Translate this page into:
Early Outcomes with Single-antenna High-powered Percutaneous Microwave Ablation for Primary and Secondary Hepatic Malignancies: Safety, Effectiveness, and Predictors of Ablative Failure

*Corresponding author: Michael J. Nisiewicz, Departments of Radiology, University of Kentucky, Lexington, Kentucky. mjnisiewicz@uky.edu
-
Received: ,
Accepted: ,
How to cite this article: Kapoor H, Nisiewicz MJ, Jayavarapu R, Gedaly R, Raissi D. Early outcomes with single-antenna high-powered percutaneous microwave ablation for primary and secondary hepatic malignancies: Safety, effectiveness, and predictors of ablative failure. J Clin Imaging Sci 2020;10:10.
Abstract
Objective:
Microwave ablation (MWA) of liver malignancies has gained much traction over the past 5 years. However, MWA carries relatively higher rates of residual disease compared to resection. Likelihood of MWA success is multifactorial and newer devices with more reliable ablation zones are being developed to overcome these drawbacks. This manuscript is a review of our first 100 liver ablations with the newer single antenna high powered MWA system.
Materials and Methods:
Retrospective chart review of patients that underwent MWA for either primary or secondary hepatic malignancies between March 2015 and July 2016 was conducted. The complete ablation rates, rate of new lesions, complications, and short-term survival were analyzed. Multiple statistical tests, including multivariate regression, were used to assess risk factors for local residual and recurrent disease.
Results:
Fifty-three patients (median age 61 ± 9 years, 39 males) underwent 100 MWAs. Of the 100 lesions ablated, 76 were hepatocellular cancers (HCCs) and 24 were metastases. Median lesion size was 16 ± 9 mm. Seventy- five of these patients had multifocal disease targeted in the same session. Seventy patients had cirrhosis (median model for end-stage liver disease score 9 ± 3; Child-Pugh B and C in 42%). An 83% complete lesion ablation rate was seen on follow-up imaging with liver protocol magnetic resonance imaging/computed tomography (median follow-up of 1 year). The minor complication rate was 9.4% with no major complications or 30-day mortality. Despite this, evidence of new foci of hepatic disease was found in 47% of patients, the majority (80%) of which were in HCC patients (P < 0.01) and most of these new lesions were in a different hepatic segment (64%). Degree of cirrhosis (P < 0.01), presence of non-alcoholic steatohepatitis (NASH) (P = 0.01) and lesion’s subcapsular location (P = 0.03) was significant predictors of residual disease. With the subset analysis of only HCC lesions larger than 1 cm, only the presence of NASH remained significant.
Conclusion:
The single probe high power MWA of malignant hepatic lesions is safe and effective with minimal morbidity. Degree of cirrhosis, NASH, and subcapsular location was associated with an increased rate of residual disease on short-term follow-up.
Keywords
Microwave ablation
Safety
Liver
Cancer
Emprint
INTRODUCTION
Percutaneous ablation techniques have been increasingly recognized for their curative success in patients with hepatocellular cancer (HCC). Surgical management in the form of liver transplantation or resection remains the best treatment option for these patients. However, only a small percentage of patients is surgical candidates, either due to HCC burden at presentation or due to cirrhosis-related morbidity.[1,2]
Percutaneous ablation has been shown to perform at par with surgery in prolonging survival, especially for lesions smaller than 2 cm, making it a first-line option for early HCC in the Barcelona Clinic Liver Cancer classification.[3,4] For larger tumors, percutaneous ablation has been known to carry a greater risk of residual disease, especially at the outer ablation rim. The recent development of newer high-powered single antenna microwave ablation (MWA) systems with Thermosphere™ technology (which promises quicker, more precise, and predictably spherical ablation zones of larger sizes compared to prior MWA technologies) holds great promise in overcoming this drawback.[5] Although thermal ablation with microwave has been theoretically shown to confer benefits such as overcoming problems of tissue charring and vascular heat sink,[6,7] no definitive evidence supports the superiority of microwave over radiofrequency ablation (RFA).[8,9] Importantly, the abovementioned practical and theoretical advantages are driving greater adoption of MWA over RFA, not only for HCC but also for liver metastases.[10,11] The interventional radiology staff at our institution unanimously switched from RFA to MWA in December 2014.
The availability of data on patient outcomes after ablation with these newer MWA devices (first FDA approved in 2014) is currently limited. Hence, we retrospectively studied our pilot experience with outcomes of the first hundred percutaneous ablations using a high-powered single antenna MWA system with Thermosphere™ technology (Emprint™ system, Covidien, Boulder, CO, USA)[5] in a mixed set of tumors (HCC, cholangiocarcinoma, and metastases) to evaluate its safety and oncologic effectiveness. Although constrained by a small sample size, we also conducted a multivariate analysis for factors that predicated residual disease (or ablative failure).
In addition, recent research has uncovered another critical obstacle against the use of percutaneous ablation – carcinogenic potentiation of the cirrhotic liver with the accelerated appearance of new lesions after ablation of the index lesion.[8,12-16] While this possibility has been reported, only few clinical studies have methodically evaluated and presented its actual incidence. Hence, as a secondary endpoint, we also evaluated the incidence of new liver lesions, close to (same-segment) or distant from (different- segment) the index lesion.
MATERIALS AND METHODS
After institutional review board approval, a prospectively maintained database of patients undergoing MWA for primary and secondary liver malignancies was queried retrospectively from March 2015 to July 2016. The endpoints of our study and parameters studied are indicated in Table 1. Ablations were performed by two interventional radiologists (3 years and 5 years of MWA experience), while the pre- and post-ablation studies were read by fellowship-trained abdominal radiologists. We excluded the first five ablations for each operator from the study, to eliminate any assumed selection bias or effect of the learning curve.[9]
| Primary endpoints – safety and effectiveness Complete ablation rate at 1 month (defined as no residual disease at 1 month) Procedurerelated mortality and major complications (major complications defined as either symptomatic hemoperitoneumor portobiliary complications) |
| Secondary endpoints – new lesions, risk factors, and minor complications Rate of new lesions (defined as resurgence of disease in a different hepatic segment or in the same segment, at least after 3 months of treatment) Risk factors for residual disease |
| Parameters studied: Patientrelated factors Age, sex, body mass index >30, comorbidities (insulindependent diabetes) |
| Parameters studied: Underlying liver disease Alcohol, nonalcoholic steatohepatitis, hepatitis B, hepatitis C, primary biliary cirrhosis Cirrhosis grading – morphologic (semiquantitative computed tomography/magnetic resonance imaging based), biochemical (ChildPugh, Model for EndStage Liver Disease) |
| Parameters studied: Target lesion related factors Size and location including proximity (1 cm) to capsule, diaphragm, heart, gallbladder and inferior vena cava, (5 mm) and vessels >3 mm Multiplicity of tumors |
| Parameters studied: Techniquerelated factors Cumulative energy applied (energy applied×time of application), total IR suite time |
Inclusion criteria
Since our primary endpoint was to evaluative ablative effectiveness based on size, we included all patients who underwent MWA in the study period (differing tumor etiologies, underlying liver disease, lesion sizes, and locations). All patients were referred and discussed at our dedicated liver tumor board for treatment eligibility assessment. We included patients who had prior locoregional interventions only if the target lesion was not located within the same segment as the prior treated lesion. Each tumor was treated as a separate data point and all imaging characteristics and treatment data were collected on a per lesion basis. For HCC, only Liver Imaging Reporting and Data System 4/5 lesions were targeted with the threshold size being <5 cm and a maximum of 4 nodules per session. For metastatic lesions and cholangiocarcinomas, the same size threshold was used.
Pre-procedural planning and laboratory data
All lesions were assessed using liver protocol dynamic contrast- enhanced cross-sectional imaging magnetic resonance imaging or computed tomography (MRI or CT) within 2 weeks before the ablation procedure. While most patients did not have liver elastography or biopsy, a semiquantitative image-based grading of cirrhosis [Figure 1] was carried out in addition to the biochemical scoring of baseline liver disease (Child-Pugh and Model for End-Stage Liver Disease [MELD] scores). The largest anterior-posterior splenic width measurement on axial images was used for “splenic size,” and a threshold of 10.5 cm was used to define splenomegaly. Our morphometric grading system was developed by adapting and combining multiple existing criteria previously published in the literature.[17-19] A 1 cm threshold was used to define proximity to larger vessels inferior vena cava or morphologic boundaries (capsule, diaphragm, pericardium, and gallbladder). A 5 mm threshold was used to define a lesion as perivascular (only for vessels >3 mm in diameter).
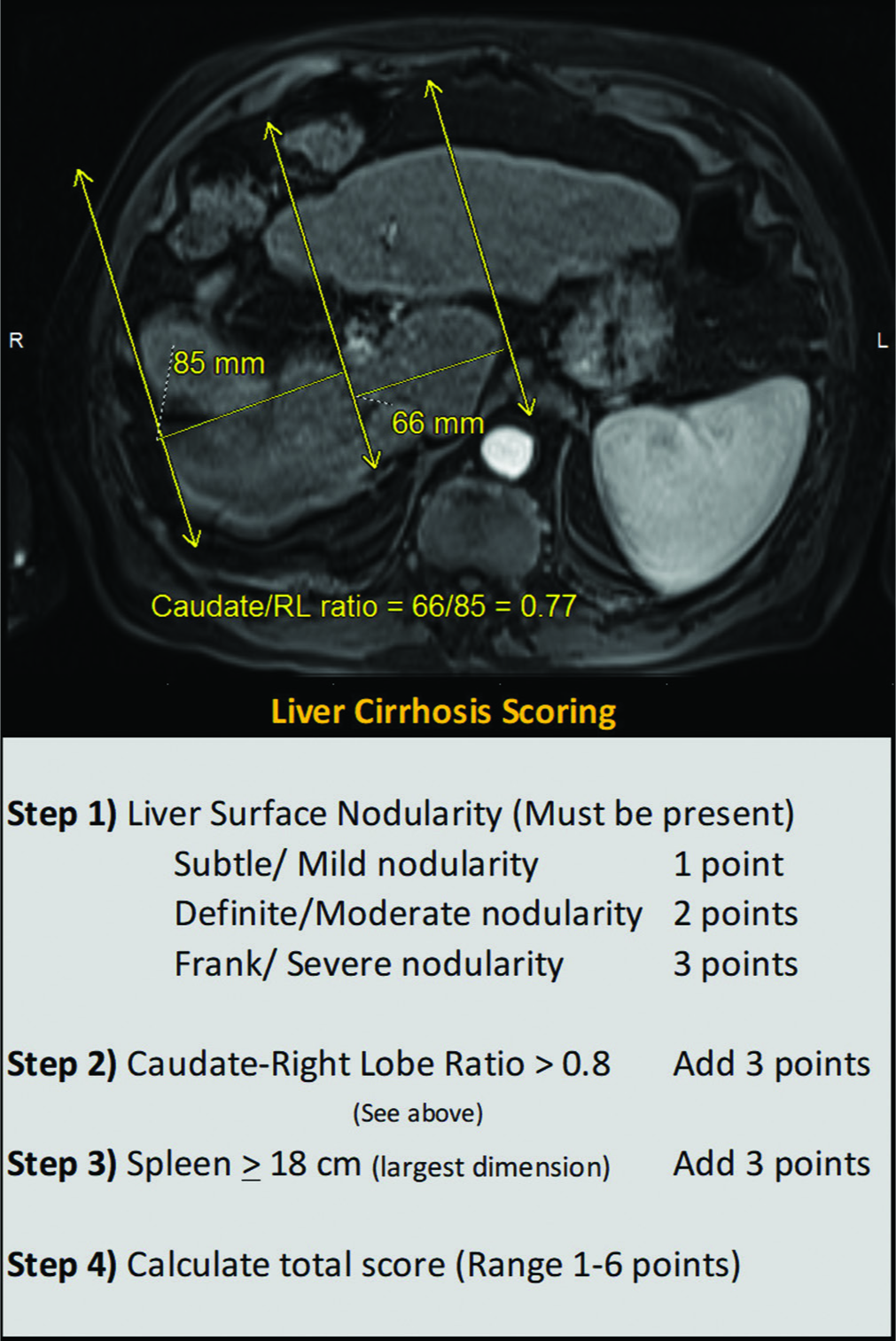
- Imaging grading for cirrhosis: T2-weighted magnetic resonance imaging through the liver demonstrating steps in the calculation of caudate to right lobe ratio is shown above and the semiquantitative morphologic cirrhosis grading used is tabulated below.
MWA technique
All procedures were performed under general anesthesia and with the use of single-dose prophylactic and post-procedure antibiotics for 5 days. CT guidance was utilized (Somatom Aera, Siemens, Erlangen, Germany). Patient positioning, use of intraprocedural contrast enhancement, and use of hydro- dissection were case based. For lesions not visible during the procedure (without contrast), lesion targeting was performed using anatomic landmarks on prior contrast-enhanced imaging. Ultrasound was not used. The Emprint probe is a 13-gauge fiberglass device that comes in 15 cm length which was used for 80% of the lesions while the rest required the use of the longer 20 cm long probe. A pre-coagulation burn with 45 W × 1 min was carried out for subcapsular lesions. An ablation margin of 1 cm was taken into account using ablation curves provided by the manufacturer for 100 W and less frequently, 75 W (choice of power was based on physician preference). The probe was retracted while ablating the tract using 45 W × 20 s every 1 cm until the liver capsule was reached. Here, reduced power and longer durations of ablation were applied to reduce the rate of gas and vapor production, thereby minimizing the likelihood of capsular burst.
Follow-up imaging
All patients had cross-sectional imaging (contrast-enhanced CT or MRI) follow-up assessment at 4–6 weeks which were evaluated using modified response evaluation criteria in solid tumors (m-RECIST) criteria. The majority of the patients had up to a year-long imaging follow-up. Patients who did not have a 1-month follow-up or had prior treatment to the same lesion/segment were excluded from the analysis.
Statistical analysis
Statistical analysis was carried out using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). Chi-square tests and t-tests were utilized to obtain basic descriptive and inferential statistics. For smaller sample sizes and skewed data, nonparametric tests were utilized. Clinically relevant variables with biologically plausible associations as well as variables that were associated with P < 0.30 on univariate analysis were introduced into a forward stepwise multivariate Cox proportional hazards model to assess for factors predicting ablative failure, i.e. presence of residual disease.
RESULTS
Demographic parameters (per-patient)
Our inclusion criteria identified 53 patients who underwent 100 MWAs. The median age of these patients was 61 ± 9 years, with the majority being male 39 (71%). Forty-one percent of these patients were obese and 18% had insulin-dependent diabetes mellitus. Patients were followed for a median of 12 months (ranging from 3 months to 2 years). Eleven patients (21%) were lost to follow-up after their first post- procedural visit at 4–6 weeks.
Tumor characteristics and imaging modality (per-lesion)
Out of the 100 lesions ablated, 76 were HCCs and 24 were metastases (colorectal metastasis – 18, neuroendocrine metastasis – 5, cholangiocarcinoma – 3, gastrointestinal stromal tumor – 1, and prostate cancer metastasis – 1). The median lesion size was 16 mm (range: 0.6–4.8 cm). The majority (70%) of the lesions were 1–3 cm in size. Seventeen tumors were sub-centimeter, while 13 tumors exceeded 3 cm. Two-thirds of the tumors (76%) were in the right lobe of the liver and a significant proportion were in the subdiaphragmatic and pericardial regions [Figure 2].
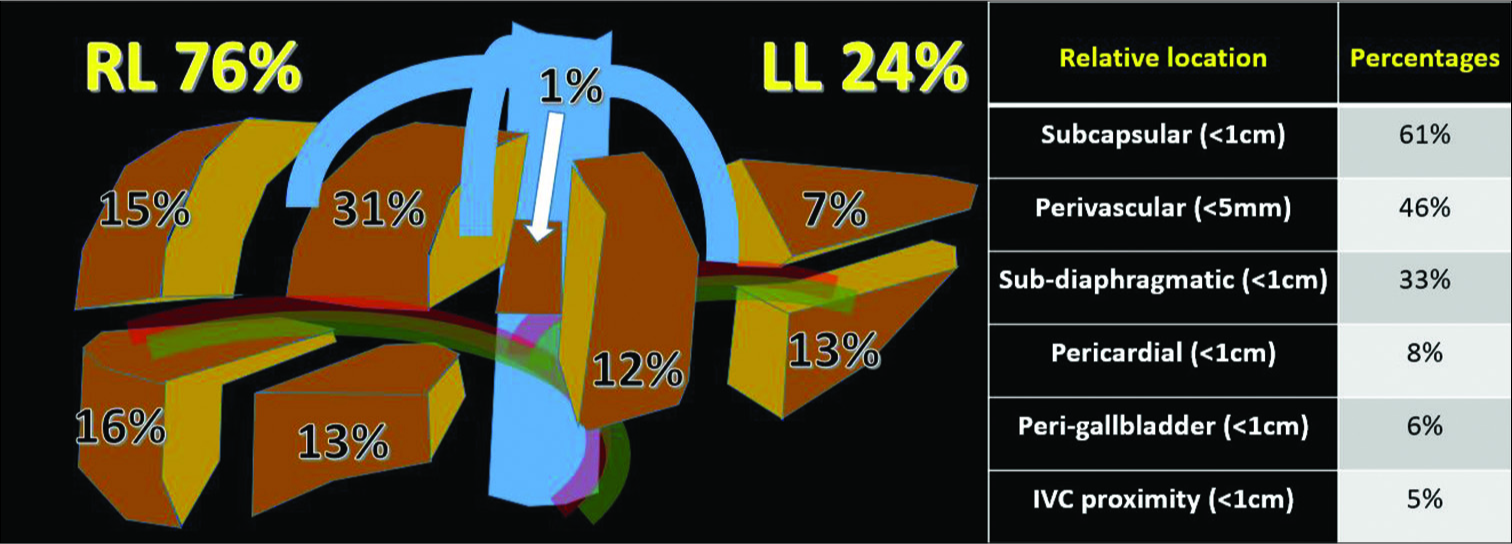
- Distribution of lesion location within the liver and in relation to vessels and anatomic structures (n = 100).
All patients had a pre-procedural liver protocol cross- sectional study (MRI: 77%, CT: 23%). The choice of imaging modality for follow-up was kept the same as that used for pre-procedural imaging.
Underlying liver disease, prior treatment, and multifocality (per-lesion)
Most HCC tumors were from livers with a prior diagnosis of cirrhosis (excluding four tumors that were in the setting of hepatitis B without cirrhosis). Percentage distribution of lesions among different background liver conditions was: Non-alcoholic steatohepatitis (NASH) (33), hepatitis C (29), alcoholic liver disease (22), hepatitis B (4), and primary biliary cirrhosis (1). The majority of the tumors occurred in patients with Child-Pugh A (58%) score, with 41% in Child-Pugh B, and 1% in Child-Pugh C. Median MELD score was 9 ± 3. A median morphological score of 2 ± 0.7 was found using the above described radio-biochemical scoring scale.
One-third of tumors (33%) targeted had some form of prior hepatic treatment (ablation, transarterial chemoembolization [TACE] or radioembolization, or resection) to a different hepatic segment from the index lesion under consideration.
Technique characteristics (per-lesion and per-patient)
The median cumulative energy used per tumor was 687 ± 210 min. The median total interventional suite time was 26 ± 15 min. Hydro-dissection was utilized in seven cases with close proximity to the bowel.
Complications and mortality (per-patient)
There were no 30-day mortality or major complications on 12-month median follow-up. The median progression-free survival was 4.3 months [Figure 3], and the median overall survival was 24.7 months [Figure 4]. A minor complication rate of 9.4% (5/53 patients) was observed. This included two patients requiring chest tubes for pneumothorax after high dome lesions were ablated. These patients remained admitted for observation until the next day. Three patients had partial portal vein thrombosis in the perilesional portal vein on follow-up imaging.
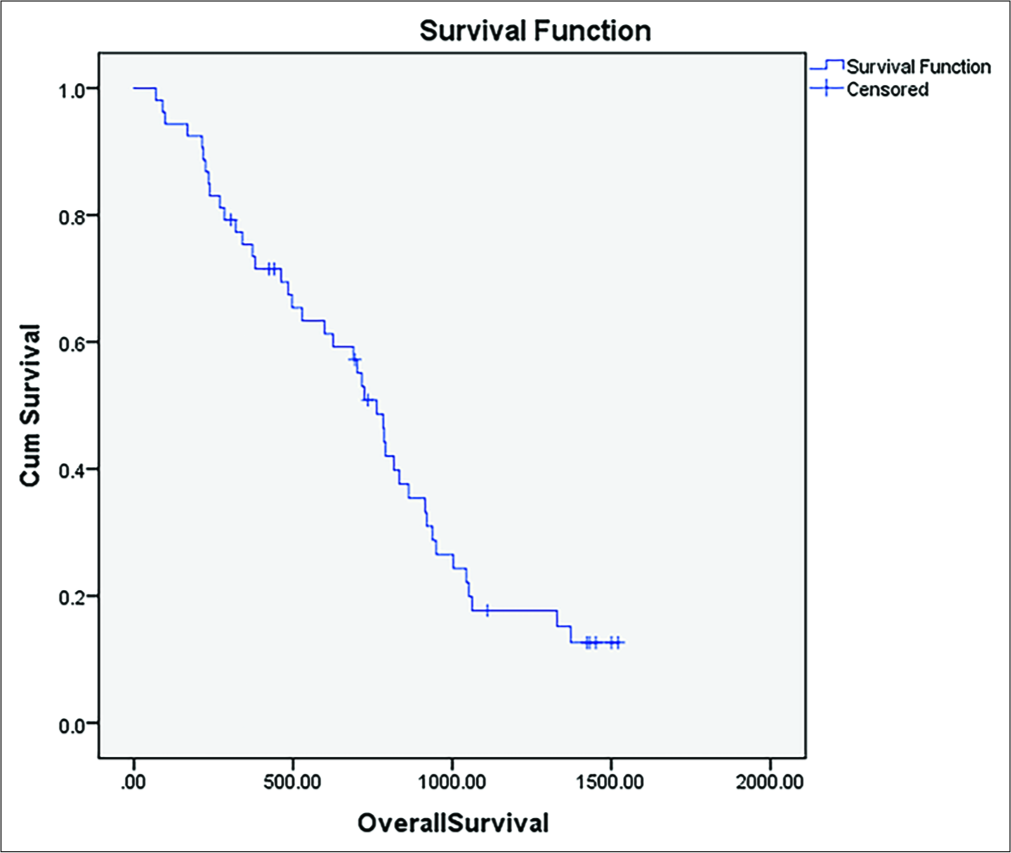
- Kaplan–Meier curve of progression-free survival following ablation.
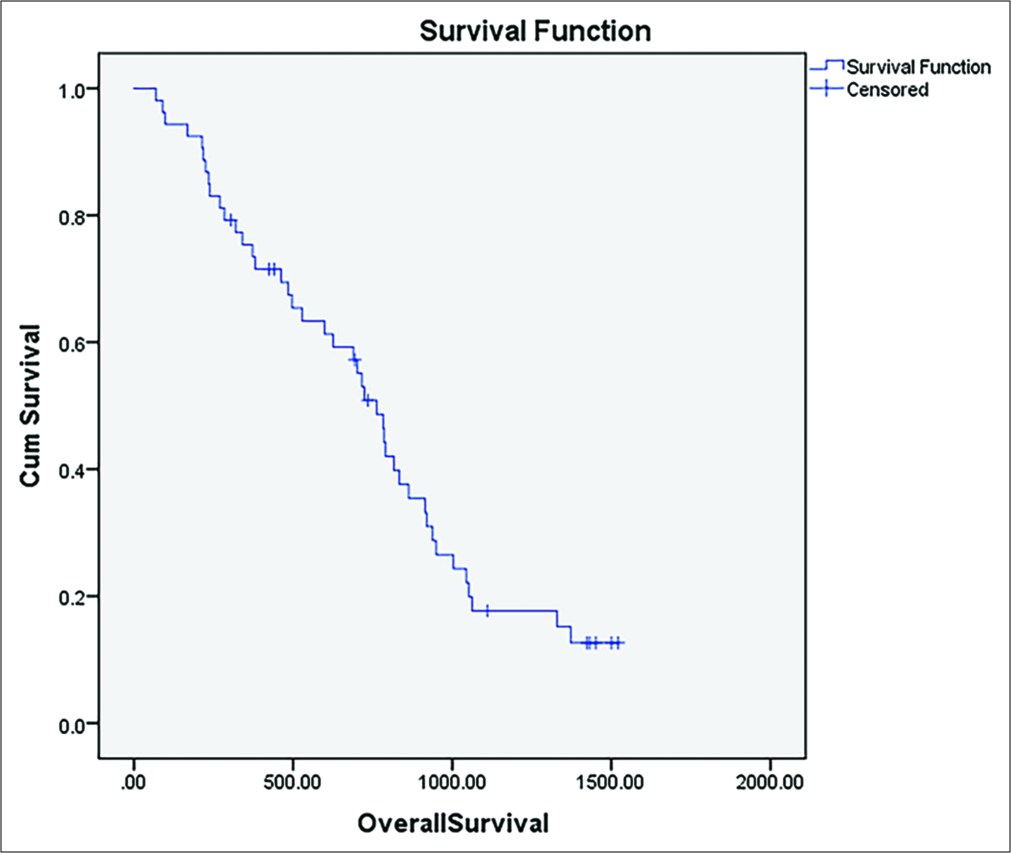
- Kaplan–Meier curve of overall survival following ablation.
Incidence of complete ablation (no residual disease)
A complete ablation rate of 83% was achieved with only 17 of the 100 lesions showing residual disease (at the margin of the index tumor) using m-RECIST criteria at the first follow-up imaging.
Incidence of new lesions
Nearly half of the patients (25/53; 47%) were found to have new foci of disease: Different-segment (16/25; 64%) and same-segment (9/25; 36%). The majority of new lesions occurred in HCC patients (80% of new lesions).
Re-interventions
Thirty-nine percent of the patients (21/53) had re- interventions. The majority (16/53; 31%) had repeat ablation of marginal residual disease or recurrence. One-third (7) of these patients had multiple repeat ablations. Eight percent of these patients were switched to transarterial therapy, either in the form of drug-eluting bead TACE) or yttrium-90 transarterial radioembolization (TARE).
Predictors of ablative failure
Factors that were included in the stepwise regression were: Age, body mass index >30, presence of cirrhosis, cirrhosis grade, etiology of cirrhosis, tumor etiology, hepatic lobar location, lesion size, prior TACE, proximity to major vessels, lesion multiplicity, and subcapsular location.
The following factors were significant predictors of ablative failure: Higher degree of morphologic cirrhosis based on our gradation (P < 0.01), NASH cirrhosis (P = 0.01), and subcapsular location of the lesion (P = 0.03).
No significant differences were found based on other lesional (lesion type, size, or multiplicity) and perilesional factors (proximity to vessels or anatomical structures).
Subgroup analysis for HCC patients with lesion sizes >1 cm
Lesions <1 cm are difficult to visualize and prone to mistargeting. To overcome this problem and to neutralize the effect of lesion type, we analyzed the subgroup of HCC lesions >1 cm in size. Sixty-four lesions from 35 patients (median age 62 years; 74% of males) had HCC lesions that were >1 cm in size. Median lesion size was 19.6 mm and 72% (46/64) were in the right lobe. A complete ablation rate of 86% (55/64) was observed in HCC patients. Forty-eight percent (17/35) of these patients had recurrent lesions and the majority 76% (13/17) of these were in a different hepatic segment. Mean time-to-recurrence for the same segment new lesions was 1.6 months while that for different segment new lesions was 2.9 months. A multiple regression analysis was carried out in this subgroup with similar thresholds for variable inclusion. As a large majority of these patients had cirrhosis, cirrhosis gradation did not hold significance. Only the presence of NASH (P = 0.03) remained statistically significant.
DISCUSSION
High-powered (2.4 GHz) MWA devices were FDA approved in 2010. The first single probe device was approved in 2014.[5,20] This small sample study demonstrates the safety and effectiveness of ablation using high-powered MWA systems with Thermosphere™ technology for liver lesions of various etiologies and sizes with a low risk of residual disease and complication rate.
At the time of the submission of this study, we are aware of one prior published study and one abstract reporting on the outcomes of percutaneous microwave liver ablation using the high-powered MWA system with Thermosphere™ technology. Imajo et al. (Japan)[20] evaluated outcomes after ablation of 21 solitary liver metastatic lesions >3 cm in size. Their rate of residual disease was 5% with no intrahepatic recurrence, but with a limited 3-month follow-up.
Pua et al. (Singapore; n = 119)[21] observed excellent overall outcomes (99% technical success; 6% residual rate; and 3% recurrence rate), but their study population included mostly solitary liver lesions and they had a higher minor complication rate with greater number of pneumothoraces and subcapsular hematomas. Their study could not be thoroughly compared with, as only an abstract was available.
Limited data are available comparing the newer 2.4 GHz and older 915 MHz MWA systems. Although theoretically, the longer wavelengths of the 915 MHz system may have better tissue penetration, the 2.4 GHz system has multiple practical advantages, reaching higher intratumoral temperatures in shorter times and more predictable time-dependent spherical ablation zones.
We found the degree of cirrhosis (P < 0.01), presence of NASH (P = 0.01), and subcapsular location of lesions (P = 0.03) to be significant predictors of residual disease after MWA. However, the degree of cirrhosis and subcapsular location was no longer significantly predictive of ablation failure when the subset of only HCC lesions was re-analyzed. These results should however be interpreted with caution, given relatively small number of target events and limitations of stepwise covariate modeling designed to avoid overfitting.
The association between the degree of cirrhosis and presence of NASH and high residual disease could be related to different tissue characteristics having differing susceptibility to ablation and tissue contraction.[22,23] This is supported by prior studies showing substantial differences in cavity sizes in patients with different degrees of cirrhosis or fatty infiltration.[21] Conventionally, subcapsular tumors were less commonly treated with thermal ablation due to technical difficulties with probe placement, fear of intraperitoneal tumor spill or hemorrhage and ready accessibility for surface wedge resection. A considerable proportion of tumors (61%) in this study were subcapsular in location. Multiple other studies were done with RFA support a higher risk of recurrence with subcapsular lesions.[24,25]
Interestingly, lesion factors such as tumor type and size as well as proximity to vessels or anatomical structures did not significantly influence ablative success in our study. This is supported by other prior studies with lower power or multi- antenna MWA systems.[16,26] This echoes the findings of multiple studies showing greater reliability of MWA systems due to a reduced heat sink effect.[6,27,28] However, contrary to our study, Zhang et al. (n = 60)[29] showed poorer outcomes with greater residual disease in lesions in proximity to “risk areas” (large bile ducts, blood vessels, etc.) using a multi- antenna 2.4 GHz MWA system.
Our recurrence rate of new disease foci within the first few months is a concerning finding. However, we believe this is most likely due to the relatively aggressive disease profile of our cases (with two-thirds of the patients having multifocal disease) and the inherent field-carcinogenesis attributable to underlying cirrhosis. However, it would be irrational to dismiss the alternative possibility of iatrogenic carcinogenic potentiation after thermal ablation. Similar phenomenon of accelerated recurrence has also been cited in the RFA and surgical literature and has been suggested to be linked to treatment-related release of inflammatory cytokines.[14,30-34] Although we predominantly used the superior contrast resolution of liver protocol MRI for both pre- and post-procedural imaging, these lesions may still represent micrometastatic disease that may have been too small to detect on pre-procedural imaging. A review of literature on rates of intrahepatic recurrence of HCC at intermediate-term follow-up revealed significant variation among reports, either post-ablation (5–42%)[8,12,13,16,35,36] or post-resection (30–50%).[37] A recent large sample study by Jung et al. (n = 628 recurrences) is an example of well- orchestrated research into characterizing and predicting HCC recurrence after curative resection. A recurrence rate of 27% was seen with solitary HCCs that were pathologically confirmed. Our recurrence rate was nearly twice this, but this can be reasonably explained by our very high rates of perivascular location (50%) and pre-treatment multifocal disease (75%). Larger prospective studies are needed to reliably decipher any association between thermal ablation and increased rates of de novo tumor recurrence. This finding highlights the need for careful patient selection and vigilant post-procedural follow-up at this time until such an association is confirmed. Furthermore, it highlights a possible future role for concomitant systemic therapies such as immunotherapy, which is currently being investigated in multiple clinical trials.[38]
Our study has several limitations. It is a retrospective, single- center analysis. We had liberal inclusion criteria with a heterogeneous group of patients and diseases. Considering pre-procedural parameters, we used data on cirrhosis from patient charts as well as qualitatively scored cirrhosis with a non-validated morphologic score. Pre-procedural and post-procedural alpha-fetoprotein measurements were not recorded since we included both primary and secondary liver malignancies. For most patients, liver elastography data or biopsy data were not available.
CONCLUSION
Single probe high-power MWA was found to be safe and effective in this pilot cohort. The higher degree of underlying cirrhosis and NASH etiology as well as subcapsular location of lesions was predictors of ablative failure at short-term follow-up. A significant rate of new lesions was observed after the ablation of HCC tumors which may or may not be linked to MWA itself. Although a causal relationship cannot be deduced from our study, this finding warrants close follow- up of these patients and need for further investigation into the possibility of iatrogenic tumor potentiation after thermal ablation and into whether concomitant systemic therapies such as immunotherapy may play a future role.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Early hepatocellular carcinoma on the procrustean bed of ablation, resection, and transplantation. Semin Liver Dis. 2014;34:415-26.
- [CrossRef] [PubMed] [Google Scholar]
- Squaring the circle of selection and allocation in liver transplantation for HCC: An adaptive approach. Hepatology. 2016;63:1707-17.
- [CrossRef] [PubMed] [Google Scholar]
- Loco-regional treatment of HCC: Current status. Clin Radiol. 2017;72:626-35.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835-53.
- [CrossRef] [PubMed] [Google Scholar]
- The emprint™ ablation system with thermosphere™ technology: One of the newer next-generation microwave ablation technologies. Semin Intervent Radiol. 2015;32:335-8.
- [CrossRef] [PubMed] [Google Scholar]
- Microwave liver ablation: Influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008;19:1087-92.
- [CrossRef] [PubMed] [Google Scholar]
- Microwave ablation: Principles and applications. Radiographics. 2005;25(Suppl 1):S69-83.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcomes following microwave ablation for liver malignancies. Br J Surg. 2015;102:85-91.
- [CrossRef] [PubMed] [Google Scholar]
- The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45:626-9.
- [CrossRef] [PubMed] [Google Scholar]
- Colorectal liver metastasis: Overview of treatment paradigm highlighting the role of ablation. AJR Am J Roentgenol. 2018;210:883-90.
- [CrossRef] [PubMed] [Google Scholar]
- Thermal ablation of colorectal liver metastases: A position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur Radiol. 2015;25:3438-54.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of microwave ablation for primary and secondary liver malignancies: A systematic review. Eur J Gastroenterol Hepatol. 2009;21:599-605.
- [CrossRef] [PubMed] [Google Scholar]
- Local recurrence after microwave thermosphere ablation of malignant liver tumors: Results of a surgical series. Surgery. 2018;163:709-13.
- [CrossRef] [PubMed] [Google Scholar]
- Accelerated perinecrotic outgrowth of colorectal liver metastases following radiofrequency ablation is a hypoxia-driven phenomenon. Ann Surg. 2009;249:814-23.
- [CrossRef] [PubMed] [Google Scholar]
- CD95 is a key mediator of invasion and accelerated outgrowth of mouse colorectal liver metastases following radiofrequency ablation. J Hepatol. 2010;53:1069-77.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrence after microwave ablation of liver malignancies: A single institution experience. HPB (Oxford). 2013;15:365-71.
- [CrossRef] [PubMed] [Google Scholar]
- Feasibility and reproducibility of liver surface nodularity quantification for the assessment of liver cirrhosis using CT and MRI. Eur J Radiol Open. 2017;4:95-100.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of splenic perfusion and spleen size using dynamic computed tomography: Usefulness in assessing degree of liver fibrosis. Hepatol Res. 2018;48:87-93.
- [CrossRef] [PubMed] [Google Scholar]
- Morphometric changes in liver cirrhosis: Aetiological differences correlated with progression. Br J Radiol. 2016;89:20150896.
- [CrossRef] [PubMed] [Google Scholar]
- New microwave ablation system for unresectable liver tumors that forms large, spherical ablation zones. J Gastroenterol Hepatol. 2018;33:2007-14.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous emprint microwave ablation of malignant liver tumors: A report of the first 100 cases. J Vasc Interv Radiol. 2018;29:S79.
- [CrossRef] [Google Scholar]
- Microwave ablation of primary and secondary liver tumours: Ex vivo, in vivo, and clinical characterisation. Int J Hyperthermia. 2017;33:34-42.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatic microwave ablation zone size: Correlation with total energy, net energy, and manufacturer-provided chart predictions. J Vasc Interv Radiol. 2016;27:1389-96.
- [CrossRef] [PubMed] [Google Scholar]
- Radiofrequency ablation of hepatocellular carcinoma: Can subcapsular tumors be safely ablated? AJR Am J Roentgenol. 2008;190:1029-34.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: Assessment of safety and therapeutic efficacy. Korean J Radiol. 2009;10:34-42.
- [CrossRef] [PubMed] [Google Scholar]
- Preliminary outcome of microwave ablation of hepatocellular carcinoma: Breaking the 3-cm barrier? J Vasc Interv Radiol. 2016;27:623-30.
- [CrossRef] [PubMed] [Google Scholar]
- MWA versus RFA for perivascular and peribiliary CRLM: A retrospective patient-and lesion-based analysis of two historical cohorts. Cardiovasc Intervent Radiol. 2016;39:1438-46.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of microwave ablation for hepatocellular carcinoma adjacent to large vessels: A propensity score analysis. Oncotarget. 2017;8:28758-68.
- [CrossRef] [PubMed] [Google Scholar]
- High-powered microwave ablation of larger hepatocellular carcinoma: Evaluation of recurrence rate and factors related to recurrence. Clin Radiol. 2015;70:1237-43.
- [CrossRef] [PubMed] [Google Scholar]
- Accelerated carcinogenesis following liver resection in chronically inflamed livers: A window of opportunity for treatment. Biomed Rep. 2017;6:545-8.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid and aggressive recurrence accompanied by portal tumor thrombus after radiofrequency ablation for hepatocellular carcinoma. Int J Clin Oncol. 2003;8:332-5.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid progression of hepatocellular carcinoma after radiofrequency ablation. World J Gastroenterol. 2004;10:1137-40.
- [CrossRef] [PubMed] [Google Scholar]
- Complications of radiofrequency thermal ablation in hepatocellular carcinoma: What about “explosive” spread? Gut. 2006;55:435-6.
- [CrossRef] [PubMed] [Google Scholar]
- Aggressive Recurrence after Radiofrequency Ablation of Liver Neoplasms. Hepatogastroenterology. 2003;50:2179-2184.
- [Google Scholar]
- Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J Am Coll Surg. 2008;207:20-9.
- [CrossRef] [PubMed] [Google Scholar]
- Aggressive Intrasegmental Recurrence of Hepatocellular Carcinoma after Radiofrequency Ablation: Risk Factors and Clinical Significance. Radiology. 2015;276:274-85.
- [CrossRef] [PubMed] [Google Scholar]
- Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69:1284-93.
- [CrossRef] [PubMed] [Google Scholar]
- Immunotherapy in hepatocellular carcinoma: Is there a light at the end of the tunnel? Cancers (Basel). 2019;11:E1078.
- [CrossRef] [PubMed] [Google Scholar]






