Translate this page into:
Dual-energy Computed Tomography Delayed Myocardial Enhancement in the Diagnostic Dilemma of True versus False Left Ventricular Aneurysm – A Case Report

*Corresponding author: Zuzana Berecova, Radiodiagnostic Clinic, University Hospital – Slovak Medical University and St. Michal Hospital, Bratislava, Slovakia, Europe. hapka78@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Berecova Z, Juskanic D, Simkova J, Simkova I. Dual-energy computed tomography delayed myocardial enhancement in the diagnostic dilemma of true versus false left ventricular aneurysm – A case report. J Clin Imaging Sci 2021;11:20.
Abstract
The aim of this case report is to show the capability of cardiac computed tomography (CT) in combination with dual-energy CT (DECT) delayed myocardial enhancement to support diagnostic decision making in the complicated differential diagnosis of true versus false left ventricle (LV) aneurysm, as well as provide additional information that can influence overall patient outcome. We present a 71-year-old obese patient with metabolic syndrome, stable chronic coronary syndrome with three-vessel disease, and recent chest discomfort. His coronary angiogram showed no significant coronary artery stenosis, but suspicion of LV apical pseudoaneurysm was expressed. Neither transthoracic nor transesophageal echocardiography was able to dismiss this suspicion. Consequently, coronary CT angiography (CCTA) followed by DECT delayed myocardial enhancement was performed. Findings on CCTA and DECT confirmed the diagnosis of a true aneurysm. Moreover, fibrotic changes within the hypertrophic myocardium were visualized. This finding will influence further patient therapy as well as the outcome. DECT delayed myocardial enhancement can be an important complementary tool for distinguishing true versus false LV aneurysms. Moreover, it can provide additional information for making complex diagnose. Adding DECT delayed myocardial enhancement to CCTA can replace cardiac magnetic resonance imaging evaluation in certain settings.
Keywords
Dual-energy computed tomography
Delayed myocardial enhancement
Left ventricle aneurysm
Left ventricle pseudoaneurysm
INTRODUCTION
Left ventricle (LV) aneurysm as well as pseudoaneurysm are usually complications of transmural myocardial infarction and have a similar clinical presentation. Untreated LV pseudoaneurysms have a 30–45% risk of rupture than a more benign natural history of true LV aneurysm.[1] Both have a similar clinical presentation. When a pseudoaneurysm cannot be confidently excluded with echocardiography, cardiac magnetic resonance imaging (MRI) with late gadolinium enhancement (LGE) is considered by the 2017 European Society of Cardiology guidelines to be the gold standard for definitive diagnosis.[2] Cardiac MRI can visualize the morphology and kinetics of LV wall bulging. However, an important aspect of MRI with LGE is its ability to identify post-ischemic fibrotic changes and show their continuity with the LV wall of the true aneurysm or discontinuity in the case of the false aneurysm. Studies have been published to show the high performance of coronary computed tomography (CT) angiography (CCTA) when differentiating true and false LV aneurysms providing the evaluation of LV morphology and kinetics in one setting.[3,4] Dual-energy CT (DECT) delayed myocardial enhancement has been reported to be a valuable diagnostic tool for the detection of post-ischemic and cardiomyopathy-associated myocardial fibrosis.[5,6] However, to the best of our knowledge, none of the published studies have demonstrated the usefulness of adding delayed DECT delayed myocardial enhancement to CCTA for the differential diagnosis of true versus false LV aneurysm, nor the feasibility of using DECT to evaluate myocardial viability or other myocardial pathology in complicated cases in one setting.
CASE REPORT
A 71-year-old obese patient with metabolic syndrome was admitted to the hospital in April 2019 for preoperative evaluation of suspected severe aortic valve stenosis recently diagnosed on echocardiography at an outside facility. He has a history of Q-wave myocardial infarction in the anterolateral wall in 2011, with stent placement in the middle left anterior descending (LAD) artery. He has documented stable chronic coronary syndrome with three-vessel disease. During admission, he complained of episodes of chest discomfort over the prior few weeks. On admission, he underwent coronary angiography, which revealed no signs of mid-LAD in-stent restenosis. Multiple stenoses were visualized on all three main coronary branches with no hemodynamical significance. Hypertrophic midventricular myocardium and suspected pseudoaneurysm of the apex were also seen. Transthoracic and transesophageal echocardiography excluded aortic valve pathology and showed mild LV systolic dysfunction with an ejection fraction of 42% and obstructive mid-ventricular hypertrophy, with subvalvular gradient localization. The LV apical remodeling was visualized with an asymmetrical thin-walled bulging of the LV apex, which showed a paradoxical motion [Figure 1]. The asymmetrical shape of the bulge, its narrowed neck, and its location combined with the patient’s history of myocardial infarction supported the suspicion of an apical LV pseudoaneurysm. We decided to perform cardiac CT instead of MRI due to its better availability, because of the possibility of a life-threatening diagnosis. CCTA was performed with retrospective ECG gating to evaluate LV function, morphology, and kinetics, and it was coupled with DECT delayed myocardial enhancement for fibrosis evaluation in one exam. CCTA confirmed multiple atherosclerotic plaques in the coronary arteries without hemodynamically significant stenosis. It also showed midventricular hypertrophy and remodeling of the apex. There was dyskinetic asymmetrical bulging of the LV apex visualized on cine multiplanar reconstruction (MPR) images and volume rendering technique reconstruction ventriculography. The bulge’s neck was moderately narrowed, which can be a morphological hallmark favoring pseudoaneurysm [Figure 2 and Video 1]. The late enhancement DECT iodine map and virtual monoenergetic imaging (VMI) reconstructions performed at 70 kV showed transmural iodine accumulation in the wall of asymmetric apical bulge extending to the apex and adjacent subendocardial portion of the interventricular septum, representing a myocardial scar [Figure 3]. Apical remodeling due to midventricular hypertrophy and post-ischemic fibrotic changes likely contributed to the bulge’s asymmetrical shape. In light of these findings and the LV morphology on MPRs, a diagnosis of false aneurysm was excluded from the study. Patchy enhancement was visualized in the hypertrophic myocardium [Figure 4], which is associated with arrhythmias and requires additional antiarrhythmic therapy. Fibrotic changes in the myocardium are considered to be a poor prognostic factor in patients with cardiomyopathies. The patient was treated conservatively, and antiarrhythmic medication was added to his treatment. He was dismissed from the hospital with scheduled routine follow-up evaluations in outpatient care. Follow-up cardiac MRI performed a few weeks later showed an apical scar, confirming the DECT diagnosis of a true aneurysm as well as fibrotic changes in the area of midventricular hypertrophy [Figures 5 and 6].
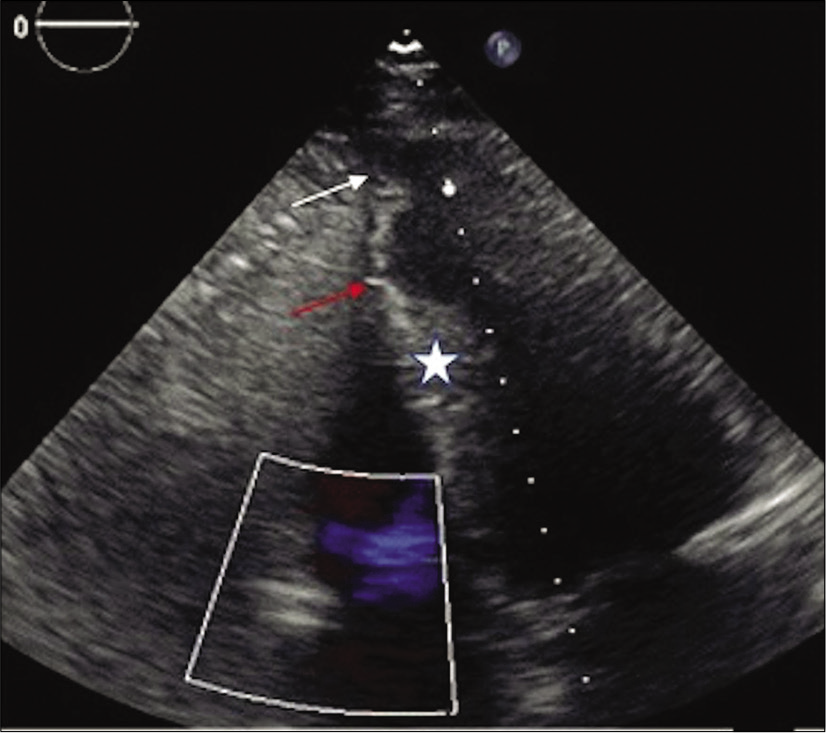
- 71-year-old male with a history of Q wave myocardial infarction and episodes of chest discomfort. Transthoracic echocardiography in four-chamber view with asymmetrical apical bulging (white arrow), midventricular hypertrophy (asterisk), and apical remodeling (red arrow).
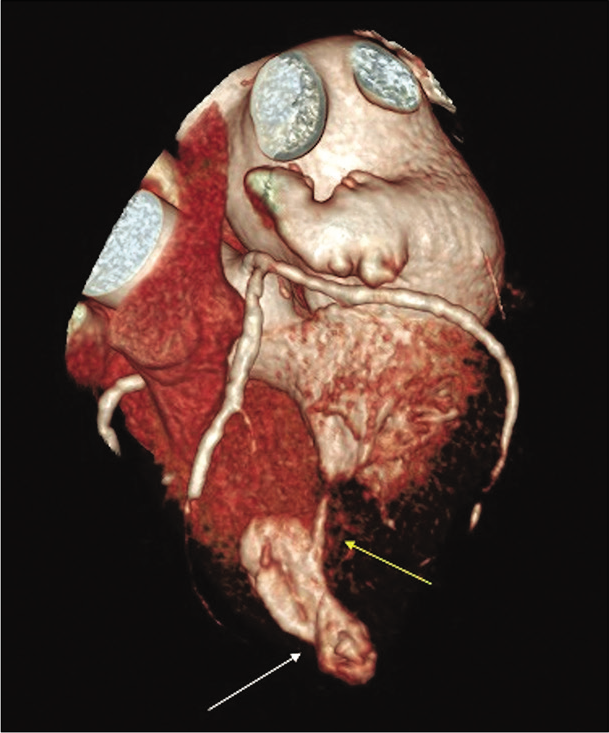
- 71-year-old male with a history of Q wave myocardial infarction who presented with episodes of chest discomfort with suspicion for an apical LV pseudoaneurysm on echocardiography. Cardiac CT angiography - volume rendering technique reconstruction in systole showing asymmetrical apical bulging with neck narrowing (white arrow). Note almost complete LV lumen obliteration due to mid-ventricular hypertrophy (yellow arrow).
Video 1:
Video 1:71-year-old male with a history of Q wave myocardial infarction and chest discomfort episodes evaluated for suspect apical LV pseudoaneurysm. Cardiac CT angiography - volume rendering technique reconstruction in cine mode. There is a paradoxical movement of asymmetrically bulged LV apex. Notice the bulging neck narrowing. There is almost complete LV lumen obliteration due to mid-ventricular hypertrophy in systole [Figure 2 – still image from Video 1].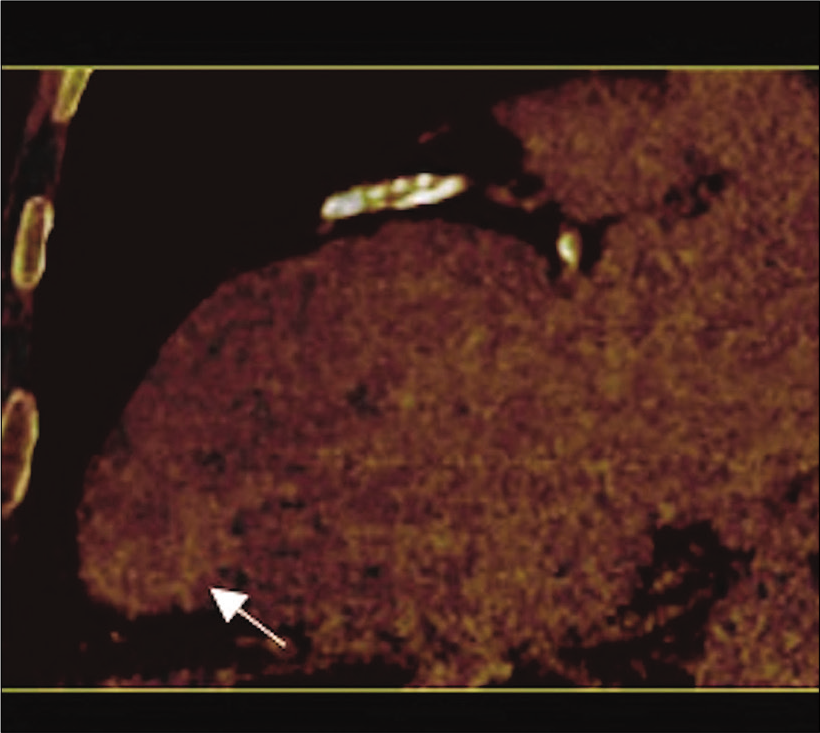
- 71-year-old male with a history of Q wave myocardial infarction and episodes of chest discomfort with suspicion for an apical LV pseudoaneurysm on echocardiography. Dual-energy CT (delayed enhancement) iodinated map in two-chamber view with increased iodine concentration (yellow arrow) in the wall of the asymmetrical bulging extending to the endocardial area (white arrow) representing myocardial wall fibrosis. These findings confirm true apical LV aneurysm.
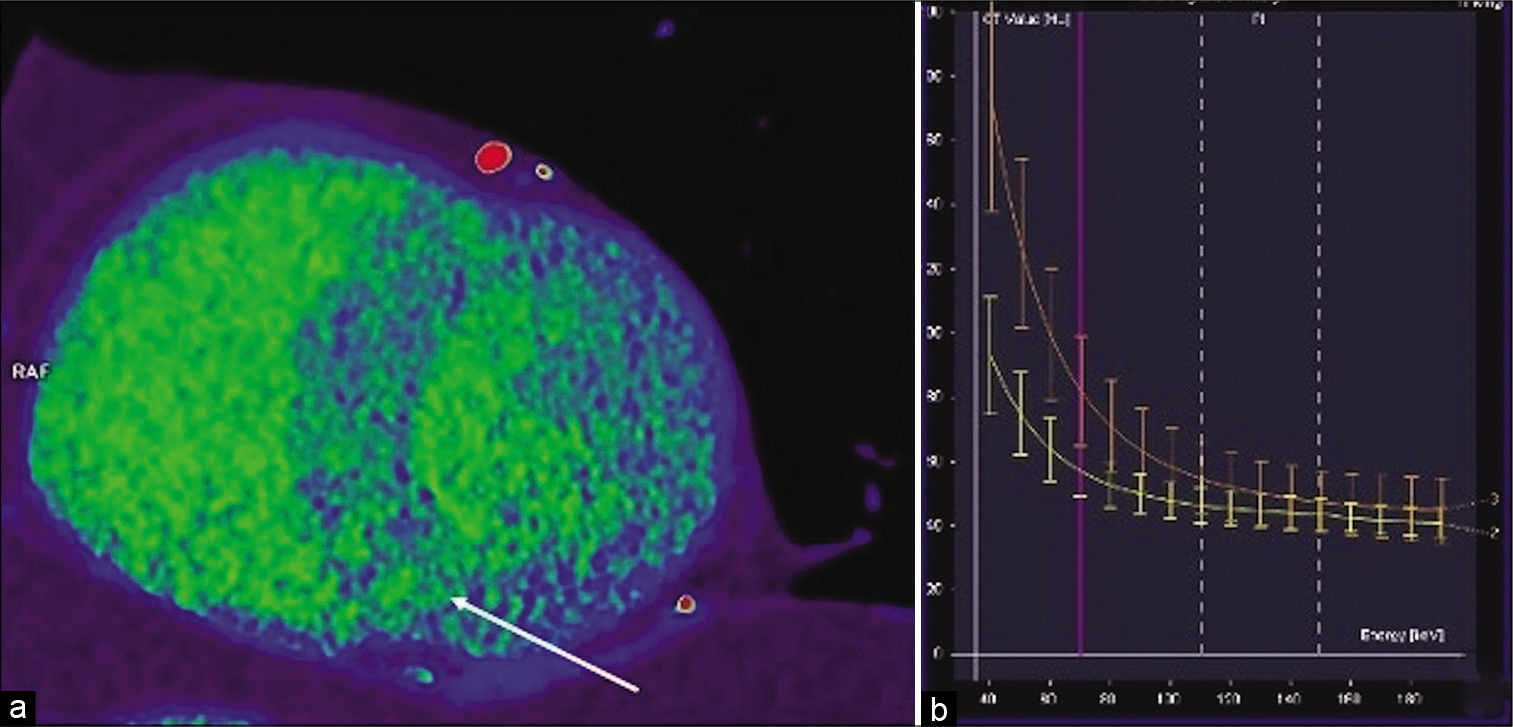
- Dual-energy CT delayed enhancement virtual monochromatic image reconstruction at 70 kV in short axis view midventricular (a) showing an inhomogeneous interventricular septum and anterior LV wall with patchy areas of delayed enhancement (arrow) representing fibrotic changes in hypertrophic cardiomyopathy. Dual-energy CT virtual monochromatic image reconstruction at 70 kV -graph of dependence of attenuation (HU) on energy (kV) (b) in normal myocardium (yellow line) and in fibrotic foci in interventricular septum (orange curve). There is an increased difference in attenuation with decreasing energy proving the iodine content and the delayed enhancement of the myocardium’s patchy areas.
![71-year-old male with a history of Q wave myocardial infarction and chest discomfort episodes evaluated for suspect apical LV pseudoaneurysm. Delayed enhancement MRI in the two-chamber view shows increased enhancement in the asymmetrical bulging wall extending to the endocardial area (white arrow). The findings represent a true apical LV aneurysm. Please note an excellent correlation with dualenergy CT image [Figure 3].](/content/12/2021/11/1/img/JCIS-11-20-g005.png)
- 71-year-old male with a history of Q wave myocardial infarction and chest discomfort episodes evaluated for suspect apical LV pseudoaneurysm. Delayed enhancement MRI in the two-chamber view shows increased enhancement in the asymmetrical bulging wall extending to the endocardial area (white arrow). The findings represent a true apical LV aneurysm. Please note an excellent correlation with dualenergy CT image [Figure 3].
![71-year-old male with a history of Q wave myocardial infarction and chest discomfort episodes evaluated for suspect apical LV pseudoaneurysm. Cardiac MRI delayed enhancement image in short axis view midventricular shows patchy areas of enhancement within interventricular septum and anterior LV wall representing fibrotic changes in hypertrophic cardiomyopathy (yellow arrow). Notice the correlation with DECT late enhancement [Figure 4a].](/content/12/2021/11/1/img/JCIS-11-20-g006.png)
- 71-year-old male with a history of Q wave myocardial infarction and chest discomfort episodes evaluated for suspect apical LV pseudoaneurysm. Cardiac MRI delayed enhancement image in short axis view midventricular shows patchy areas of enhancement within interventricular septum and anterior LV wall representing fibrotic changes in hypertrophic cardiomyopathy (yellow arrow). Notice the correlation with DECT late enhancement [Figure 4a].
DISCUSSION
Cardiac CT generally has better availability and allows for faster evaluation compare to MRI. It enables the analysis of the coronary tree, cardiac function, cardiac morphology, and kinetics; by adding late-phase DECT images, it can also visualize myocardial fibrotic changes as with MRI. Dual-energy technology employs the evaluation with two different energy levels using a two source-detector system, a single X-ray tube switching between energies, or special detector technology depending on a manufacturer. Attenuation data from a second X-ray energy spectrum allows for material decomposition. The presence of materials with high atomic numbers, such as iodine, can be quantified and shown on the iodine map. Moreover, reconstructed VMI at lower energy can better visualize enhancement and is not as affected by beam–hardening artifacts as a single-energy CT.[7] The pooling of iodine in the interstitial space of the myocardial scar follows the same principles as the pooling of gadolinium on LGE MRI images. The ability to construct an iodine map and increased attenuation on VMI reconstructions contributes to better visualization of fibrotic changes on DECT than on single-energy CT.[8]
The reported radiation dose of cardiac DECT is 0.5–4.7 mSv ± 0.10–4.7 mSv depending on the DECT technique used; this dose is lower than or close to the overall annual nonmedical effective dose radiation per capita (3 mSv).[9,10] Other known limitations of DECT are high body mass index, pronounced arrhythmia, and incorrigible high heart rate. DECT might potentially be particularly useful for imaging patients with claustrophobia, implantable cardiac devices, impaired breath-holding capabilities, or other situations when MRI is either contraindicated or not feasible. Indeed, recent concerns, even if they are overemphasized, regarding delayed enhancement cardiac MRI safety, including reports of DNA double-strand breaks and gadolinium-associated nephrogenic systemic fibrosis and deposition in the skin and brain, might encourage the role of DECT delayed enhancement as an alternative for delayed enhancement MRI in selected patients. Late enhancement visualization of post-ischemic fibrotic changes can be useful, especially in ambiguous cases where morphology does not show the typical signs of a true or false aneurysm. It is also important to note that patients with suspected LV pseudoaneurysm have a history of MI and usually have multi-vessels coronary disease. DECT delayed enhancement can, in these cases, offer additional information about other LV segments viability and possibly guide future revascularization therapy. Revascularization can consequently improve an overall LV systolic function and further patient outcome. DECT-delayed myocardial enhancement is also feasible for revealing other myocardial pathologies, such as fibrotic changes in the hypertrophic myocardium, which were found in our case.
CONCLUSION
Adding a DECT delayed myocardial enhancement protocol to CCTA can emphasize the role of CT in the complex diagnosis of true versus false LV aneurysms. It can also allow the CT to serve as a one-stop-shop for evaluation of coronary arteries as well as morphologic and structural changes of the myocardium in complicated patients with the diagnostic dilemma of LV aneurysm versus pseudoaneurysm in potentially urgent cases. Finally, we hope that this case report might support the role of DECT delayed myocardial enhancement as a potentially safe, time, and cost-saving alternative to MRI in certain settings.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Left ventricular pseudoaneurysm versus aneurysm a diagnostic dilemma. Ann Card Anaesth. 2016;19:169-72.
- [CrossRef] [PubMed] [Google Scholar]
- 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119-77.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiac CT angiography: Normal and pathological anatomical features a narrative review. Cardiovasc Diagn Ther. 2020;10:1918-45.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of left ventricular myocardial diseases with cardiac computed tomography. Korean J Radiol. 2019;20:335-51.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of myocardial infarction using delayed enhancement dual-energy CT in stable patients. AJR Am J Roentgenol. 2017;209:1023-32.
- [CrossRef] [PubMed] [Google Scholar]
- Myocardial delayed enhancement CT for the evaluation of heart failure: Comparison to MRI. Radiology. 2018;288:682-91.
- [CrossRef] [PubMed] [Google Scholar]
- Incremental value of myocardial perfusion over coronary angiography by spectral computed tomography in patients with intermediate to high likelihood of coronary artery disease. Eur J Radiol. 2015;84:637-42.
- [CrossRef] [PubMed] [Google Scholar]
- Update on cardiovascular applications of multienergy CT. Radiographics. 2017;37:1955-74.
- [CrossRef] [PubMed] [Google Scholar]
- CAD detection in patients with intermediate-high pre-test probability: Low-dose CT delayed enhancement detects ischemic myocardial scar with moderate accuracy but does not improve performance of a stress-rest CT perfusion protocol. J Am Coll Cardiol Cardiovasc Imaging. 2013;6:1062-71.
- [CrossRef] [PubMed] [Google Scholar]
- First-arterial-pass dual-energy CT for assessment of myocardial blood supply: Do we need rest, stress, and delayed acquisition? Comparison with SPECT. Radiology. 2014;270:708-16.
- [CrossRef] [PubMed] [Google Scholar]






