Translate this page into:
Endovascular management of HIV vasculopathy

*Corresponding author: Jack B. Newcomer, University of Kentucky College of Medicine, Lexington, Kentucky, United States. jbne222@uky.edu
-
Received: ,
Accepted: ,
How to cite this article: Newcomer JB, Chishti EA, Raissi D. Endovascular management of HIV vasculopathy. J Clin Imaging Sci 2022;12:9.
Abstract
HIV is a multisystem disease process that can affect the cardiovascular system resulting in vasculopathy. As highly active anti-retroviral therapy has allowed patients to live longer with the disease, vascular complications such as aneurysms, occlusive disease, spontaneous arteriovenous fistulae, and arterial dissections have been described. The pathogenesis of vascular-related complications in HIV is poorly understood but is thought to involve an interplay between viral-induced inflammation, vascular smooth muscle changes, endothelial alterations, and circulating blood factors. The most well-described management strategies for symptomatic aneurysm-related complications are surgical in nature, with mostly anecdotal reports of endovascular intervention. We present a case of a 24-year-old male who was found to have findings consistent with HIV vasculopathy on angiography after presenting with acute GI hemorrhage secondary to left gastric artery bleeding. Our patient was managed with endovascular embolization. Although studies have shown promise regarding the endovascular management of HIV-related aneurysmal complications in the short term, more research is needed to evaluate the long-term success of these interventions.
Keywords
HIV
AIDS
Kaposi sarcoma
Vasculopathy
Embolization
INTRODUCTION
HIV is a multisystem disease process that can affect the cardiovascular system, especially as highly active anti-retroviral therapy (HAART) has allowed patients to live longer with the disease. Vasculopathy in HIV patients can present in a variety of different ways, including aneurysms, occlusive disease, spontaneous arteriovenous fistulae (AVF), and arterial dissections. The pathogenesis of how vascular complications arise in the setting of HIV is not completely understood but is thought to involve multiple interconnected processes including viral-induced inflammation and vascular smooth muscle changes. We present a case of a patient with acute GI hemorrhage secondary to left gastric artery (LGA) pseudoaneurysm, with findings consistent with HIV vasculopathy seen on catheter angiography.
CASE REPORT
A 24-year-old male with a past medical history of HIV/AIDS on HAART consisting of bictegravir/emtricitabine/tenofovir (most recent absolute CD4-cell count of 79/μL with HIV-RNA of 280), disseminated CMV infection, latent syphilis infection, latent pneumocystis jirovecii infection, latent tuberculosis infection, and Kaposi sarcoma with visceral involvement was referred to interventional radiology service for suspected GI bleeding after endoscopic ultrasound (EUS) guided transgastric liver biopsy and endoscopic retrograde cholangiopancreatography (ERCP). The patient initially presented to the emergency department for intractable hematemesis and epistaxis attributed to nasopharyngeal and oropharyngeal Kaposiform lesions; this was treated with embolization of the bilateral ascending pharyngeal arteries and left internal maxillary artery. Following this, the Kaposiform lesions were treated with external beam radiation therapy. During his hospitalization, the patient developed epigastric abdominal pain and jaundice with persistently elevated total bilirubin (highest of 12.8 mg/dL) and liver function tests, notably alkaline phosphatase (highest of 998 U/L) and gamma-glutamyl transferase (highest of 540 U/L). Magnetic resonance cholangiopancreatography (MRCP) was obtained which showed discrete T2 hyperintense nodules in a periportal distribution with intense arterial enhancement in the liver (consistent with Kaposi sarcoma) and multifocal stenosis of the intrahepatic bile ducts with beaded appearance (suggestive of AIDS cholangiopathy). To further investigate the etiology of hyperbilirubinemia, EUS-guided liver biopsy and ERCP with sphincterotomy were performed. Liver biopsy revealed focal areas of vascular proliferation in the parenchyma with endothelial cells staining positive for HHV-8 immunostain, further confirming a diagnosis of Kaposi sarcoma. ERCP demonstrated segmental rarefaction and beading of the right and left intrahepatic biliary ductal branches, suggestive of AIDS cholangiopathy. Following EUS/ERCP, the patient became hypotensive with a hemoglobin of 4.8 g/dL (normal range 13.7–17.5 g/dL) and hematocrit of 13% (normal range 40–51%). After the patient went into cardiac arrest, the return of spontaneous circulation was achieved. Massive transfusion protocol was initiated, and the patient received 9 units of packed red blood cells, 6 units of fresh frozen plasma, 2 units of platelets, and 1 unit of cryoprecipitate. Following this, abdominal CTA was obtained, revealing a large abdominal hematoma and evidence of active contrast extravasation from the LGA [Figure 1]. Emergent catheter angiography and embolization were planned.
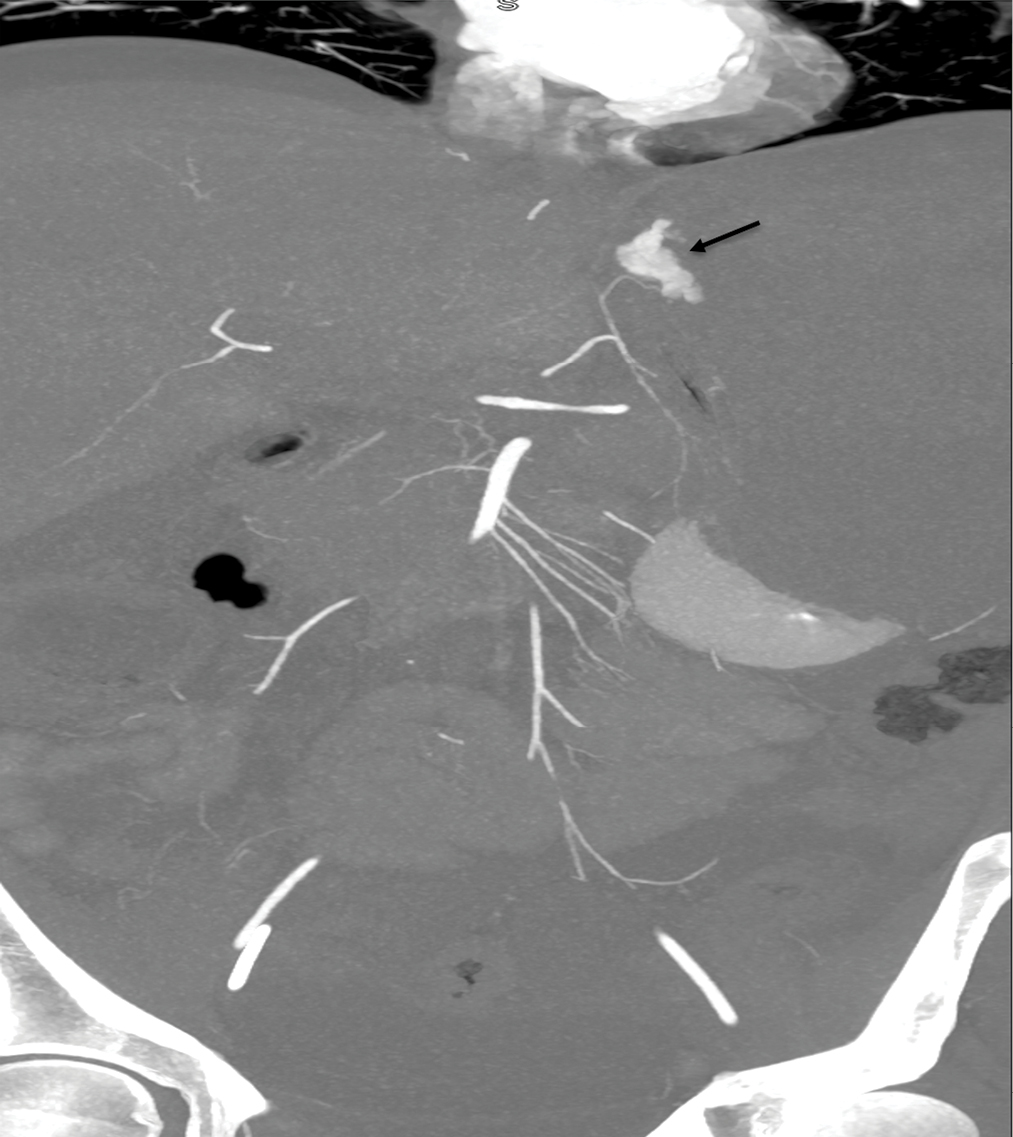
- CT angiography 10 mm slab coronal reconstruction showing active extravasation from distal fundal branch of the LGA (arrow).
The patient was transferred to the angiography suite after being intubated and sedated by the ICU team. The micropuncture technique was used to access the right common femoral artery under ultrasound (US) guidance. A 5-French reverse curve catheter was used to select the origin of the celiac artery, and a celiac angiogram was performed. Celiac angiography revealed fusiform dilation of the gastroduodenal and left gastric arteries [Figure 2], as well as diffuse sequential beaded aneurysmal dilations of common hepatic and proper hepatic arteries extending into the proximal left and right hepatic arteries [Figure 3]. Subsequently, the LGA was selected with the use of a 2.4 French microcatheter via the 5 French catheters, without evidence of contrast extravasation. However, given ominous findings seen on the abdominal CTA, prophylactic embolization of the LGA was performed under fluoroscopic guidance using Gelfoam slurry. Postembolization angiography demonstrated complete stasis of the LGA [Figure 4].
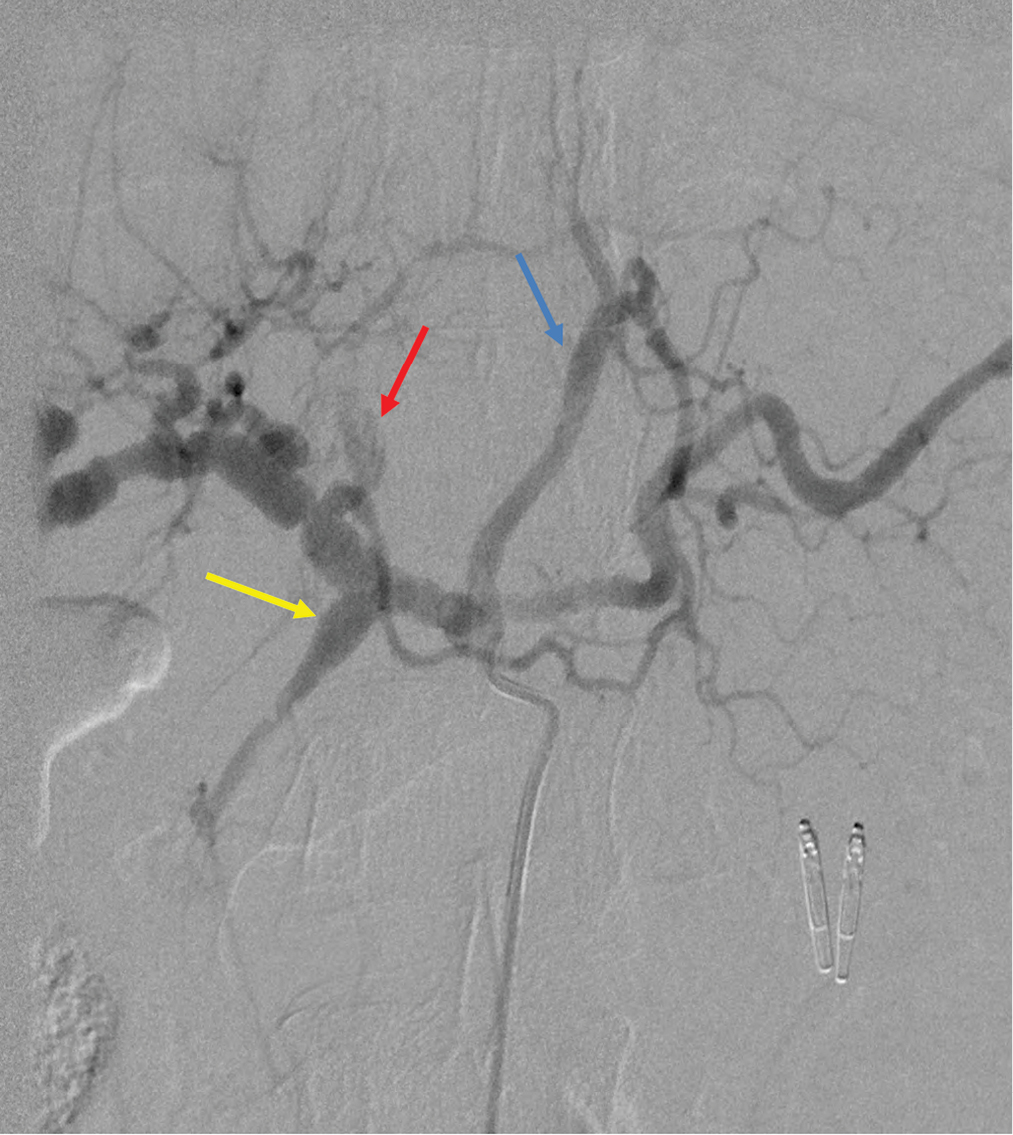
- Digital subtraction angiography of celiac trunk showing fusiform dilation of the gastroduodenal (yellow arrow) and left gastric (blue arrow) arteries. The left hepatic artery pseudoaneurysm can also be seen (red arrow).
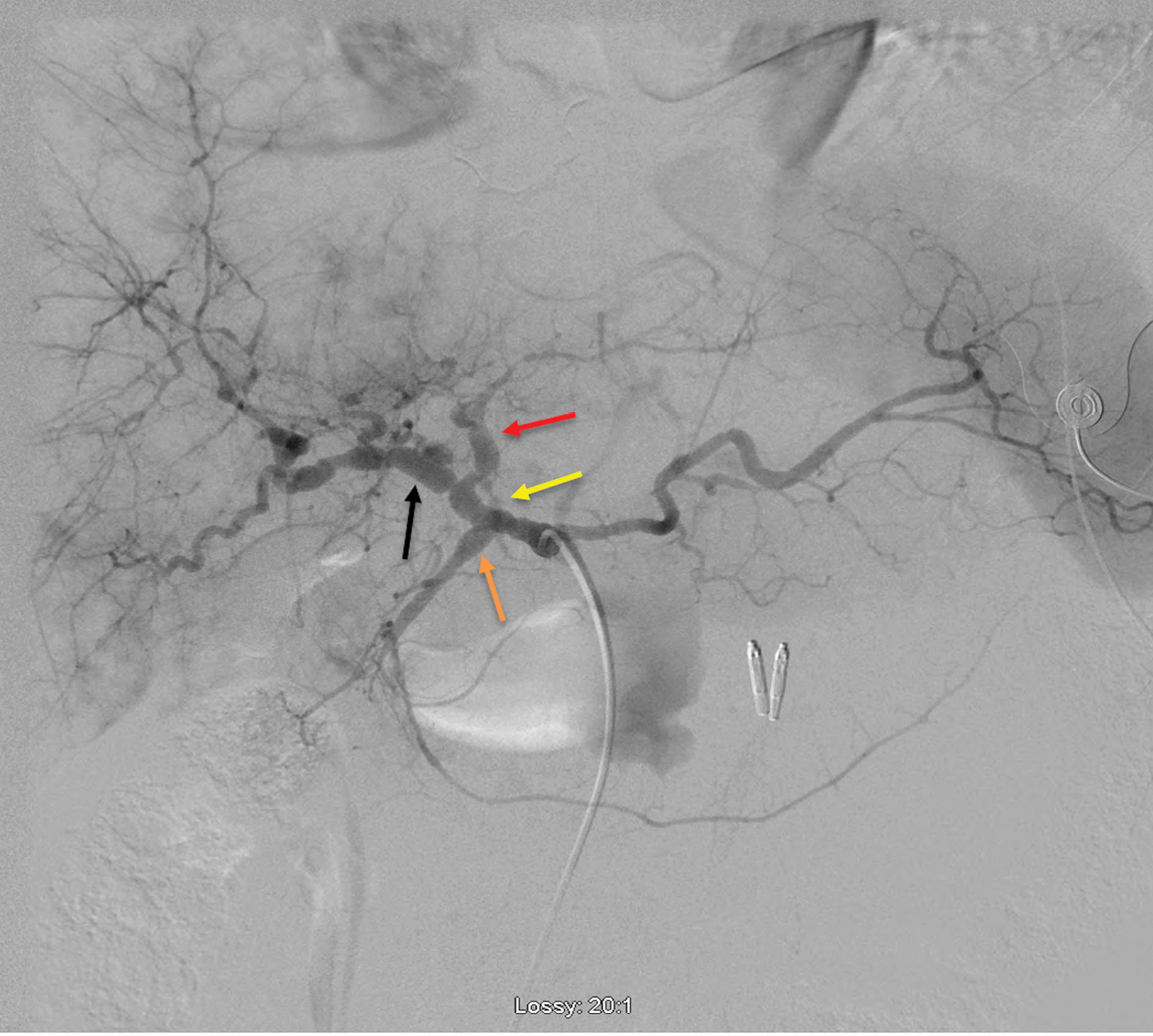
- Digital substraction angiography of celiac trunk demonstarting diffuse sequential beaded aneurysmal dilations of common hepatic and proper hepatic (yellow arrow) arteries extending into the proximal left (red arrow) and right hepatic (black arrow) arteries. Also, fusiform dilation of the gastroduodenal artery (orange arrow) can be seen.
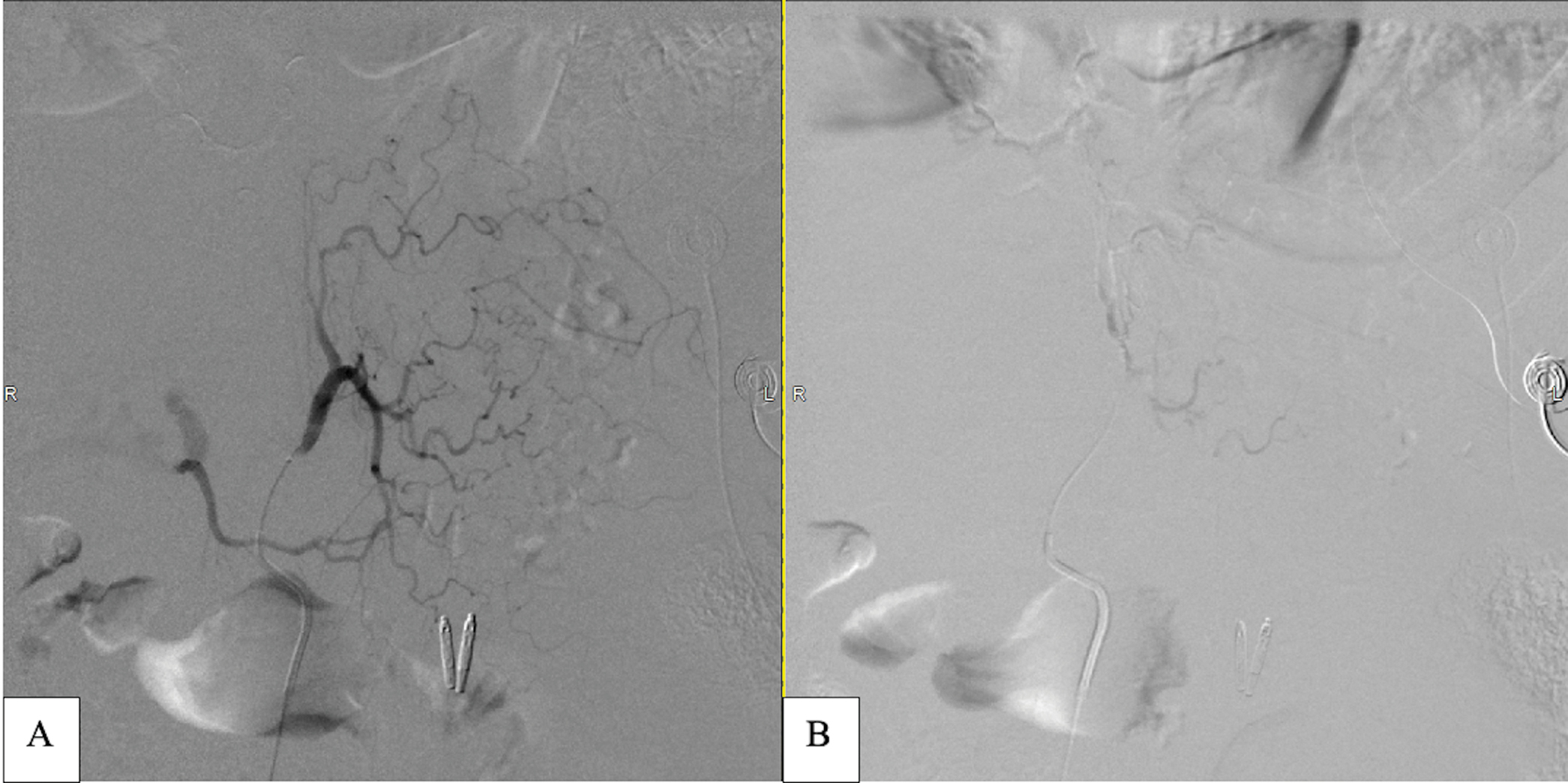
- Digital subtraction angiography of pre and post embolization images of the LGA. (a) LGA showing no active extravasation. (b) Post Gelfoam slurry prophylactic embolization of the LGA showing complete stasis.
The microcatheter was then advanced into the common hepatic artery. Common hepatic angiography demonstrated left hepatic artery (LHA) pseudoaneurysmal formation with delayed drainage into the left portal vein [Figure 5a]. The LHA was selected and multiple detachable microcoils were used to embolize the LHA pseudoaneurysm. Postembolization LHA angiography revealed complete embolization of the LHA pseudoaneurysmal formation and resolution of the arterioportal AV fistula [Figure 5b].
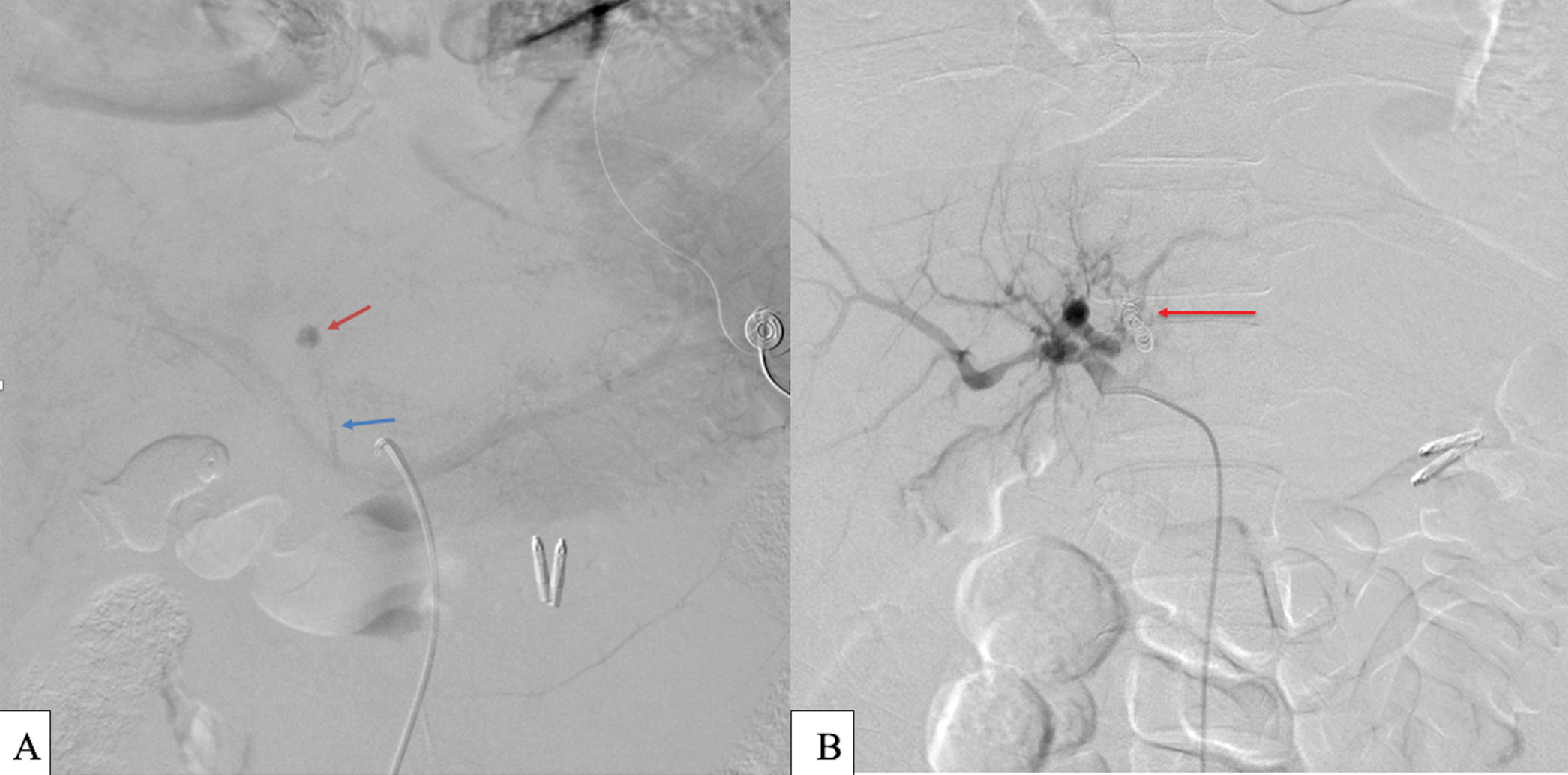
- Digital subtraction angiography of the proper hepatic artery: (a) Left hepatic artery round pseudoaneurysm (red arrow) seen on delayed venous phase of celiac trunk angiography with portal venous drainage (blue arrow). No active extravasation was seen during angiography. (b) Final digital subtraction angiography of the proper hepatic artery showing successful endovascular coiling of the LHA pseudoaneurysm with resolution of the AV fistula (red arrow).
There were no immediate post-procedural complications, however, the patient developed abdominal pain and distension with hemoglobin dropping from 8.7 to 5.2 g/dL on day 4. At this time, he was administered 6 units of packed red blood cells and 2 units of fresh frozen plasma. A paracentesis was performed removing 4L of the bloody fluid. Abdominal CTA was obtained and revealed a small focus of contrast extravasation along the inferior margin of the left lateral hepatic segment [Figure 6a]. Given this finding, repeat embolization was performed.
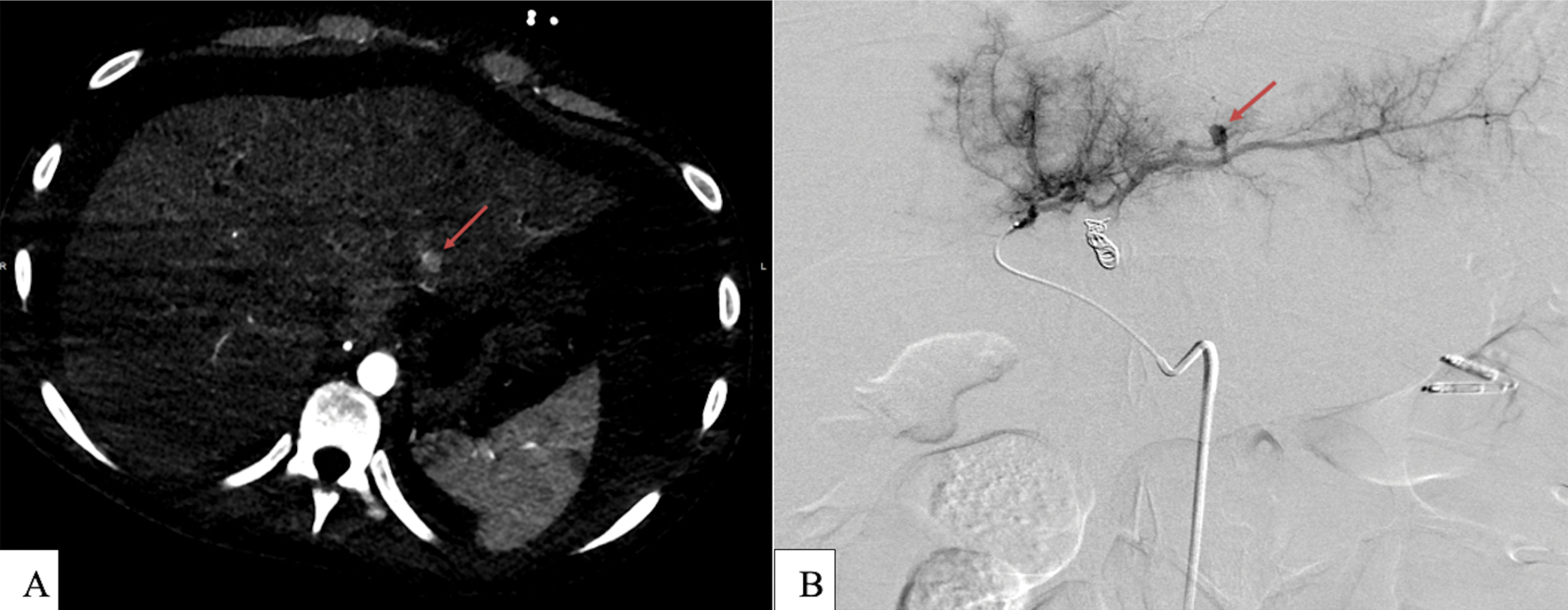
- (a) Abdominal CTA revealing a small focus of contrast enhancement off from LHA branch (red arrow). (b) Selective LHA angiography showing 7 mm pseudoaneurysm off segment II LHA (red arrow).
Using the same technique described above, celiac angiography demonstrated no active contrast extravasation. Selective left hepatic artery angiography revealed a new 7 mm pseudoaneurysm arising from the hepatic segment II arterial branch [Figure 6b]. Microcatheter selection of this branch was performed followed by Gelfoam slurry embolization. Post-embolization angiography of the LHA demonstrated successful embolization of segment II branch pseudoaneurysm with a preferential filling of segment VI branch. A final celiac angiogram showed no active contrast extravasation and complete stasis within segment II. The patient tolerated the procedure well with no immediate complications.
The patient’s condition improved, and his hemoglobin remained stable throughout the remainder of his hospital stay. CT liver with contrast on day 6 after repeat-embolization showed no areas of active contrast extravasation. At the time of discharge (day 7), his hemoglobin was 10.5 g/dL.
DISCUSSION
HIV vasculopathy is characterized by both aneurysmal and occlusive diseases.[1] The exact pathogenesis is unknown, but it is thought to arise from interactions between a variety of different processes including viral-induced inflammation, vascular smooth muscle proliferation, endothelial changes, and circulating blood factors affecting the remodeling of the vessel wall that may lead to subsequent symptomatic clinical disease.[2] HAART is thought to contribute to some of these vascular complications of HIV via immune activation and reconstitution[3] and the development of early atherosclerosis.
The aneurysmal disease often involves the aorta, carotid, popliteal, and femoral vessels[4] and usually arises in the advanced stages of the disease, correlating with lower CD4 counts. Aneurysms can manifest with a variety of clinical symptoms, including pain, pulsatile mass, or venous thrombosis if the aneurysm causes adjacent venous compression. Aneurysms found at one location should guide the screening for aneurysms at other locations, as they are usually multiple.
Imaging findings of aneurysmal disease have been best described with the use of duplex ultrasound, which shows a typical yin-yang pattern within the pseudoaneurysm, mixed echogenicity, and turbulent flow with a vessel wall defect. The aneurysmal pathology can coexist with otherwise normal-looking vasculature proximally and distally; angiographically, these uninvolved arterial segments appear pristine with smooth vessel contours.
In our patient, the typical changes of HIV vasculopathy were seen on initial celiac angiography and hepatic angiogram which revealed diffuse sequential fusiform aneurysmal dilations of the LGA, gastroduodenal, common hepatic, and proper hepatic arteries extending to proximal left and right hepatic arteries [Figures 2 and 3]. These changes are reminiscent of the classic beaded appearance of HIV cholangiopathy.[5]
There are currently no universal guidelines regarding the treatment of aneurysmal disease in the setting of HIV vasculopathy. In symptomatic aneurysms, the most well-described interventions in the literature involve either surgical ligation of vessels or resection and restoration of arterial continuity following aneurysmal excision.[6] Endovascular intervention is a well-accepted alternative, although there are mostly anecdotal reports of endovascular management in the literature.[7] One retrospective analysis evaluating an endovascular intervention for HIV vasculopathy showed short-term success. In this study by Pillay et al.,[8] a group of 23/24 patients with HIV-related nontraumatic aneurysmal disease was treated with stents grafts; only one asymptomatic stent graft occlusion was noted at 30-day follow-up. However, only 11 of these patients returned for their late surveillance, and 9/11 had confirmed stent graft patency. The results of this study suggest that an “endovascular first” strategy was associated with good short-term outcomes in HIV patients with nontraumatic aneurysmal disease, with a lack of follow-up limiting conclusions about long-term success. Our patient was treated urgently with embolization, as he developed hemorrhagic shock secondary to bleeding from LGA and possibly from the LHA. At a 2-month follow-up, the patient did present with bright red blood per rectum but was discharged as the bleeding resolved with no surgical or endovascular intervention needed.
CONCLUSION
HIV vasculopathy is a complex disease process to manage with the pathogenesis of disease being largely theoretical. HAART has allowed patients with HIV to live longer with better quality of life but has also led to an increase in cardiovascular complications such as early-onset atherosclerosis and the development of atypical aneurysmal disease. Although preliminary studies have shown promise regarding the efficacy of endovascular intervention vs. surgical repair for symptomatic HIV-related aneurysmal complications, more research is needed to evaluate the long-term success of these interventions.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- HIV-associated large-vessel vasculopathy: A review of the current and emerging clinicopathological spectrum in vascular surgical practice. Cardiovasc J Afr. 2015;26:70-81.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular surgical complications of the acquired immunodeficiency syndrome. Eur J Vasc Endovasc Surg. 2002;24:473-79.
- [CrossRef] [PubMed] [Google Scholar]
- HIV-associated vascular diseases: Structural and functional changes, clinical implications. Int J Cardiol. 2009;133:293-306.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical Profile of HIV–related aneurysms. Eur J Vasc Endovasc Surg. 2000;20:235-40.
- [CrossRef] [PubMed] [Google Scholar]
- HIV-related/AIDS cholangiopathy: Pictorial review with emphasis on MRCP findings and differential diagnosis. Clin Imaging. 2013;37:219-26.
- [CrossRef] [PubMed] [Google Scholar]
- Management of HIV vasculopathy – a South African experience. Eur J Vasc Endovasc Surg. 2010;39:S25-S31.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple HIV-related aneurysms: Open and endovascular treatment. J Endovasc Ther. 2005;12:405-10.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular therapy for large vessel vasculopathy in HIV-infected patients. Eur J Vasc Endovasc Surg. 2016;52:343-51.
- [CrossRef] [PubMed] [Google Scholar]






