrectomy and percutaneous cryoablation of clinical T1 renal cell carcinoma – A prospective study. J Clin Imaging Sci. 2023;13:37. doi: 10.25259/JCIS_66_2023
Abstract
Objectives:
The objective of this study was to investigate renal function after robot-assisted partial nephrectomy (RAPN) and percutaneous cryoablation (PCA) in clinical stage T1 (cT1) renal cell carcinoma (RCC) and evaluate the relationship between baseline renal function and renal functional outcome.
Material and Methods:
Patients with cT1 RCC treated with RAPN or PCA were prospectively enrolled between June 2019 and January 2021. Renal function was evaluated using estimated glomerular filtration rate, Tc-99m diethylenetriamine-pentaacetate plasma clearance, Tc-99m mercaptoacetyltriglycine renography, and renal volume at baseline and 6 months after treatment.
Results:
Fifty-six patients were included (18 RAPN, 38 PCA). PCA patients had a significantly higher age (68.5 years; P = 0.019) and Charlson comorbidity index (3.0; P = 0.007). Tumor characteristics did not differ significantly between RAPN and PCA. Total renal volume decreased significantly after PCA (−18.2 cm3; P = 0.001). Baseline chronic disease stage IIIb–IV leads to a greater reduction in renal volume (−31.8 cm3; P = 0.003) but not other renal function measures. Renal function declined significantly after either treatment without significant differences between RAPN and PCA.
Conclusion:
This study found a small, similar decrease in renal function 6 months after RAPN or PCA, despite significant differences in baseline patient characteristics. Reduced renal function at baseline did not lead to a worse renal functional outcome.
Keywords
Kidney function
Kidney neoplasms
Minimally invasive surgical procedures
Ablation
nephron-sparing treatment
INTRODUCTION
The incidence of renal cell carcinoma (RCC) has been increasing for decades, making RCC one of the most rapidly increasing cancer diagnoses in the developed world.[1] The primary treatment for localized T1 RCC is surgery, and partial nephrectomy (PN) is the gold standard due to its nephron-sparing quality and similar oncological outcome to radical nephrectomy (RN).[2,3]
RCC primarily occurs in the elderly, complicating treatment since elderly patients have abundant comorbidities, including renal functional impairment. Therefore, nephron-sparing treatment (NST) and minimally invasive treatments such as PN and thermal ablation (TA) are preferred to minimize the risk of the development or progression of chronic kidney disease (CKD).[2-4] The long-term consequences of CKD include increased risk of cancer, cardiovascular events, hospitalization, and death.[5] Over 25% of RCC patients have impaired renal function before treatment,[6,7] and they are at risk of CKD progression following surgery or TA.[8]
Robot-assisted PN (RAPN) and laparoscopic PN (LPN) are the preferred minimally invasive treatments with equal oncological outcomes.[2,3,9,10] RAPN has a shorter warm ischemia time (WIT) and a lower conversion rate to RN.[9] Renal functional outcome after different types of PN has been extensively studied.[11-14]
TA is an increasingly prioritized minimally invasive treatment of T1 RCC offered to comorbid patients with no other treatment options. Studies have indicated a lower risk of complications after TA compared to PN.[15,16] Furthermore, the 5-year RCC-specific survival in selected patients after TA to those treated with PN.[16] However, renal functional outcome after TA has not been thoroughly investigated, especially the impact of percutaneous cryoablation (PCA).[17,18]
This study investigated renal function after RAPN and PCA in clinical stage T1 (cT1) RCC and evaluated the relationship between baseline renal function and renal functional outcome.
MATERIAL AND METHODS
This non-randomized single-center prospective cohort study was approved by the Danish National Data Protection Agency (19/8784) and the Regional Committee on Health Research Ethics for Southern Denmark (S-20180080NA) with approval of an updated version in June 2020. Patients participated after informed written and oral consent.
Patient selection and data collection
Patients with cT1 RCC treated with RAPN or PCA at Odense University Hospital were prospectively and consecutively enrolled from Region of Southern Denmark between June 2019 and January 2021. Exclusion criteria were being under 18 years old at the time of treatment, presence of a benign tumor, conversion of RAPN to open surgery, conversion of RAPN to RN, salvage treatment within 6 months after primary treatment, missing baseline examinations, or multiple treatments in cases of multiple tumors [Figure 1].
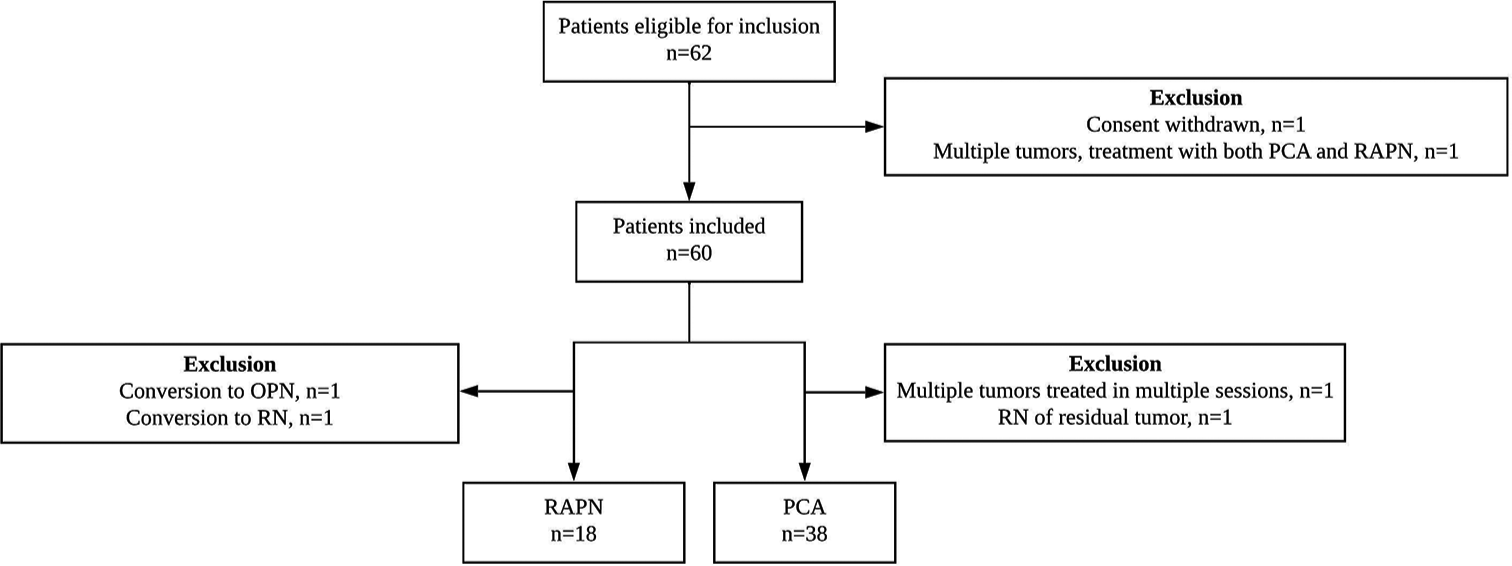
- Diagram of the inclusion process of patients undergoing nephron-sparing RCC treatment. RCC: Renal cell carcinoma, PCA: Percutaneous cryoablation, RAPN: Robot-assisted partial nephrectomy, OPN: Open partial nephrectomy, RN: Radical nephrectomy.
The treatment options for individual patients were discussed in multidisciplinary team conferences with the participation of pathologists, radiologists, oncologists, and urologists. When NST was considered suitable, the final treatment decision was based on tumor characteristics, age, health status, renal function, and patient preference, following the principle of shared decision-making.[19]
Data were collected from the picture archiving and communication systems (PACS) and electronic patient records and stored in a REDCap database. Findings were reported according to the “Strengthening the Reporting of Observational Studies in Epidemiology” guidelines.[20]
Treatment
Two senior radiologists with 2 and 8 years of experience with computed tomography (CT) intervention performed the CT-guided PCA. When possible, patients were sedated using intravenous dexmedetomidine and remifentanil and local anesthesia with lidocaine and bupivacaine otherwise. When required, adjacent tissues were protected by hydro displacement with an 18G percutaneous entry thin wall needle (Cook; Bloomington, IN, USA) with 2% iodine-based saline solution.[21] The cryoprobes were inserted percutaneously under CT-fluoroscopy guidance (Siemens Somatom Flash system with 2 × 128-channel/slice; Siemens Healthcare, Erlangen, Germany). An argon-based cryoablation system (IceFx system; Boston Scientific, MN, USA) with 17G or 14G sealed cryoprobes was used. PCA was performed with a double-freeze-thaw cycle of 10 min freeze and 8 min thaw. Sequential CT scans were performed 4 and 8 min into each freezing cycle and after completion of the procedure.
RAPN was performed under general anesthesia by four board-certified urologists with 3–7 years of experience with RAPN. The four-armed Da Vinci Si robotic surgical system (Intuitive Surgical; Sunnyvale, CA, USA) was used. Patients were placed in the full flank position, and tumors were located and marked with TilePro integrated endoscopic ultrasound. The renal artery was clamped with Bulldog clamps, and WIT was registered. The tumor was excised with cold scissors leaving a healthy tissue margin evaluated by endoscopic ultrasound, and the parenchyma was closed with 3-0 V-lock sutures.
Variables of interest
The surgical complexity of tumors was evaluated with the Radius-Endophytic-Nearness-Anterior-Location nephrometry score.[22] All tumors were histologically diagnosed with biopsy before treatment. The histological subtype was determined from the biopsy report. Tumors that underwent RAPN had an additional pathology report from the excised specimen. Tumors were staged according to the RCC tumor node metastasis staging system, clinically from imaging (CT stage) based on contrast-enhanced CT or magnetic resonance imaging of the kidneys and chest CT, and pathologically from the pathology report after RAPN (pT stage).[23] The American Society of Anesthesiologists score was assessed before treatment.[24] The Charlson comorbidity index (CCI) was calculated based on information from the electronic medical record and cross-checked in patient interviews.[25]
Renal function was evaluated at baseline and 6 months after treatment. The following variables were collected: P-creatinine, estimated glomerular filtration rate (eGFR; CKDEPI formula), and renal scintigraphy with 99mTechnetiummercaptoacetyltriglycine (MAG3) to evaluate the split function. A single sample isotopic technique GFR method using 99mTechnetium-diethylenetriamine pentaacetate (DTPA) was performed as the reference method for GFR measurement. Standard GFR was calculated by the plasmatic DTPA depuration using a 3-h post-injection blood sample. The patient height and weights were measured for body surface area adjustment.
The volumes of the tumor and kidneys were measured with an integrated volume measurement tool in Philips Vue PACS (Philips Medical Systems; Best, the Netherlands), excluding non-functional areas such as cysts. CKD stages were defined according to KDIGO guidelines:[26] CKD I (GFR: 390 mL/min/1.73 m2), CKD II (GFR: 60–89 mL/min/1.73 m2), CKD IIIa (GFR: 45–59 mL/min/1.73 m2), CKD IIIb (GFR: 30– 44 mL/min/1.73 m2), CKD IV (GFR: 15–29 mL/min/1.73 m2), and CKD V (GFR: <15 mL/min/1.73 m2) and categorized based on eGFR and DTPA. Normal renal function was defined as CKD I–II and impaired renal function as CKD IIIa–V.
Due to low interest in participation in the study, patients were offered inclusion without DTPA clearance from June 2020.
Statistical analysis
Continuous variables were described as median with interquartile range and categorical variables as frequencies. Normality was assessed by the Shapiro–Wilk W-test. Renal functional outcome was calculated with a paired t-test to evaluate mean differences between baseline and 6 months post-treatment for normally distributed continuous variables. The Wilcoxon signed-rank test was used for categorical and ordinal variables and non-normal continuous variables. The primary outcome was assessed with a linear mixed effect model (maximum likelihood estimation) with patients as random effects and renal volume, eGFR or DTPA-GFR, and visits (baseline and 6 months) as fixed effects. Standard model selection was performed using the likelihood ratio test. Statistical significance was considered as P < 0.05. All analyses were performed using the STATA 16 software (release 16; StataCorp LLC; College Station, TX, USA).
RESULTS
A total of 56 patients were included in this study: 38 PCA and 18 RAPN [Figure 1]. We found a significantly higher age (68.5 vs. 57.5 years, P = 0.019) and CCI (3.0 vs. 2.0, P = 0.007) in patients undergoing PCA compared to RAPN [Table 1]. Three patients had a solitary kidney, and all underwent PCA. All patients with baseline CKD IIIa–IV underwent PCA. One patient underwent PCA of two tumors. Therefore, 57 tumors were assessed in this study. Tumor characteristics in patients undergoing RAPN and PCA did not differ significantly [Table 2]. Four tumors were downgraded from cT1b to pT1a, and one tumor was upgraded from cT1b to pT2.
| Patient characteristic | All, n=56 | RAPN, n=18 | Cryoablation, n=38 | P-value |
|---|---|---|---|---|
| Sex, male/female, n(%) | 40/16 (71.4/28.6) | 13/5 (72.2/27.8) | 27/11 (71.1/29.0) | 0.928 |
| Age, years, median (IQR) | 66.5 (56.5–74.0) | 57.5 (53.0–69.0) | 68.5 (61.0–76.0) | 0.019* |
| Smoking | ||||
| Yes | 11 (19.6) | 5 (27.8) | 6 (15.8) | 0.573 |
| No | 31 (55.4) | 9 (50.0) | 22 (57.9) | |
| Former | 14 (25.0) | 4 (22.2) | 10 (26.3) | |
| ASA score | ||||
| 1. Healthy, n(%) | 3 (5.4) | 2 (11.1) | 1 (2.6) | 0.249 |
| 2. Mild systemic disease, n(%) | 28 (50.0) | 10 (55.6) | 18 (47.4) | |
| 3. Severe systemic disease, n(%) | 25 (44.6) | 6 (33.3) | 19 (50.0) | |
| 4. Severe systemic disease that is a constant threat to life, n(%) | 0 (0) | 0 (0) | 0 (0) | |
| CCI, median (IQR) | 3.0 (1.5–4) | 2.0 (1.0–3.0) | 3.0 (2.0–5.0) | 0.007* |
| BMI, median (IQR) | 29.4 (27.1–32.4) | 29.4 (27.4–32.5) | 29.4 (26.9–32.4) | |
| <18.5: Underweight, n(%) | 0 (0) | 0 (0) | 0 (0) | 0.806 |
| 18.5–24.9: Normal, n(%) | 8 (14.3) | 3 (16.7) | 5 (13.2) | |
| 25-29.9: overweight, n(%) | 22 (39.3) | 7 (38.9) | 15 (39.5) | |
| ≥30: obesity, n(%) | 26 (46.4) | 8 (44.4) | 18 (47.4) | |
| Other renal diseases yes/no, n(%) | 8/48 (14.3/85.7) | 0/18 (0.0/100.0) | 8/30 (21.1/78.9) | |
| History of urolithiasis, n(%) | 4 (7.1) | 0 (0.0) | 4 (10.5) | 0.044* |
| Solitary kidney, n(%) | 3 (5.4) | 0 (0.0) | 3 (7.8) | |
| Renal impairment due to side effects of medication | 1 (1.8) | 0 (0.0) | 1 (2.6) | |
| Synchronous cancer | ||||
| None, n(%) | 41 (73.2) | 16 (88.9) | 25 (65.8) | 0.152 |
| Ongoing treatment, n(%) | 3 (5.4) | 0 (0.0) | 3 (7.9) | |
| Follow-up/surveillance, n(%) | 12 (21.4) | 2 (11.1) | 10 (26.3) | |
| Diabetes | ||||
| None, n(%) | 43 (76.8) | 14 (77.8) | 29 (76.3) | 1.000 |
| Without complications, n(%) | 12 (21.4) | 4 (22.2) | 8 (21.1) | |
| Organ damage, n(%) | 1 (1.8) | 0 (0.0) | 1 (2.6) |
| Tumor characteristics | All, n=57 | RAPN, n=18 | Cryoablation, n=39A | P-value |
|---|---|---|---|---|
| Tumor size, (cm) | ||||
| Median (IQR) | 3.3 (2.5–3.9) | 3.6 (2.5–4.3) | 3.0 (2.5–3.9) | 0.376 |
| Tumor volume, (cm3) | ||||
| Median (IQR) | 13.6 (7.2–23.2) | 22.1 (8.2–31.5) | 13.4 (6.4–22.1) | 0.167 |
| Tumor placement, n(%) | ||||
| Right/left | 27/30 (47.4/52.6) | 10/8 (55.6/44.4) | 17/22 (43.6/56.4) | 0.400 |
| Exophytic/endophytic, n(%) | ||||
| ≥50% Exophytic | 24 (42.1) | 9 (50.0) | 15 (38.5) | 0.456 |
| <50% Exophytic | 29 (50.1) | 9 (50.0) | 20 (51.3) | |
| 100% Endophytic | 4 (7.0) | 0 (0.0) | 4 (10.3) | |
| Nearness to sinus or collecting system, n(%) | ||||
| ≥7 mm | 10 (17.5) | 3 (16.7) | 7 (18.0) | 1.000 |
| >4 mm, <7 mm | 5 (8.8) | 1 (5.6) | 4 (10.3) | |
| ≤4 mm | 42 (73.7) | 14 (77.8) | 28 (71.8) | |
| RENAL score | ||||
| Median (IQR) | 7 (7–8) | 7 (6–8) | 8 (7–8) | 0.618 |
| RENAL score group, n(%) | ||||
| 4–6 (low) | 14 (24.6) | 5 (27.8) | 9 (23.1) | 0.895 |
| 7–9 (medium) | 39 (68.4) | 12 (66.7) | 27 (69.2) | |
| 10–12 (high) | 4 (7.0) | 1 (5.6) | 3 (7.7) | |
| Clinical tumor stage, n(%) | ||||
| cT1a, n(%) | 46 (80.7) | 12 (66.7) | 34 (87.2) | 0.068 |
| cT1b, n(%) | 11 (19.3) | 6 (33.3) | 5 (12.8) | |
| Pathological tumor stage, n (%)B | ||||
| pT1a | 16 (88.9) | 16 (88.9) | N/A | N/A |
| pT1b | 1 (5.6) | 1 (5.6) | ||
| pT2a | 1 (5.6) | 1 (5.6) | ||
| Histological subtype, n(%) | ||||
| Unclassified RCC | 2 (3.5) | 0 (0.0) | 2 (5.1) | 0.708 |
| Clear cell | 37 (64.9) | 12 (66.7) | 25 (64.1) | |
| Papillary | 14 (24.6) | 4 (22.2) | 10 (25.6) | |
| Chromophobe | 2 (3.5) | 1 (5.6) | 1 (2.6) | |
| Multilocular cystic clear cell neoplasm | 1 (1.8) | 0 (0.0) | 1 (2.6) | |
| Epithelioid angiomyolipomaC | 1 (1.8) | 1 (5.6) | 0 (0.0) |
[Supplementary Table 1] shows treatment characteristics for RAPN and PCA. In RAPN, WIT was ≤30 min in all cases, and in 17 of 18 patients (94%), WIT was ≤25 min.
Renal function was assessed in 56 patients and decreased significantly after both treatment types regarding median p-creatinine, eGFR, eGFR-CKD, and DTPA, but not in DTPA-CKD [Table 2].
Total renal volume change in patients was significant in patients who underwent PCA but not RAPN. Both treatment groups had a significant reduction in the ipsilateral volume but not a corresponding increase in the contralateral volume. The ipsilateral renal area decreased significantly in both treatment groups. The contralateral renal area also significantly decreased 6 months after PCA, but no significant change was found after RAPN. The split renal function of the ipsilateral kidney decreased significantly in both groups [Tables 3, and 4]. No patients developed end-stage renal disease or required dialysis within 6 months of follow-up.
| Variable | Baseline | 6 months | Mean difference (95% CI)# | P-value |
|---|---|---|---|---|
| P-creatinine, μmol/L, median (IQR) 1 missing at 6 months | 73.0 (69.0–83.0) | 85.0 (73.0–92.0) | 6.5 (2.2;10.8) | 0.007* |
| eGFR, mL/min/1.73 m2, median (IQR) 1 missing at 6 months | 91.0 (74.0–102.0) | 83.0 (68.0–92.0) | −6.5 (−10.5; −2.4) | 0.008* |
| DTPA-GFR, mL/min/1.73m2, median (IQR) 4 missing | 90.0 (83.0–104.0) | 89.5 (71.0–90.0) | −8.0 (−12.6; −3.4) | 0.002* |
| eGFR CKD, n (%) | ||||
| I | 10 (55.6) | 6 (35.3) | N/A | 0.046* |
| II 1 missing at 6 months | 8 (44.4) | 11 (64.7) | ||
| DTPA-CKD, n (%) | ||||
| I | 9 (64.3) | 7 (50.0) | N/A | 0.103 |
| II | 5 (35.7) | 5 (35.7) | ||
| IIIa | 0 (0.0) | 2 (14.3) | ||
| IIIb | ||||
| IV | ||||
| V 4 missing | ||||
| Renal parenchymal volume, cm3, median (IQR) | ||||
| Total | 376.3 (329.7–414.5) | 382.5 (320.5–414.2) | 1.6 (−24.1;27.3) | 0.393 |
| Ipsilateral | 190.7 (161.2–207.8) | 172.9 (148.1–197.9) | −15.0 (−23.1; −7.0) | 0.003* |
| Contralateral 2 missing at 6 months | 194.8 (170.4–225.2) | 199.1 (170.4–232.7) | 5.3 (−1.6;12.1) | 0.177 |
| Renal area (cm2) | ||||
| Ipsilateral, median (IQR) | 63.0 (54.0–73.0) | 55.0 (46.0–66.0) | −9.3 (−17.8; −0.7) | 0.023* |
| Contralateral, median (IQR) | 67.6 (60.0–77.0) | 66.5 (57.0–75.0) | −2.1 (−7.7;3.6) | 0.453 |
| Split renal function, % | ||||
| Ipsilateral, median (IQR) | 47.0 (45.0–50.0) | 42.5 (35.0–48.0) | −4.7 (−1.1; −8.3) | 0.008* |
| Contralateral, median (IQR) | 53.0 (50.0–55.0) | 57.5 (53.0–65.0) | 4.8 (1.2;8.3) | 0.008* |
IQR: Interquartile range, RAPN: Robot-assisted partial nephrectomy, eGFR: Estimated glomerular filtration rate (CKD-EPI), DTPA-GFR: Tc-99m-diethylenetriamine pentaacetic acid clearance, CKD stages: I (GFR: ≥90 mL/min/1.73 m2), II (GFR: 60–89 mL/min/1.73 m2), IIIa (GFR: 45–59 mL/min/1.73 m2), IIIb (GFR: 30–44 mL/min/1.73 m2), IV (GFR: 15–29 mL/min/1.73 m2), V (GFR<15 mL/min/1.73 m2). Split renal function: Tc-99-MAG3-renography, Ipsilateral: Tumor-bearing kidney, Contralateral: Opposite kidney as tumor-bearing kidney, *P<0.05, statistical significance
| Variable | Baseline | 6 months | Mean difference (95%CI)# | P-value |
|---|---|---|---|---|
| P-creatinine, μmol/L, median (IQR) 1 missing at 6 months | 76.5 (68.0–99.0) | 88.0 (74.0–113.0) | 9.2 (4.4;14.0) | <0.001* |
| eGFR, mL/min/1.73m2, median (IQR) 1 missing at 6 months | 71.0 (64.0–94.0) | 73.0 (52.0–91.0) | −5.5 (−8.7; −2.3) | <0.001* |
| DTPA-GFR, mL/min/1.73m2, median (IQR) 11 missing |
72.0 (58.0–101.0) | 66.5 (51.5–96.5) | −5.2(−8.8; −1.6) | 0.006* |
| eGFR CKD, n (%) | ||||
| I | 13 (34.2) | 11 (29.7) | N/A | 0.011* |
| II | 18 (47.4) | 14 (37.8) | ||
| IIIa | 2 (5.3) | 7 (18.9) | ||
| IIIb | 2 (5.3) | 4 (10.8) | ||
| IV 1 missing at 6 months | 3 (7.9) | 1 (2.7) | ||
| DTPA-CKD, n (%) | ||||
| I | 8 (29.6) | 9 (32.1) | N/A | 0.317 |
| II | 12 (44.4) | 10 (35.7) | ||
| IIIa | 4 (14.8) | 5 (17.9) | ||
| IIIb | 2 (7.4) | 3 (10.7) | ||
| IV 11 missing at baseline, 10 at 6 months | 1 (3.7) | 1 (3.6) | ||
| Renal parenchymal volume, cm3, median (IQR) | ||||
| Total | 351.6 (297.0–401.8) | 327.7 (273.6–390.7) | −18.2 (−28.4; −8.1) | 0.001* |
| Ipsilateral | 184.8 (147.9–207.9) | 160.7 (127.9–199.5) | −24.6 (−32.9; −16.2) | <0.001* |
| Contralateral, median | 181.3 (155.8–213.1) | 192.7 (154.5–218.2) | 5.1 (−3.0;13.1) | 0.098 |
| Renal area (cm2) | ||||
| Ipsilateral, median (IQR) 1 missing | 63.0 (55.0–82.0) | 58.0 (49.0–65.0) | −6.8 (−11.8; −1.9) | 0.006* |
| Contralateral, median (IQR) 4 missing | 66.5 (53.0–80.0) | 60.5 (53.0–72.0) | −5.0 (−9.6; −0.4) | 0.033* |
| Split renal function, % | ||||
| Ipsilateral, median (IQR) | 48.0 (44.0–52.0) | 43.5 (40.0–48.0) | −4.8 (−6.6; −3.1) | <0.001* |
| Contralateral, median (IQR) 2 missing | 52.0 (48.0–56.0) | 57.5 (54.0–60.5) | 5.1 (3.3;6.9) | <0.001* |
Baseline CKD IIIb–IV leads to a significantly greater reduction in renal volume at 6 months after treatment compared to patients with baseline normal renal function CKD I–II (P = 0.003; [Supplementary Table 2a]). In addition, median total renal volume and area were slightly lower at baseline and 6 months after PCA with a higher range compared to RAPN [Figure 2]. Median eGFR and DTPA-GFR were lower and their range was higher at both baseline and 6 months after PCA compared to RAPN [Figure 2]. Changes in median DTPA-GFR and eGFR are illustrated with the baseline CKD group and treatment type [Figure 3]. Median GFR was stable or decreased, except for patients with baseline DTPA-CKD IIIb in whom GFR increased at the 6-month follow-up [Figure 3]. Nevertheless, changes in renal function after PCA were not significantly different from those after RAPN with any of the measured parameters [Supplementary Table 2b].

- Boxplots of renal functional change from baseline to 6 months after RAPN and PCA. (a) Total renal volume. (b) Renal area of the tumor-bearing (ipsilateral) and non-tumor bearing (contralateral) kidneys as determined by renography. (c) eGFR. (d) DTPA-GFR. RAPN: Robotic-assisted partial nephrectomy, PCA: Percutaneous cryoablation, eGFR: Estimated glomerular filtration rate (CKD-EPI), mL/min/1.73m2, DTPA-GFR: Tc-99m-diethylenetriamine pentaacetic acid clearance, mL/min/1.73m2. Black dots are outliers. Gray box: Box plot of the total renal function.
![Illustrations of the change in median GFR from baseline to 6 months post-treatment. Color represents renal function baseline CKD-group. CKD stages defined according to KDIGO guidelines:[26] CKD I (GFR: 390 mL/min/1.73 m2), CKD II (GFR: 60–89 mL/min/1.73 m2), CKD IIIa (GFR: 45–59 mL/min/1.73 m2), CKD IIIb (GFR: 30–44 mL/min/1.73 m2), CKD IV (GFR: 15–29 mL/min/1.73 m2), CKD V (GFR: <15 mL/min/1.73 m2). (a) Change in DTPA GFR in patients treated with RAPN. Blue: Baseline CKD I (9 patients); red; baseline CKD II (5 patients). (b) Change in eGFR in patients treated with RAPN. Blue: Baseline CKD I (10 patients); red: Baseline CKD II (8 patients). (c) Change in DTPA GFR in patients treated with PCA. Blue: Baseline CKD I (8 patients); red: Baseline CKD II (12 patients); green: Baseline CKD IIIa (4 patients); yellow: Baseline CKD IIIb (2 patients); gray: baseline CKD IV (1 patient). (d) Change in eGFR in patients treated with PCA. Blue: Baseline CKD I (13 patients); red: Baseline CKD II (18 patients); green: Baseline CKD IIIa (2 patients); yellow: Baseline CKD IIIb (2 patients); gray: Baseline CKD IV (3 patients). GFR: Glomerular filtration rate, eGFR: Estimated glomerular filtration rate, CKD: Chronic kidney disease, DTPA GFR: Tc-99m-diethylenetriamine pentaacetic acid clearance, RAPN: Robot-assisted partial nephrectomy, PCA: Percutaneous cryoablation.](/content/12/2023/13/1/img/JCIS-13-37-g003.png)
- Illustrations of the change in median GFR from baseline to 6 months post-treatment. Color represents renal function baseline CKD-group. CKD stages defined according to KDIGO guidelines:[26] CKD I (GFR: 390 mL/min/1.73 m2), CKD II (GFR: 60–89 mL/min/1.73 m2), CKD IIIa (GFR: 45–59 mL/min/1.73 m2), CKD IIIb (GFR: 30–44 mL/min/1.73 m2), CKD IV (GFR: 15–29 mL/min/1.73 m2), CKD V (GFR: <15 mL/min/1.73 m2). (a) Change in DTPA GFR in patients treated with RAPN. Blue: Baseline CKD I (9 patients); red; baseline CKD II (5 patients). (b) Change in eGFR in patients treated with RAPN. Blue: Baseline CKD I (10 patients); red: Baseline CKD II (8 patients). (c) Change in DTPA GFR in patients treated with PCA. Blue: Baseline CKD I (8 patients); red: Baseline CKD II (12 patients); green: Baseline CKD IIIa (4 patients); yellow: Baseline CKD IIIb (2 patients); gray: baseline CKD IV (1 patient). (d) Change in eGFR in patients treated with PCA. Blue: Baseline CKD I (13 patients); red: Baseline CKD II (18 patients); green: Baseline CKD IIIa (2 patients); yellow: Baseline CKD IIIb (2 patients); gray: Baseline CKD IV (3 patients). GFR: Glomerular filtration rate, eGFR: Estimated glomerular filtration rate, CKD: Chronic kidney disease, DTPA GFR: Tc-99m-diethylenetriamine pentaacetic acid clearance, RAPN: Robot-assisted partial nephrectomy, PCA: Percutaneous cryoablation.
DISCUSSION
This study found that renal function significantly decreased 6 months after RAPN and PCA of cT1 RCC, reflected in multiple renal function parameters. Pre-existing CKD IIIb–IV was predictive of a significantly greater reduction in total renal volume after PCA compared to patients with a normal baseline renal function. This reduction was not accompanied by significant decreases in eGFR, DTPA, or split renal function. Despite significant differences in baseline health and renal function between patients who underwent RAPN and PCA, the change in renal function was comparable in both treatment groups.
Evaluating renal function after RCC treatment is important because the RCC population is typically elderly with abundant comorbidities.[6,7] In addition, all-cause mortality for patients with pre existing CKD is significantly higher than for patients with normal renal function.[5] In living kidney donors, neither postoperative GFR nor CKD development is associated with reduced survival compared to patients with impaired renal function due to medical conditions.[13] This lack of association is likely explained by the remaining parenchyma taking over the surgically removed nephrons and compensatory mechanisms in healthy kidneys.[27] In this study, patients treated with PCA were significantly older, prone to other renal diseases, and had significantly higher CCI compared to those who underwent RAPN. Furthermore, all patients with pre-existing CKD were treated with PCA. The contralateral renal volume in patients who underwent PCA did not increase sufficiently to preserve the total renal volume, as opposed to patients who underwent RAPN. This finding can be explained by the presence of preexisting impaired renal function reducing the regenerative mechanisms of the non-malignant renal parenchyma, indicating that the quantity and quality of the remaining renal tissue are crucial factors for preserving renal function.[27,28]
A statistically significant change in eGFR-CKD was found in patients treated with PCA and RAPN with a net worsening in the CKD stage. In patients undergoing PCA, a fluctuation in eGFR-CKD and DTPA-CKD stages was observed, with some patients upstaging and others downstaging after treatment. A similar inconsistency in CKD stage changes was found by Wehrenberg-Klee et al., who retrospectively studied a small group of patients with baseline CKD III–V undergoing radiofrequency ablation (RFA) and PCA of RCC.[28] Despite changes in CKD stage, they found no significant change in eGFR 1-year post-treatment with RFA or PCA. Consequently, CKD changes could indicate significant changes in renal function that cannot be detected with other variables. Therefore, the role of baseline CKD on renal functional outcome after RCC NST remains unclear and should not be considered of high value without other renal functional parameters.
WIT was found to be crucial for renal functional outcomes after PN.[29] In a systematic review comparing LPN and RAPN, a lower WIT and a favorable postoperative eGFR rate were found after RAPN.[9] A WIT cutoff of 20–25 min has been proposed to be an important predictor of adverse renal outcomes for all levels of baseline renal function.[29] In this study, ischemic time was minimal, with a median WIT of 17.5 min, and no patients had WIT >30 min. Six months after treatment, no RAPN patients had new-onset CKD IIIb–V. Therefore, the shorter WIT used here is beneficial for renal functional outcomes. However, it should be noted that all patients treated with RAPN in this study had normal baseline renal function (eGFR ≥60 mL/min/1.73 m2; CKD I-II).
This study found a significant reduction in renal function after both RAPN and PCA and no significant differences between RAPN and PCA. Bhindi et al. evaluated eGFR changes in 118 patients with a solitary kidney undergoing PN or PCA of RCC,[10] finding no significant difference in eGFR drop from baseline to 3 months post-treatment between PCA and PN. Similarly, in a systematic literature review, Uhlig et al. found that PN, PCA, RFA, and microwave ablation of renal tumors were all associated with a net decrease in renal function.[18] However, no significant differences between PN and PCA were observed.
The findings of this study are limited by several factors. For example, as a single-center study with no randomization, it is sensitive to selection bias in the recruitment process. The majority of patients did not wish to participate in the study. This could be explained by the fact that treatment was performed in a fast-track setting at a highly specialized hospital with a large uptake area. Patients should attend the hospital for baseline DTPA examination in the short time frame from diagnosis to treatment. Furthermore, the inclusion period of the study was during the COVID-19 pandemic, and research examinations were temporarily shutdown. The small sample size introduces a degree of uncertainty to its statistical findings and the conclusions based on them. Moreover, because patients were recruited from a large geographical area, many refused to participate due to supplementary examinations requiring an additional hospital visit within the short period between diagnosis and treatment. Finally, its use of a single post-treatment renal function measurement will miss longitudinal fluctuations in changes in renal function. Because CKD is defined as a permanent decrease in renal function after 90 days,[26] a 6-month follow-up was chosen to mitigate the potential impact of longitudinal variability on the data collected.
CONCLUSION
This study found a decrease in renal function 6 months after both RAPN and PCA. Patients with CKD IIIb–IV, all treated with PCA, showed a greater decline in renal volume than patients with baseline normal renal function. The renal functional changes were similar for RAPN and PCA patients.
Ethical approval
This non-randomized single-center prospective cohort study was approved by the Danish National Data Protection Agency (19/8784) and the Regional Committee on Health Research Ethics for Southern Denmark (S-20180080NA) with approval of an updated version in June 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Duus LA: Grant for participation in international conference: Boehringer-Ingelheim. Graumann, O: Speaker honoraria, Advisory Board member, and Research grant: Boston Scientific. Junker T: Research grant: Boston Scientific.
References
- Epidemiology of renal cell carcinoma. World J Oncol. 2020;11:79-87.
- [CrossRef] [PubMed] [Google Scholar]
- European association of urology guidelines on renal cell carcinoma: The 2022 update. Eur Urol. 2022;82:399-410.
- [CrossRef] [PubMed] [Google Scholar]
- Renal mass and localized renal cancer: Evaluation, management, and follow-up: AUA guideline: Part I. J Urol. 2021;206:199-208.
- [CrossRef] [Google Scholar]
- Comparison of renal parenchymal volume preservation between partial nephrectomy, cryoablation, and radiofrequency ablation using 3D volume measurements. J Endourol. 2015;29:948-55.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-305.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative CT volumetry of estimated residual kidney for prediction of postoperative chronic kidney disease in patients with renal cell carcinoma. Clin Exp Nephrol. 2021;25:315-21.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study. Lancet Oncol. 2006;7:735-40.
- [CrossRef] [PubMed] [Google Scholar]
- Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58-66.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of perioperative outcomes between robotic and laparoscopic partial nephrectomy: A systematic review and meta-analysis. Eur Urol. 2015;67:891-901.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes after cryoablation versus partial nephrectomy for sporadic renal tumors in a solitary kidney: A propensity score analysis. Eur Urol. 2018;73:254-9.
- [CrossRef] [PubMed] [Google Scholar]
- Progression to chronic kidney disease in patients undergoing nephrectomy for small renal masses: A price to pay for a therapeutic success? Postgrad Med. 2018;130:613-20.
- [CrossRef] [PubMed] [Google Scholar]
- Perioperative and long-term outcomes of robot-assisted partial nephrectomy: A systematic review. Am Surg. 2021;87:21-9.
- [CrossRef] [PubMed] [Google Scholar]
- Surgically induced chronic kidney disease may be associated with a lower risk of progression and mortality than medical chronic kidney disease. J Urol. 2013;189:1649-55.
- [CrossRef] [PubMed] [Google Scholar]
- Who really benefits from nephron-sparing surgery? Urology. 2014;84:860-7.
- [CrossRef] [PubMed] [Google Scholar]
- Safety, efficacy, and mid-term oncological outcomes of computed tomography-guided cryoablation of T1 renal cancer. Acta Radiol. 2023;64:814-820.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous ablation versus partial and radical nephrectomy for T1a renal cancer: A population-based analysis. Ann Intern Med. 2018;169:69-77.
- [CrossRef] [PubMed] [Google Scholar]
- Renal functional outcomes after surgery, ablation, and active surveillance of localized renal tumors: A systematic review and meta-analysis. Clin J Am Soc Nephrol. 2017;12:1057-69.
- [CrossRef] [PubMed] [Google Scholar]
- Partial nephrectomy versus ablative techniques for small renal masses: A systematic review and network meta-analysis. Eur Radiol. 2019;29:1293-307.
- [CrossRef] [PubMed] [Google Scholar]
- Person-centred shared decision making. J Eval Clin Pract. 2019;25:1057-62.
- [CrossRef] [PubMed] [Google Scholar]
- The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007;4:e296.
- [CrossRef] [PubMed] [Google Scholar]
- Image-guided tumor ablation: Standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273:241-60.
- [CrossRef] [PubMed] [Google Scholar]
- The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844-53.
- [CrossRef] [PubMed] [Google Scholar]
- Renal cell carcinoma staging: Pitfalls, challenges, and updates. Histopathology. 2019;74:18-30.
- [CrossRef] [PubMed] [Google Scholar]
- American society of Anesthesiologists classification In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2021.
- [Google Scholar]
- A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-83.
- [CrossRef] [PubMed] [Google Scholar]
- The 2021 KDIGO blood pressure target and the progression of chronic kidney disease: Findings from KNOW-CKD. J Intern Med. 2023;294:653-64.
- [CrossRef] [PubMed] [Google Scholar]
- Compensatory changes in the retained kidney after nephrectomy in a living related donor. Transplant Proc. 2012;44:2901-5.
- [CrossRef] [PubMed] [Google Scholar]
- Impact on renal function of percutaneous thermal ablation of renal masses in patients with preexisting chronic kidney disease. J Vasc Interv Radiol. 2012;23:41-5.
- [CrossRef] [PubMed] [Google Scholar]
- Renal function after partial nephrectomy: Effect of warm ischemia relative to quantity and quality of preserved kidney. Urology. 2012;79:356-60.
- [CrossRef] [PubMed] [Google Scholar]
SUPPLEMENTARY TABLES
| RAPN, n= 18 | Cryoablation, n= 39A | |
|---|---|---|
| Anesthesia, n(%) | ||
| Sedation | N/A | 37 (97.4) |
| GA | 18 (100) | 1 (2.6) |
| Type of cryoprobes | ||
| Force | N/A | 26 (68.4) |
| Rod | 2 (5.3) | |
| Pearl | 9 (23.7) | |
| Mix | 1 (2.6) | |
| Number of probes per tumor, n(%) | ||
| 2 | N/A | 12 (31.6) |
| 3 | 14 (36.8) | |
| 4 | 7 (18.4) | |
| 5 | 3 (7.9) | |
| 6 | 1 (2.6) | |
| 7 | 1 (2.6) | |
| Hydro displacement, n(%) | ||
| Yes | 27 (71.1) | |
| No | 11 (28.9) | |
| Dose, mL | ||
| Median (IQR) | N/A | 320 (280–510) |
| Mean (±SD) | 457.9 (308.9) | |
| Range | 122–1,360 | |
| Knife time, min | ||
| Median (IQR) | 175 (160–214) | 60 (49.3–83.0) |
| Mean (±SD) | 181.1 (46.3) | 68.8 (24.5) |
| Range | 79–269 | 38–138 |
| WIT, min | ||
| Median (IQR) | 17.5 (12–20) | N/A |
| Mean (±SD) | 17.3 (5.6) | |
| Range | 11–30 | |
| Blood loss, mL | ||
| Median (IQR) | 100 (100–237.5) | N/A |
| Mean (±SD) | 183.3 (158.1) | |
| Range | 0–600 | |
| Blood transfusion, n(%) | ||
| Yes | 1 (5.3) | N/A |
| No | 18 (94.3) |
RAPN: Robot-assisted partial nephrectomy, RCC: Renal cell carcinoma, GA: General anesthesia, WIT: Warm ischemia time, N/A: Not applicable, IQR: Interquartile range, SD: Standard deviation. AOne patient underwent cryoablation of two RCC
| Change baseline to 6 months (95% CI) |
Standard error | P-value | |
|---|---|---|---|
| CKD IIIa–IV versus CKD I–II (reference) | |||
| Total renal volume (cm3) | −30.3 (−66.4;5.7) | −1.7 | 0.100 |
| eGFR (mL/min/1.73 m2) | 1.8 (−5.5:9.2) | 3.8 | 0.624 |
| DTPA-GFR (mL/min/1.73 m2) | 2.3 (−6.1;10.8) | 4.3 | 0.588 |
| Ipsilateral function (%) | −3.0 (−8.0;2.0) | 2.5 | 0.239 |
| Contralateral function (%) | 5.2 (−0.4;10.8) | 2.9 | 0.071 |
| Ipsilateral renal area (cm2) | 5.4 (−6.8;17.7) | 6.3 | 0.386 |
| Contralateral renal area (cm2) | 8.0 (−3.2;19.1) | 5.7 | 0.161 |
| CKD IIIb–IV versus CKD I–II (reference) | |||
| Total renal volume (cm3) | −31.8 (−63.0; −0.7) | 15.9 | 0.045* |
| eGFR (mL/min/1.73 m2) | −1.1 (−10.4;8.1) | 4.7 | 0.810 |
| DTPA-GFR (mL/min/1.73 m2) | 1.7 (−7.7;11.1) | 4.8 | 0.722 |
| Ipsilateral function (%) | 2.5 (−2.1;7.0) | 2.3 | 0.294 |
| Contralateral function (%) | −0.4 (−6.8;5.9) | 3.2 | 0.879 |
| Ipsilateral renal area (cm2) | 3.3 (−10.6;17.1) | 7.1 | 0.642 |
| Contralateral renal area (cm2) | 3.8 (−10.1;17.7) | 7.1 | 0.593 |
Mixed linear regression model estimating the total renal volume, eGFR, and DTPA-GFR 6 months after treatment, compared to baseline values, CKD: Chronic kidney disease, at CKD I–II versus CKD IIIa–IV and CKD IIIb–IV, respectively. NST: Nephron sparing treatment, eGFR: Estimated glomerular filtration rate, DTPA-GFR: Tc-99m-diethylenetriamine pentaacetic acid clearance, ACKD stages: I (eGFR: ≥90 mL/min/1.73 m2), II (eGFR: 60–89 mL/min/1.73 m2), IIIa (eGFR: 45–59 mL/min/1.73 m2), IIIb (eGFR: 30–44 mL/min/1.73 m2), IV (eGFR: 15–29 mL/min/1.73 m2), V (eGFR<15 mL/min/1.73 m2). BAll patients with CKD IIIa-V (n=7) and IIIb–V (n=5) were treated with cryoablation. CNormal function defined as CKD I–II. *P<0.05, statistical significance
| Change baseline to 6 months (95% CI) | Standard error | P-value | |
|---|---|---|---|
| Total renal volume (cm3) | −18.1 (−44.4;8.2) | 13.4 | 0.178 |
| eGFR (mL/min/1.73 m2) | 4.0 (−1.4;9.4) | 2.8 | 0.148 |
| DTPA-GFR (mL/min/1.73 m2) | 3.7 (−2.6;10.0) | 3.2 | 0.249 |
| Ipsilateral function (%) | 0.6 (−2.9;4.2) | 1.8 | 0.726 |
| Contralateral function (%) | −0.7 (−4.2;2.9) | 1.8 | 0.701 |
| Ipsilateral renal area (cm2) | 2.6 (−7.0; 12.2) | 4.9 | 0.594 |
| Contralateral renal area (cm2) | −2.9 (−9.8;3.9) | 3.5 | 0.399 |
Mixed linear regression model estimating the impact on renal function (total renal volume, eGFR, DTPA-GFR) 6 months after cryoablation compared to baseline values and RAPN. RAPN: Robot-assisted partial nephrectomy, eGFR: Estimated glomerular filtration rate, DTPA-GFR: Tc-99m-diethylenetriamine pentaacetic acid clearance, CI: Confidence interval







