Translate this page into:
Incidental Cystic Lymphangioma of the Small Bowel Mesentery
Address for correspondence: Dr. Brindley David Cupido, Department of Radiology and Diagnostic Imaging, University of Alberta Hospital, 2A2.41 WMC, 8440-112 Street, Edmonton, Alberta - T6G 2B7, Canada. E-mail: brindley.cupido@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Lymphangiomas are benign lesions of mesenchymal origin. Although more commonly encountered in the head and neck, intra-abdominal lymphangiomas are a rare entity that typically present as multiloculated intra-abdominal cystic lesions that are often incidentally discovered on imaging. This case report discusses such a case.
Keywords
Cystic lesions
magnetic resonance imaging
mesenteric lymphangioma

INTRODUCTION
Lymphangiomas are lesions of vascular origin with lymphatic differentiation. Approximately 95% occur in the neck and axilla,[1] while the remaining 5% are found in the chest and abdomen. Lymphangiomas of the abdomen are rare (accounting for 1 per 100,000 hospital admissions),[2] but have been reported in the mesentery, retroperitoneum, gastrointestinal tract and intra-abdominal solid viscera. The clinical presentation of lymphangiomas varies from incidental discovery on imaging to presenting with an acute abdomen. Most, however, remain asymptomatic until they grow to a large size.[3] Mesenteric lymphangiomas, in particular, can result in complications such as intestinal obstruction or volvulus leading to infarction. This case report documents a lymphangioma of the small bowel mesentery, incidentally discovered on pelvic ultrasound and initially thought to represent a complex cystic adnexal mass. The mass was subsequently delineated on further imaging.
CASE REPORT
A 42-year-old female presented with a history of chronic iron deficiency anemia and menorrhagia for several years. An ultrasound of the pelvis was requested to determine the cause for menorrhagia. Ultrasound was technically difficult, but demonstrated multiple fibroids [Figure 1] and a simple cyst within the left ovary. The right ovary was not visualized, but ultrasound demonstrated a complex cystic right “adnexal mass” measuring 9.7 × 5.2 × 6.3 cm that was presumed to originate from the right ovary [Figure 2].
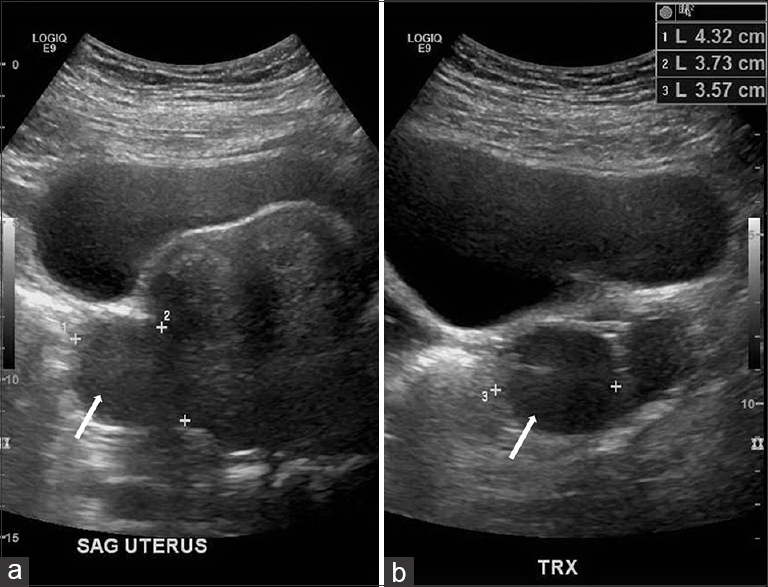
- 42-year-old female with a history of chronic iron deficiency anemia and menorrhagia for several years and later diagnosed with cystic lymphangioma of the small bowel mesentery. Transabdominal ultrasound (a) sagittal and (b) transverse scans demonstrate a bulky uterus with a subserosal fibroid (white arrows) seen in both sagittal and transverse planes.
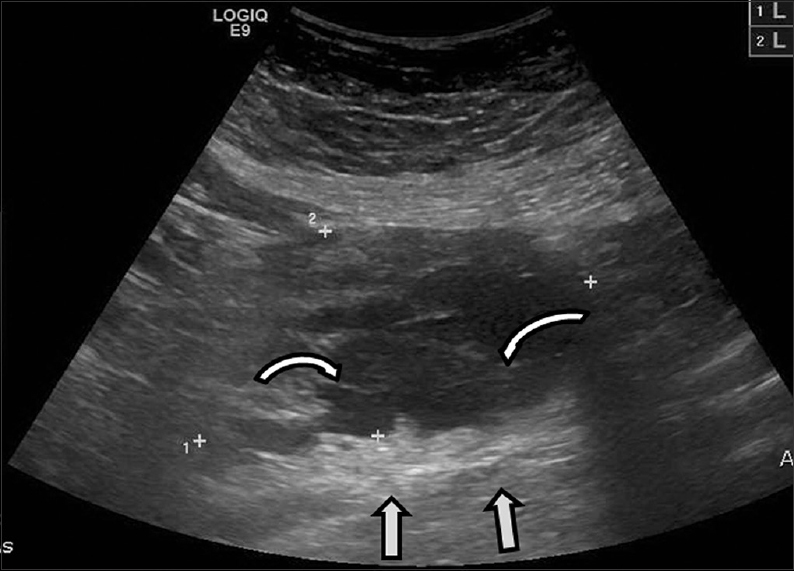
- 42-year-old female with a history of chronic iron deficiency anemia and menorrhagia for several years and later diagnosed with cystic lymphangioma of the small bowel mesentery underwent pelvic ultrasound for investigation of menorrhagia and was found to have a complex cystic right adnexal mass. Transabdominal ultrasound demonstrates a cystic lesion with numerous thin septae (curved arrows) and good through transmission (straight arrows).
An MRI was advised for definitive characterization. This confirmed multiple uterine fibroids, T1 bright foci within the left ovary suggestive of an ovarian endometrioma, as well as a dilated left fallopian tube with T1 high signal consistent with a hematosalpinx. The right “adnexal lesion” was only partially visualized on the pelvic MRI, but was deemed to originate from the abdomen rather than the ovary, which was tethered to the uterus [Figure 3]. A gadolinium-enhanced MRI of the abdomen was requested to delineate the mass.
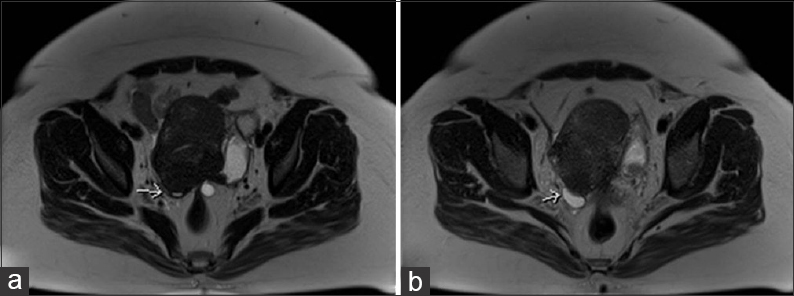
- 42-year-old female with a history of chronic iron deficiency anemia and menorrhagia for several years and later diagnosed with cystic lymphangioma of the small bowel mesentery. Axial T2 images (a and b) demonstrate tethering of right ovary (arrows) to the uterus posteriorly.
A gadolinium-enhanced MRI of the abdomen was performed 2 weeks later. This study demonstrated a cystic mesenteric lesion with high T2 signal and low T1 signal and measuring 17.6 × 6.8 × 8.7 cm. The lesion insinuated around adjacent vessels within the small bowel mesentery and demonstrated multiple thin internal septations [Figure 4a]. No solid components, calcifications, or enhancement were demonstrated. Mass effect was notably absent, and the cisterna chyli and thoracic duct were not enlarged. The lesion was considered to represent a mesenteric lymphangioma.
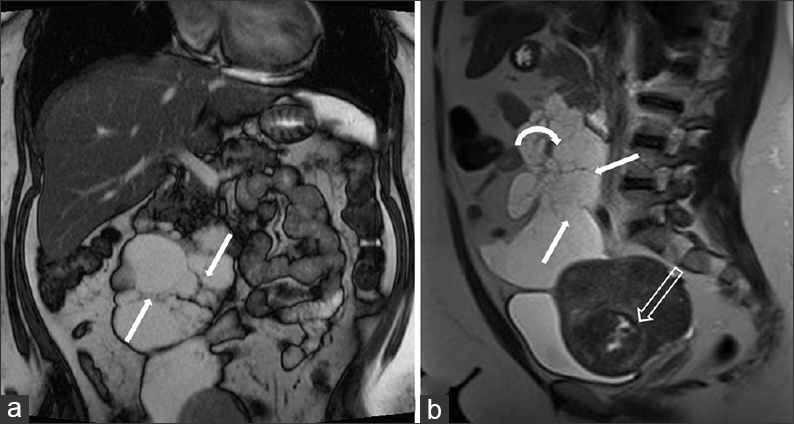
- 42-year-old female with a history of chronic iron deficiency anemia and menorrhagia for several years and later diagnosed with cystic lymphangioma of the small bowel mesentery. (a) Coronal fast imaging with steady-state precession (FISP) demonstrates a multiloculated cystic lesion with numerous septae (straight arrows), lacking mass effect. (b) Sagittal T2 (13 months later) of lesion, shows increase in size of lesion with thin-walled septae (straight arrows) and insinuating around the mesenteric vessels (curved arrow). Note the uterine fibroid with evidence of cystic degeneration (hollow arrow).
A 13-month follow-up MRI examination showed that the lymphangioma had increased in size compared to the initial examination, measuring 17.6 × 8.4 × 11.4 cm and extending from the uncinate process of the pancreas to the uterine fundus [Figure 4b]. The increase in size prompted a surgical referral. Furthermore, the cystic fluid of the lymphangioma demonstrated signal loss on the T1 out-of-phase sequence compared to the T1 in-phase sequence, consistent with microscopic lipid content. The signal intensity on T1 in-phase was 78.4 and on T1 out-of-phase was 56.2, thus denoting a 28.3% signal intensity loss between phases. This finding is compatible with the presence of chylous fluid and is a very suggestive feature of a lymphangioma [Figure 5].
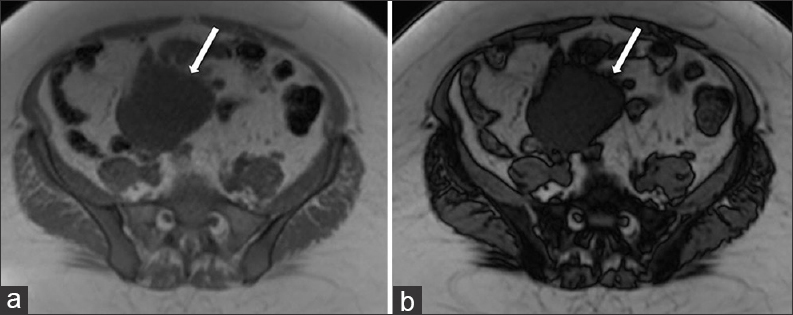
- 42-year-old female with a history of chronic iron deficiency anemia and menorrhagia for several years and later diagnosed with cystic lymphangioma of the small bowel mesentery. Corresponding axial T1 (a) in-phase and (b) out-of-phase MR images demonstrate the cystic lymphangioma (straight arrows) with signal intensity loss on the out-of-phase image (b) compared to the in-phase image, indicative of microscopic lipid content of chylous fluid.
DISCUSSION
Lymphangiomas are low-flow vascular malformations that develop as a result of failure of communication of lymph sacs with the venous drainage system. It is postulated that these lesions arise secondary to inflammatory processes, surgery, or radiotherapy that lead to lymphatic obstruction.[2]
Lymphangiomas are histologically divided into several types: Simple capillary, cavernous, and cystic lymphangiomas. The cystic lymphangioma consists of lymphatic spaces with no connection to the adjacent lymphatics. Cysts may contain serous, chylous, bloody fluid or even purulent fluid in the case of secondary infection.[3] The presence of emulsified fats within chylous fluid most likely explains the signal loss on the T1 out-of-phase MR sequence compared to the T1 in-phase MR sequence in our study.
Intra-abdominal lymphangiomas mostly occur in the mesentery followed by the omentum, mesocolon, and retroperitoneum.[4] Involvement of solid viscera has been described in the literature in the liver, spleen, pancreas, adrenals, kidneys, bladder, and the ovaries.[5]
The clinical symptoms of a mesenteric lymphangioma are non-specific and include abdominal pain, vomiting, and constipation,[6] making a specific diagnosis practically impossible on clinical grounds. Imaging thus plays an indispensable role in achieving diagnosis. The majority of lymphangiomas are discovered incidentally on imaging for the investigation of unrelated clinical indications. The differential diagnosis includes a wide range of cystic intra-abdominal lesions, ranging from pancreatic pseudocysts to abdominal tuberculosis, hydatid disease, or malignancies such as mucinous carcinomatosis. The patient did not have a history of previous pancreatitis to suggest pseudocysts or risk factors for pancreatitis such as gallstones or alcohol use. Abdominal tuberculosis and malignancy were excluded largely on clinical grounds, as the patient was relatively healthy with no clinical complaints other than menorrhagia. The patient did not have constitutional symptoms or weight loss. There were no features to suggest infection on laboratory tests. Hydatid disease was unlikely, given the absence of daughter cysts and calcifications, and the insinuating nature of the lesion lacking mass effect despite its size. The absence of aggressive features on imaging, such as invasion or solid component, made malignancy unlikely. A combination of imaging modalities may be helpful in confirming the diagnosis, with MRI being the preferred examination of choice.
Typical imaging findings are those of a thin-walled, multiloculated cystic lesion lacking solid components or mural nodularity. Calcifications may occur, but are uncommon.[5] The walls and septae are typically non-enhancing or minimally enhancing. Lymphangiomas typically show T2 high signal fluid, while signal loss may be visualized on chemical shift T1-weighted imaging due to microscopic fat content from chylous fluid – this is a very suggestive finding for a lymphangioma occurring in 20–30% of cases, although rarely it may be found in other limited differentials such as lymphoceles, lymphoepithelial cysts, and pancreatic pseudocysts. Lymphangiomas may either be stable in size or show slow progressive growth. They typically show little in the way of mass effect and generally insinuate around adjacent structures. Lymphangiomas are not associated with local invasion, enlarged adenopathy, or organ metastases. The imaging algorithm for the work-up of lymphangiomas would depend on the local resources and availability of imaging modalities such as US, CT, and MRI. In our institution, cystic lesions that are either indeterminate or suspicious for lymphangiomas on US or CT are referred to MRI for further characterization. On MRI, the presence of a thin-walled, multiloculated cystic lesion in the abdomen, without mass effect on adjacent structures, is suggestive of the diagnosis after relevant differential diagnoses have been excluded on clinical, laboratory, and imaging grounds. The presence of signal loss on chemical shift imaging is very suggestive of the diagnosis. If definitive confirmation is required, imaging-guided diagnostic aspiration of the cyst fluid could be performed. Lymphangiomas can often be managed conservatively by routine clinical and imaging follow-up. Rare cases that are symptomatic or show rapid growth may merit surgery.
Although often asymptomatic, complications can be life-threatening. Secondary infection, bowel obstruction, volvulus, and rupture with hemorrhage are a few recognized complications. The development of symptoms is often related to the size of the lymphangioma.[3] Thus, definitive management becomes relevant should surveillance demonstrate significant growth.
Treatment options include fine needle aspiration, sclerotherapy, or surgical excision. Fine needle aspiration, however, does carry a risk of infection. So, to date, radical excision remains the definitive treatment, in particular, to prevent recurrence.[6]
CONCLUSION
In conclusion, intra-abdominal lymphangiomas are rare entities, which although are typically asymptomatic, can result in life-threatening complications. In our case, the lesion was discovered incidentally on imaging for an unrelated clinical indication and demonstrated interval growth at 1 year warranting a surgical referral. As of the present time, the surgical consultation has not yet been conducted and our diagnosis is based on imaging. Our findings were those of a thin-walled multiloculated cystic lesion, lacking both mass effect and enhancement, and which insinuated around vessels within the small bowel mesentery. The cyst fluid showed signal loss on chemical shift T1-weighted MR imaging due to the microscopic fat content of chylous fluid – a very suggestive finding for a lymphangioma that helps to differentiate it from other etiologies of cystic lesions in the abdomen.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2015/5/1/55/166358
REFERENCES
- Cystic lymphangioma of the small-bowel mesentery: Case report and a review of the literature. Pathol Oncol Res. 2000;6:146-8.
- [Google Scholar]
- Cystic lymphangioma of the jejunal mesentery presenting with acute abdomen in an adult. Can J Surg. 2009;52:E286-8.
- [Google Scholar]
- Correlative imaging of cystic lymphangiomas: Ultrasound, CT and MRI comparison. Acta Radiol Open. 2015;4:2047981614564911.
- [Google Scholar]
- Abdominal lymphangiomas: Imaging features with pathologic correlation. AJR Am J Roentgenol. 2004;182:1485-91.
- [Google Scholar]
- A case of mesenteric cystic lymphangioma in an adult which caused duodenal stenosis after resection. Int J Surg Case Rep. 2013;4:212-5.
- [Google Scholar]






