Translate this page into:
Role of Ultrasound Acoustic Radiation Force Impulse in Differentiating Benign from Malignant Superficial Lymph Nodes

*Corresponding author: Vinu Moses, Department of Radiology, Christian Medical College, Vellore, Ida Scudder Road, Vellore - 632 004, Tamil Nadu, India. vinu@cmcvellore.ac.in
-
Received: ,
Accepted: ,
How to cite this article: Chanda R, Kandagaddala M, Moses V, Sigamani E, Keshava SN, Janakiraman R. Role of ultrasound acoustic radiation force impulse in differentiating benign from malignant superficial lymph nodes. J Clin Imaging Sci 2020;10:18.
Abstract
Objective:
The purpose of this study was to evaluate the diagnostic performance of acoustic radiation force impulse (ARFI) imaging in differentiating benign from malignant peripheral lymphadenopathy.
Materials and Methods:
This was a prospective study approved by the Institutional Review Board with financial grant for the same. Ultrasound and ARFI imaging of peripheral lymph nodes were performed and correlated with pathological results, which were used as the reference standard. The virtual touch tissue imaging and virtual touch tissue quantification parameters of ARFI were analyzed in 86 lymph nodes, of which 78 were included in the study. Using receiver operating characteristic curve analysis, the diagnostic usefulness of ARFI values were evaluated with respect to their sensitivity, specificity, and area under the curve.
Results:
The mean area ratio of benign lymph nodes was 0.88 (±0.2) and that of malignant lymph nodes was 1.17 (±0.14). The mean shear wave velocities (SWV) of benign and malignant lymph nodes were 2.02 m/s (±0.94) and 3.7 m/s (±2.27), respectively. The sensitivity and specificity of virtual touch imaging area ratio in differentiating benign from malignant lymph nodes was 97% and 77%, of SWV was 71% and 70%, and of SWV ratio was 68% and 79%, respectively.
Conclusion:
As ARFI was found to have a superior diagnostic performance over conventional ultrasound and color Doppler in the characterization of lymph nodes, we recommend its routine use in differentiating benign from malignant nodes.
Keywords
Acoustic radiation force impulse
Ultrasound
Elastography
Lymph node
INTRODUCTION
The etiology for palpable enlarged lymph nodes can range from reactive changes to inflammatory or infective conditions to more sinister causes such as malignancy and metastasis. Ultrasound has a high sensitivity in detecting abnormal lymph nodes. However, a single grey scale or Doppler criteria is yet to be found that can satisfactorily differentiate malignant from benign lymph node.[1]
Acoustic radiation force impulse (ARFI) is a novel technique that can be used to perform both qualitative and quantitative measurements of tissue stiffness and is independent of the amount of manual compression. This study was aimed at assessing the diagnostic accuracy of ARFI elastography in comparison to clinical palpation, ultrasound (US), and Doppler in differentiating benign and malignant superficial lymph nodes using histopathological diagnosis as the reference standard.
MATERIALS AND METHODS
Study population recruitment
This institutional review board approved prospective study was performed at a tertiary hospital in India.
The study was conducted on 86 patients who were referred to the Department of General Surgery and Department of Radiodiagnosis for biopsy/fine-needle aspiration cytology (FNAC) (84 biopsy, two converted to FNAC) of peripheral lymph nodes between September 2015 and August 2016. An informed consent was obtained from all patients before the study. Lymph nodes <7 mm, heterogeneous nodes with large areas of necrosis or calcification precluding virtual touch quantification (VTQ) assessment were not included in the study. US and ARFI of peripheral lymph nodes were performed before any intervention using a high multi- frequency linear probe (4–9 MHz) on a Siemens ACUSON S2000TM. Time between the index study (ARFI) and the reference standard was a maximum of 2 weeks. Eight patients lacking confirmatory tissue diagnosis were excluded from the study. Flowchart showing patient flow during the study is depicted in Figure 1.
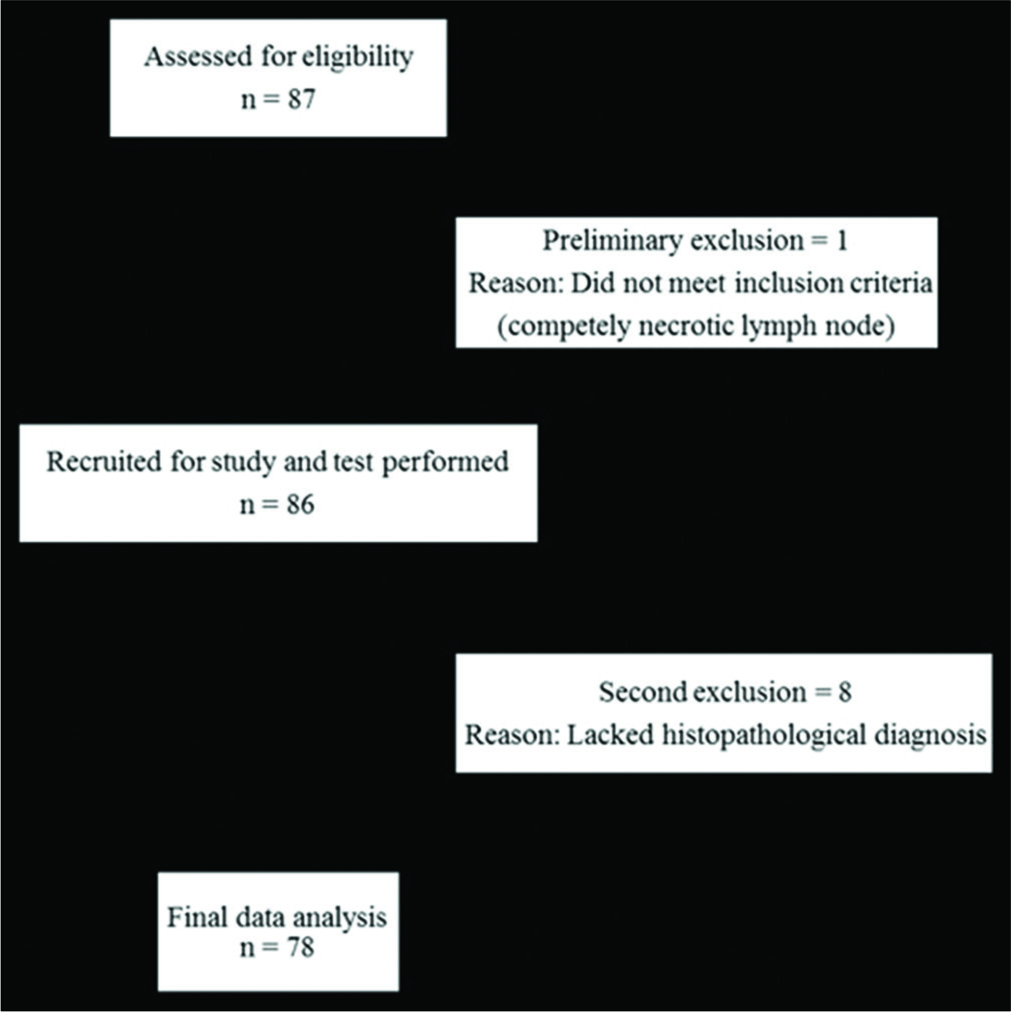
- Schematic representation of patient flow through the study.
Technique
After clinical palpation for consistency, B-mode ultrasonography was performed and the single most representative node was isolated based on size (largest and most accessible node). The selected lymph node was examined for site, size – long axis diameter, short axis diameter, ratio of long axis to short axis, shape, margins, echogenicity, presence or absence of hilum, and calcification and liquefaction, if any. Nodal vascularity was assessed using Color Doppler with a low scale (1–8 cm/s).
Using light pressure and suspended respiration, virtual touch imaging (VTI) was performed. With the VTI mode turned on, once the lesion was entirely placed within the region of interest (ROI), a B-mode image was obtained on the left hand side of the screen, and VTI image was obtained on the right hand side. The area of the lesion was measured on both B-mode and VTI and area ratio calculated.
For VTQ, the ROI box was placed within the lesion and shear wave velocity (SWV) measured 5 times. SWV of perilesional soft tissue at the same depth was also measured and the ratio of both calculated. In five cases, a VTQ “X.XX m/s” was repeatedly obtained, implying that the VTQ values were outside the machine’s range of assessment. Such lymph nodes were quantified based on their appearance on grey scale, as “too soft” for cystic lesions and “too hard” for entirely solid lesions. These five cases were excluded from the VTQ analysis but included in the study for the assessment of other parameters, namely, US and VTI, to determine whether VTI had any benefit over VTQ in such cases.
Final diagnosis
Each imaging parameter was interpreted individually and compared with the cytological/histopathological diagnosis.
RESULTS
Statistical analysis was performed using SPSS® v. 24.0 software (SPSS Inc., Chicago, IL). Receiver operating characteristic curves were employed to compare the diagnostic performances of ultrasound, Color Doppler, and both VTI and VTQ parameters of ARFI. P < 0.01 was considered statistically significant. Positive predictive values (PPV), likelihood ratios and odds ratios for malignant and benign lymph nodes were determined.
A total of 86 patients were enrolled in this study, of which 78 were included in the final analysis. There were 32 men and 46 women. The average age of the patients was 36 years. Tuberculosis was present in a significant proportion of benign lymph nodes (20 of 43). Lymphoma was the most common etiology of nodal involvement by neoplastic process (19 of 35). Histopathology of the analyzed lymph nodes is detailed in Tables 1 and 2.
| Histopathology | Number of nodes (%) |
|---|---|
| Reactive change/hyperplasia | 16 (37.2) |
| Tuberculosis | 20 (46.5) |
| Granulomatous inflammation | 6 (14) |
| Kikuchi disease | 1 (2.3) |
| Total | 43 |
| Histopathology | Number of nodes (%) |
|---|---|
| Lymphoma | 19 (54.3) |
| Metastasis | |
| Adenocarcinoma | 4 (11.4) |
| Squamous cell carcinoma | 2 (5.7) |
| Breast | 6 (17.1) |
| Papillary thyroid | 2 (5.7) |
| Ewing | 1 (2.9) |
| Urothelial | 1 (2.9) |
| Total | 35 |
The sensitivity of clinical palpation in determining the presence of malignancy in a lymph node was 37.5% (low Chi-square statistic, P > 0.01).
Ultrasound and color Doppler characteristics
Detailed assessment of each parameter is provided in Table 3 and the corresponding sensitivity and specificity in Figure 2. Using short axis diameter of ≥1 cm predicted malignancy with a sensitivity of 60%, specificity of 49%, and an accuracy of 54%. Alhough an altered nodal echotexture had a sensitivity of 77%, a large number of benign nodes also appeared hypoechoic. The presence of intra nodal necrosis or calcification was highly indicative of a pathological process; however, these were not specific to any benign or malignant entity. The diagnostic accuracy of L-S ratio was 56.4%, with a sensitivity of 71.4%, specificity of 44.2%, PPV of 51%, and negative predictive values of 66%. Overall, diagnostic accuracy of US parameters ranged from 49% to 58%. An altered hilar vascularity favored malignancy with a sensitivity of 86%, specificity of 44%, and accuracy of 63%. A combination of US and CD increased the specificity to 69% and the overall diagnostic accuracy to 65.4%.
| Benign (n=43) | Malignant/Metastatic (n=35) | |
|---|---|---|
| Short axis diameter (%) | ||
| ≤1 cm | 21 (48.8) | 14 (40) |
| >1 cm | 22 (51.2) | 21 (60) |
| Long axis to short axis ratio (%) | ||
| <2 | 24 (55.8) | 25 (71.5) |
| >2 | 19 (44.2) | 10 (28.5) |
| Hilum (%) | ||
| Present | 23 (53.5) | 17 (48.6) |
| Absent | 20 (46.5) | 18 (51.4) |
| Necrosis (%) | ||
| Absent | 37 (86.1) | 34 (97.1) |
| Present | 6 (13.9) | 1 (2.9) |
| Calcification (%) | ||
| Absent | 37 (86.1) | 34 (97.1) |
| Present | 6 (13.9) | 1 (2.9) |
| Shape (%) | ||
| Normal (oval) | 30 (69.8) | 20 (57.1) |
| Abnormal (other) | 13 (30.2) | 15 (42.9) |
| Margin (%) | ||
| Normal (circumscribed) | 37 (86.1) | 30 (85.7) |
| Abnormal (other) | 6 (13.9) | 5 (14.3) |
| Echo pattern (%) | ||
| Normal | 14 (32.6) | 8 (22.8) |
| Abnormal | 29 (67.4) | 27 (77.2) |
| Hilar vascularity (%) | ||
| Normal | 19 (44.2) | 5 (14.3) |
| Abnormal | 24 (55.8) | 30 (85.7) |
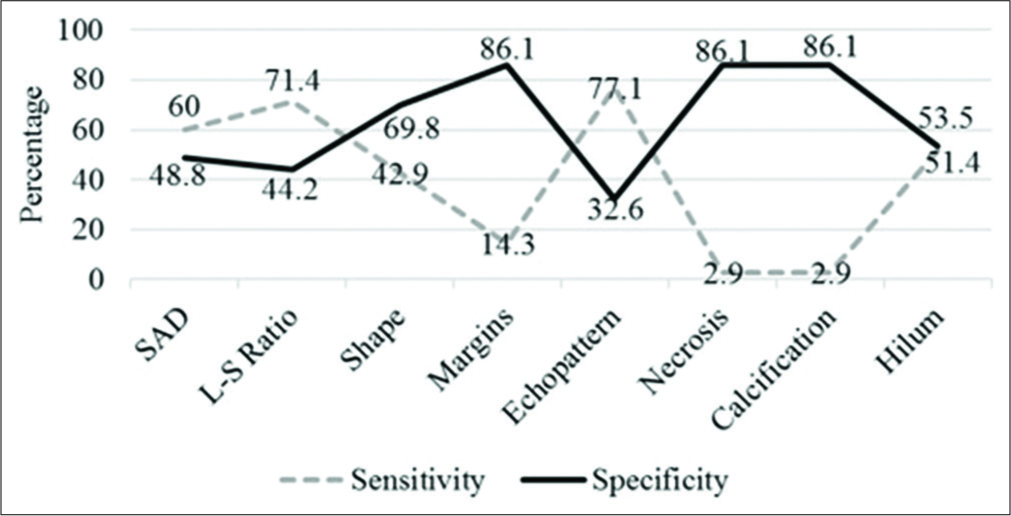
- Statistical parameters of ultrasound characteristics.
ARFI characteristics
The mean lymph nodal area on VTI was 1.5 cm2 (±1.08) for benign and 2.6 cm2 (±1.74) for malignant nodes. VTI area ≥1.4 cm2 had a sensitivity of 74%, specificity of 63%, and accuracy of 68% in predicting malignancy. Cutoff area ratio of ≥1 had an improved sensitivity of 97%, specificity of 77%, accuracy of 86%, and area under curve (AUC) of 0.86 (P < 0.01) [Figure 3a]. Benign nodes had a mean area ratio of 0.88 (standard deviation [SD] ± 0.2) while for malignant node the mean ratio was 1.17 (SD ± 0.14) [Figure 4]. VTI brightness pattern detected malignancy with a sensitivity of 74%, specificity of 77%, and diagnostic accuracy of 76% [Figures 5 and 6]. Combining both VTI parameters increased the sensitivity to 99% and specificity to 95%.
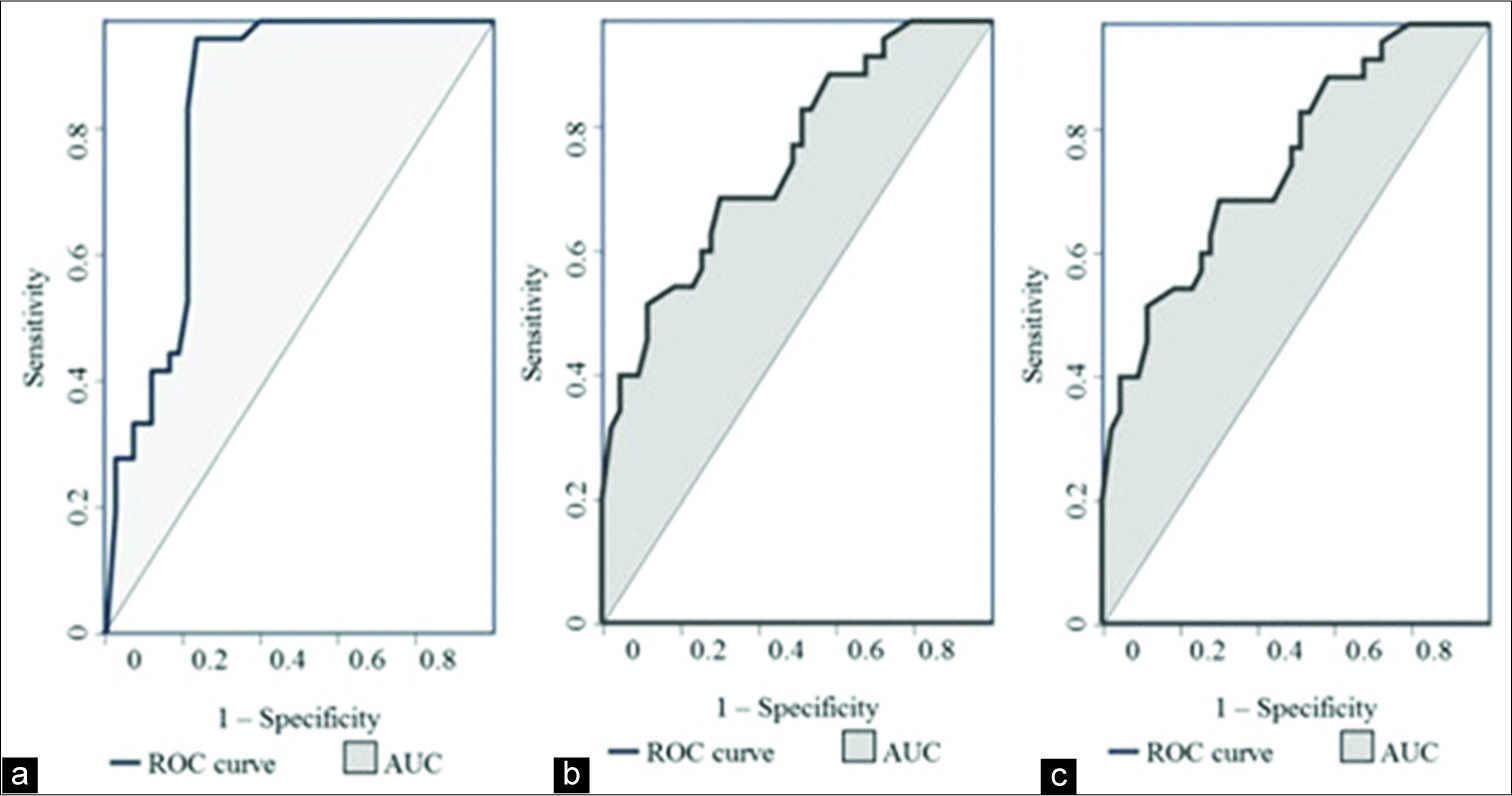
- Receiver operating characteristic curves of (a) area ratio on virtual touch imaging, (b) mean shear wave velocity of lymph node on VTQ and (c) mean SWV ratio of lymph node to surrounding tissue on VTQ.
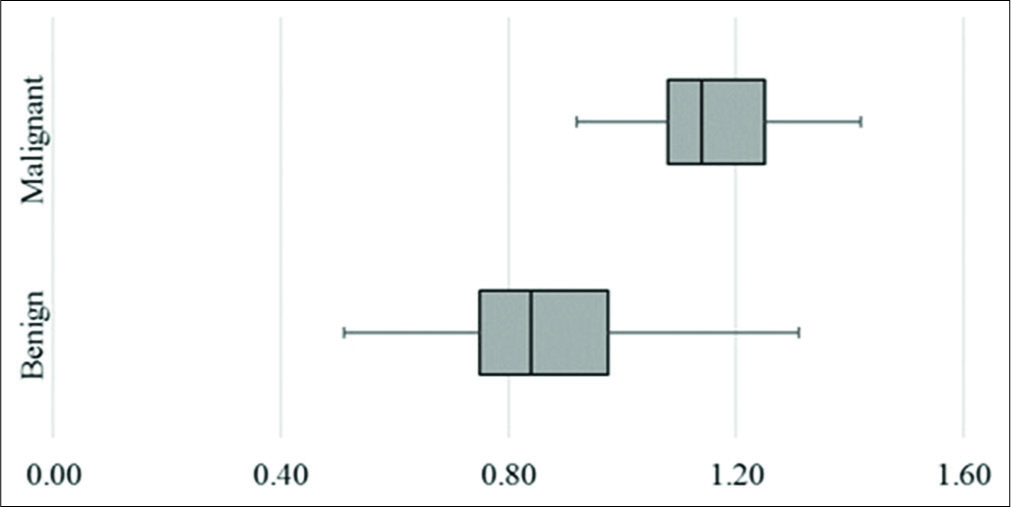
- Box and whisker plot of area ratio of lymph nodes on virtual touch imaging.
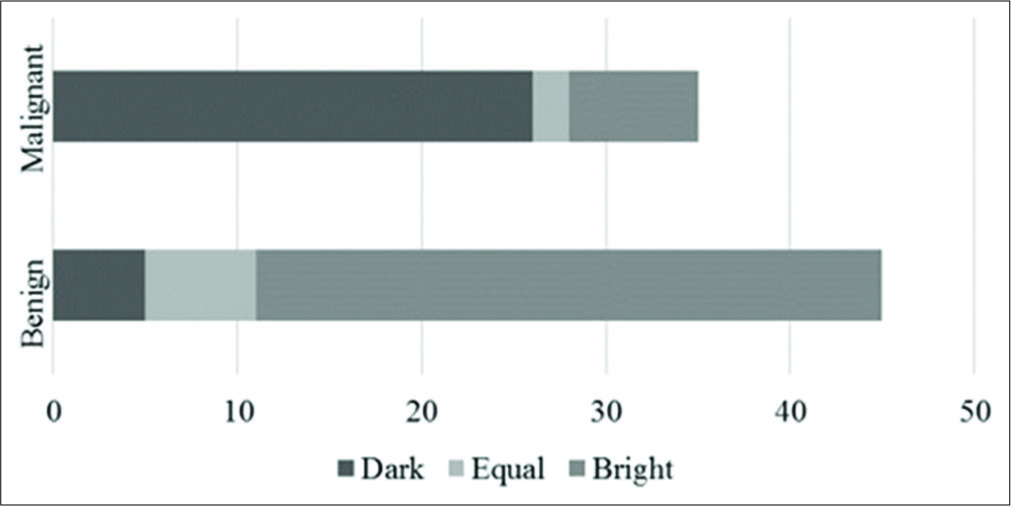
- Brightness pattern of lymph nodes on virtual touch imaging.
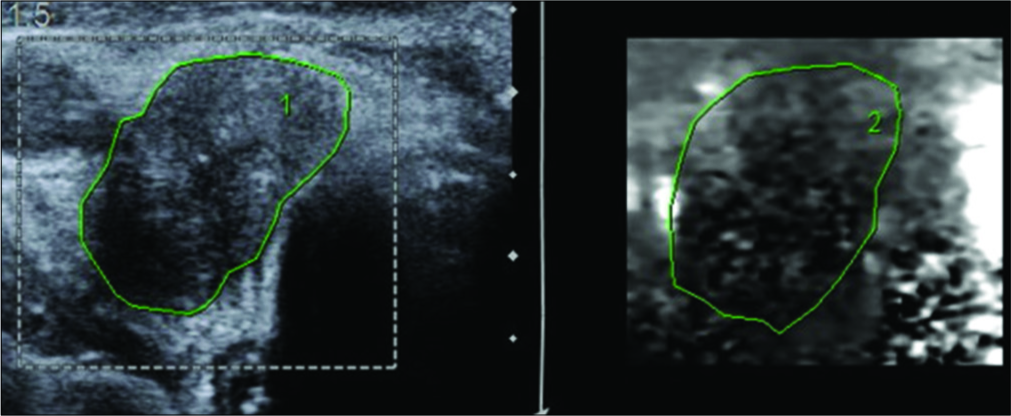
- A 62-year-old male presented with swelling of the thyroid gland and palpable cervical lymph nodes. Ultrasonography and corresponding virtual touch imaging image of a malignant lymph node demonstrating that the node is darker than the surrounding tissue. Histopathological examination was reported as papillary thyroid carcinoma with nodal involvement.
On VTQ, the mean SWV of benign lesions was 2.02 m/s (SD ± 0.94) and of malignant lesions was 3.7 m/s (SD ± 2.27) [Figure 7]. Cutoff value of ≥2.4 cm2 predicted malignancy with a sensitivity of 71%, specificity of 70%, accuracy of 70%, and AUC 0.78 (P < 0.01) [Figure 3b]. The mean SWV ratio (SWVR) of benign nodes was 1.1 m/s (SD ± 0.64) and of malignant nodes was 2.4 m/s (SD ± 1.71) [Figure 8]. Using ≥1.5 cutoff SWVR had a sensitivity of 68%, specificity of 79%, accuracy of 74%, and AUC 0.82 (P < 0.01) [Figure 3c]. Concomitant use of both SWV of lesion and SWVR increased the sensitivity to 91% and specificity to 94%.
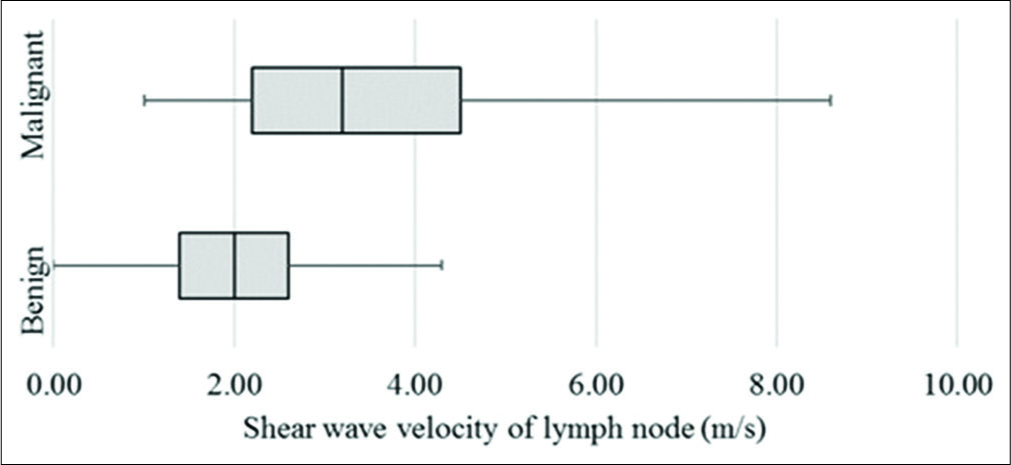
- Box plot of shear wave velocity of lymph nodes on virtual touch quantification.
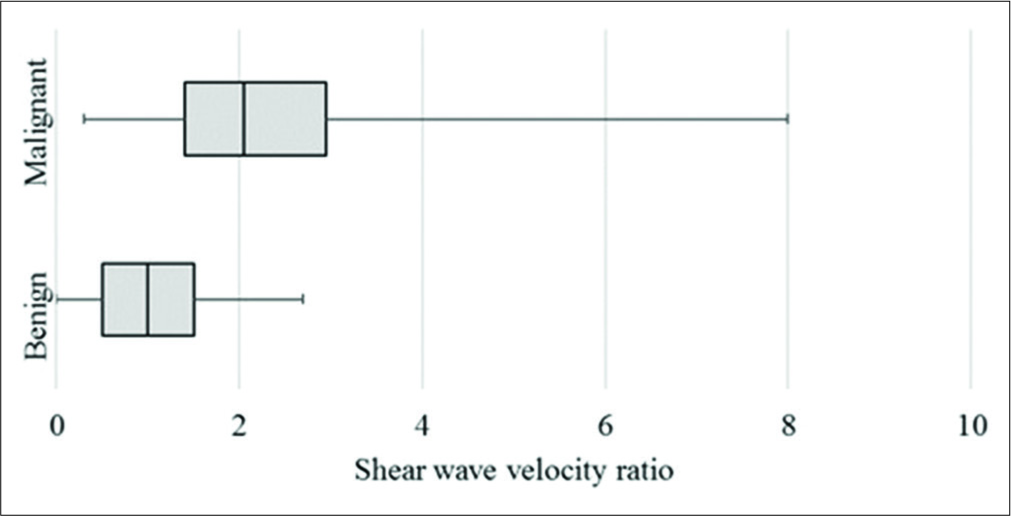
- Box and whisker plot of shear wave velocity ratio between lymph node and surrounding tissue.
Amongst the benign category of lymph nodes, VTI area ratio of reactive hyperplasia was 0.77 (SD ± 0.14) and of tuberculous nodes was 0.90 (SD ± 0.23). Mean SWV of reactive and tuberculous nodes were 2.47 m/s (SD ± 0.98) and 1.21 (SD ± 0.5) and SWVR was 1.71 m/s (SD ± 1.09) and 0.96 (SD ± 0.96), respectively. None of the ARFI parameters could reliably differentiate among the malignant lymph node subgroups, namely, lymphoma and metastatic nodes, though all five nodes with indeterminate SWV values were solid on grey scale and metastatic on histopathological examination (HPE). ARFI characteristics of the examined lymph nodes are described in Table 4.
| ARFI Parameter | Benign (n=43) | Malignant/Metastatic (n=35) |
|---|---|---|
| VTI area (%) | ||
| <1.4 | 25 (58.1) | 8 (22.9) |
| ≥1.4 | 18 (41.9) | 27 (77.1) |
| VTI area ratio (%) | ||
| <1 | 28 | 1 (2.9) |
| ≥1 | 15 (34.9) | 34 (97.1) |
| VTI brightness pattern (Compared to surrounding) (%) | ||
| Less than surrounding | 10 (23.3) | 26 (74.3) |
| More than surrounding | 33 (76.7) | 9 (25.7) |
| VTQ SWV of lesion (%) | ||
| <2.4 | 30 (69.8) | 11 (31.5) |
| ≥2.4 | 13 (30.2) | 24 (68.5) |
| VTQ SWV ratio (%) | ||
| <1.5 | 28 (65.1) | 10 (28.5) |
| ≥1.5 | 15 (34.9) | 25 (71.5) |
ARFI: Acoustic radiation force impulse, VTI: Virtual touch imaging, VTQ: Virtual touch quantification, SWV: Shear wave velocities
Figure 9 shows US and ARFI images of a 55-year-old female patient who presented with fever, loss of weight, loss of appetite, and lymphadenopathy for few months. She had cervical lymphadenopathy on the clinical examination. Final diagnosis of tuberculosis was made on HPE.

- A 55-year-old woman with constitutional symptoms for few months. An enlarged cervical lymph node was imaged before biopsy. (a) US: Hypoechoic oval lymph node with slightly lobulated contour. (b) Doppler: Significantly increased nodal and perinodal vascularity. (c) VTQ: SWV of lymph node 1.5 m/s. (d) VTQ: SWV of surrounding tissue is 2.3 m/s. (e) VTI: Brightness pattern similar to surrounding with decreased area ratio. (f) Large areas of caseous necrosis, H&E, ×40. (g) Epithelioid granu-lomas with Langhan’s giant cells, H&E, ×400. Final diagnosis: Tuberculosis. US: Ultrasonography, VTQ: Virtual touch quantification, SWV: Shear wave velocities, VTI: Virtual touch imaging.
DISCUSSION
Ultrasonography (US) is a good and cost-effective tool which evaluates a multitude of parameters such as size, shape, borders, as well as internal architecture to discern whether a node is malignant or not.[2-4] Lyshchik et al. found that short-to-long-axis diameter ratio >0.5 was the best grey scale criterion with 75% sensitivity, 81% specificity, and 79% overall accuracy.[5] A multivariate analysis of US findings performed by Chikui et al. suggested that the only sonographic features such as increase in short axial diameter, presence or absence of hilar echoes, and the presence of normal hilar flow were predictive of reactive (presence of hilar flow and hilar echoes) and metastatic (increase in short axis length) lymph nodes.[6]
Although conventional lymph node elastography improves the specificity of lymph node characterization, the amount of manual compression applied in it is operator dependent and hence variable.[7-11]
ARFI based elasticity imaging methods use operator independent short duration push pulses that cause transient dynamic tissue displacement which can be measured and reconstructed to provide both qualitative images and quantitative elasticity metrics.[12,13] Although the application of ARFI imaging in the liver has been standardized for a while now, its use in other organs is still limited to research phase, with very few studies being currently available on the evaluation of peripheral lymph nodes.[14]
Fujiwara et al. used ARFI on 42 lymph nodes and found that reactive lymph nodes had a SWV of 1.52 ± 0.48 m/s, while SWV of metastatic/malignant lymph nodes was 2.46 ± 0.75 m/s. A cutoff SWV > 1.9 m/s predicted metastatic lymph nodes with 81.8% sensitivity, 95.0% specificity, and 88.0% overall accuracy. The area under the ROC curve was 0.923.[15] Meng et al. evaluated VTQ component of ARFI in 181 cervical lymph nodes. Using a cutoff value of 2.595 m/s, the mean VTQ values of the benign lesions were found to be 2.01 ± 0.95 m/s and that of malignant lesions were 4.61 ± 2.56 m/s. Malignancy could be predicted with 82.9% sensitivity, 93.1% specificity.[16] VTI was analyzed by Che et al. in 81 lymph nodes (36 benign nodes and 45 metastatic nodes). In their study, most metastatic nodes were darker than the surrounding tissue and a cutoff area ratio of 1.16 predicted malignancy with 91.1% sensitivity and 83.3% specificity.[17] In a multivariate analysis by Xu et al., among US, SE, and ARFI, VTI area ratio >1 emerged to be the best predictor for central lymph node metastasis with sensitivity, specificity, and AUC of 0.784, 83.0%, and 73.9%, respectively. Combining US characteristics increased the specificity to 100.0%.[18] A recent study by Sudhir et al. on 126 nodes used a higher SWV cutoff of 2.45m/s that predicted malignancy with sensitivity of 83.6%, specificity of 91%, and accuracy of 93.7%.[19]
Our investigation incorporated a multitude of US and CD parameters as well as both VTI and VTQ components of ARFI for lymph node assessment. We found that concurrent use of US parameters and CD improved the sensitivity to 96% and specificity to 69% while combining VTI and VTQ increased the sensitivity to 99.9% and specificity to 99.7%. Three parameters, LS ratio on US, hilar vascularity, and brightness pattern, when used together, had a sensitivity of 99% in differentiating benign from malignant lymph nodal pathology. This was also the least time-consuming method which did not require repeat imaging values (as occurs in mean VTQ) or any form of mathematical calculation (as required for AR in VTI or SWVR in VTQ).
An in-depth analysis revealed that though ARFI parameters could reliably differentiate between benign and malignant lymph nodes, the same statement does not hold true in differentiating simple reactive nodal hyperplasia from other benign yet sinister pathologies.
Limitations
ARFI presented challenges in evaluating small sized nodes (<8 mm) as well as very superficial lymphadenopathy. In addition, VTQ values of X.XX m/s were assigned stiffness values based on their appearance on grey scale and VTI as the real stiffness could not be determined.
The high prevalence of tuberculosis in India was reflected in the patient population visiting our set-up.[20] In this study, tuberculous lymph nodes were found to have US features similar to malignant nodes while their ARFI parameters overlapped with benign reactive nodes, lowering the mean nodal stiffness of benign nodes. The number of malignant lymph nodes in our study was not large enough to define conclusively the role of ARFI in differentiating between lymphoma and metastatic nodes.
CONCLUSION
Complementing US with ARFI in peripheral lymph nodes increases the specificity of predicting malignancy as compared to a US alone from 69% to 99.7%. We therefore recommend using ARFI along with US in the evaluation of all peripheral enlarged lymphadenopathy in differentiating benign from malignant etiology.
Recommendations
We propose the combination of LS ratio, vascularity on CD and brightness pattern on VTI as a standard US protocol in differentiating benign from malignant lymph nodes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Sonography of soft tissue masses of the neck. J Clin Ultrasound. 2002;30:356-73.
- [CrossRef] [PubMed] [Google Scholar]
- Sonography of the neck: Current potentials and limitations. Ultraschall Med Stuttg Ger 1980. 2005;26:185-96.
- [CrossRef] [PubMed] [Google Scholar]
- Sonographic appearance of normal lymph nodes. J Ultrasound Med. 1985;4:417-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sonography of neck lymph nodes. Part II: abnormal lymph nodes. Clin Radiol. 2003;58:359-66.
- [CrossRef] [Google Scholar]
- Cervical lymph node metastases: Diagnosis at sonoelastography initial experience. Radiology. 2007;243:258-67.
- [CrossRef] [PubMed] [Google Scholar]
- Multivariate feature analysis of sonographic findings of metastatic cervical lymph nodes: Contribution of blood flow features revealed by power Doppler sonography for predicting metastasis. AJNR Am J Neuroradiol. 2000;21:561-7.
- [Google Scholar]
- Accuracy of sonographic elastography in the differential diagnosis of enlarged cervical lymph nodes: Comparison with conventional B-mode sonography. Am J Roentgenol. 2008;191:604-10.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound elastography: Its potential role in assessment of cervical lymphadenopathy. Acad Radiol. 2010;17:849-55.
- [CrossRef] [PubMed] [Google Scholar]
- Value of ultrasound elastography in assessment of enlarged cervical lymph nodes. Asian Pac J Cancer Prev. 2012;13:2081-5.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound elastography in the head and neck. Part II. Accuracy for malignancy. Cancer Imaging. 2013;13:260-76.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound elastography: Review of techniques and clinical applications. Theranostics. 2017;7:1303-29.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound elastography: Principles and applications. Diagnostic Radiology: Recent Advances and Applied Physics in Diagnostic Imaging. New Delhi: JP Medical Ltd.;
- [CrossRef] [Google Scholar]
- Acoustic radiation force-based elasticity imaging methods. Interface Focus. 2011;1:553-64.
- [CrossRef] [PubMed] [Google Scholar]
- New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850-60.
- [CrossRef] [PubMed] [Google Scholar]
- Acoustic radiation force impulse imaging for reactive and malignant/ metastatic cervical lymph nodes. Ultrasound Med Biol. 2013;39:1178-83.
- [CrossRef] [PubMed] [Google Scholar]
- Initial experience of acoustic radiation force impulse ultrasound imaging of cervical lymph nodes. Eur J Radiol. 2013;82:1788-92.
- [CrossRef] [PubMed] [Google Scholar]
- Differentiation of metastatic cervical lymph nodes with ultrasound elastography by virtual touch tissue imaging: Preliminary study. J Ultrasound Med. 2015;34:37-42.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction of cervical lymph node metastasis in patients with papillary thyroid cancer using combined conventional ultrasound, strain elastography, and acoustic radiation force impulse (ARFI) elastography. Eur Radiol. 2016;26:2611-22.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of acoustic radiation force impulse elastography in differentiation of benign and malignant lymph nodes. Asian J Oncol. 2017;3:106.
- [CrossRef] [Google Scholar]
- 2025 too short time to eliminate tuberculosis from India. Lung India. 2017;34:409-10.
- [CrossRef] [PubMed] [Google Scholar]






