Translate this page into:
The Global Nonalcoholic Fatty Liver Disease Epidemic: What a Radiologist Needs to Know
Address for correspondence: Dr. Keith Pereira, C-252,1611 NW 12th Ave, Miami, FL 33136, USA. E-mail: keithjppereira@gmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Nonalcoholic fatty liver disease (NAFLD) represents a spectrum of disorders from a benign steatosis to hepatocellular carcinoma (HCC). Metabolic syndrome, mainly obesity, plays an important role, both as an independent risk factor and in the pathogenesis of NAFLD. With the progressive epidemics of obesity and diabetes mellitus, the prevalence of NAFLD and its associated complications is expected to increase dramatically. Therapeutic strategies for treating NAFLD and metabolic syndrome, particularly obesity, are continuously being refined. Their goal is the prevention of NAFLD by the management of risk factors, prevention of progression of the disease, as well as management of complications, ultimately preventing morbidity and mortality. Optimal management of NAFLD and metabolic syndrome requires a multidisciplinary collaboration between the government as well as the health system including the nutritionist, primary care physician, radiologist, hepatologist, oncologist, and transplant surgeon. An awareness of the clinical presentation, risk factors, pathogenesis, diagnosis, and management is of paramount importance to a radiologist, both from the clinical perspective as well as from the imaging standpoint. With expertise in imaging modalities as well as minimally invasive percutaneous endovascular therapies, radiologists play an essential role in the comprehensive management, which is highlighted in this article, with cases from our practice. We also briefly discuss transarterial embolization of the left gastric artery (LGA), a novel method that promises to have an enormous potential in the minimally invasive management of obesity, with details of a case from our practice.
Keywords
Hepatocellular carcinoma
left gastric artery embolization
metabolic syndrome
nonalcoholic fatty liver disease
INTRODUCTION

Defining the “NAFLD spectrum”
Nonalcoholic fatty liver disease (NAFLD) is an umbrella classification that represents a spectrum of disorders characterized by the accumulation of fat in the liver, usually as a result of insulin resistance, without significant alcohol use. This spectrum ranges from simple steatosis to lobular inflammation with variable degrees of fibrosis leading to cirrhosis and even hepatocellular carcinoma (HCC).[12] In a majority of the patients, NAFLD is associated with metabolic risk factors such as obesity, diabetes mellitus (DM), and dyslipidemia. Figure 1 represents the NAFLD spectrum.
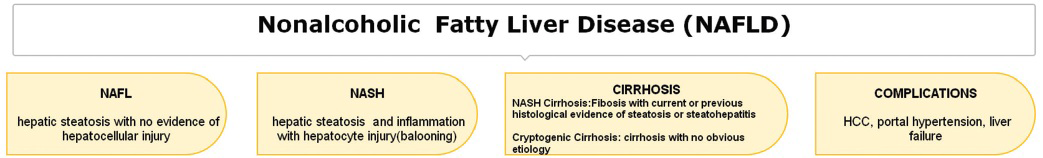
- Defining the NAFLD spectrum.
The impact
-
Incidence: Worldwide, the prevalence of NAFLD in the general population ranges from 9 to 37%.[1234] NAFLD is the most common etiology of chronic liver disease in the US and other developed countries.[56] While the reported incidence is higher in Japan[7] and lower in England,[8] in the US, recent estimates suggest that NAFLD affects 30% of the general population, 58% of overweight people, and 90% of the morbidly obese.[9] Nonalcoholic steatohepatitis (NASH) has been estimated to affect 5–7% of the general population and as much as 34–40% of patients who have elevated liver enzymes.[10111213]
-
Metabolic syndrome (obesity, DM, hyperlipidemia) is an established risk factor for primary NAFLD.[12] More than one-third of adults and 17% of youth in the US are obese.[14] Obesity is the fifth leading risk factor for mortality globally.[15]
-
Economic impact: Finkelstein et al., estimated that in the US, the medical cost to treat obesity may be as high as $147 billion per year or roughly 9% of annual medical expenditures.[16] A 33% increase in the prevalence of obesity over the next two decades will hinder the efforts of healthcare cost containment.[17] An expert panel convened by the National Institutes of Health (NIH) stated that for the first time in history, the steadily improving worldwide life expectancy could level off or even decline as a result of increasing obesity.[1819]
Natural history of NAFLD
Progression of NAFLD: NAFLD was historically thought to be of little importance. Although a majority of patients with NAFL remain stable, 25% of these patients can progress to NASH.[2021] Of patients with NASH, the disease remains stable in 34–50%, histology improves in 18–29%, the progression of fibrosis occurs in 26–37%, and fibrosis progresses to cirrhosis in 9–25%.[22] Once cirrhosis develops in patients with NASH, their clinical course is comparable to patients with other causes of cirrhosis.[23] NASH is proposed as a probable cause of cryptic cirrhosis (CC), even though most of the histologic hallmarks of NASH are not present in CC, probably due to overlapping risk factors like DM and obesity.[242526] NASH cirrhosis can result in liver failure, portal hypertension (HTN), and HCC. NASH cirrhosis results in about 30–40% of deaths due to liver failure. Figure 2 is a graphic illustration of the natural history of NAFLD.
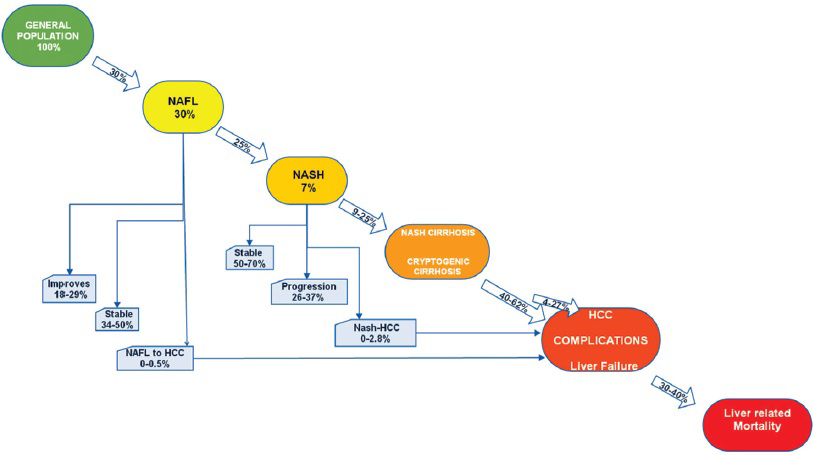
- The natural history of NAFLD.
NAFLD and HCC: Experimental studies, retrospective as well as prospective studies showed that NAFLD is a risk factor for HCC. Different stages of NAFLD, simple hepatic steatosis (0–0.5%), NASH (0–2.8%), and cirrhosis (40–62%) can progress to HCC. Higher risk is associated with advanced fibrosis and cirrhosis.[2427] The mechanisms by which NASH promotes HCC are beginning to be understood. The same insulin resistance and its subsequent inflammatory cascade and gut-derived inflammation contribute to NASH-driven HCC. Several risk factors are independently associated with the development of HCC. These include age of the patient, obesity, DM, and superimposed hepatitis C virus (HCV).[2328]
METABOLIC SYNDROME – AN INDEPENDENT RISK FACTOR FOR NAFLD
Metabolic syndrome (obesity, DM, hyperlipidemia) has been established as the risk factor for primary NAFLD.[12] Obesity is also definitively associated with a 1.5–4 times increased risk of development of HCC. This risk is conferred by two factors: The increased risk for NAFLD with subsequent progression and the carcinogenic potential of obesity alone.[2021]
Obesity can alter the hepatic pathology, metabolism, and promote inflammation, leading to NAFLD and the progression to the most severe form, NASH. NASH is characterized by prominent steatosis and inflammation, and can lead to HCC.[22232425] Figure 3 elucidates the role of obesity and metabolic syndrome as risk factors in the NAFLD spectrum.
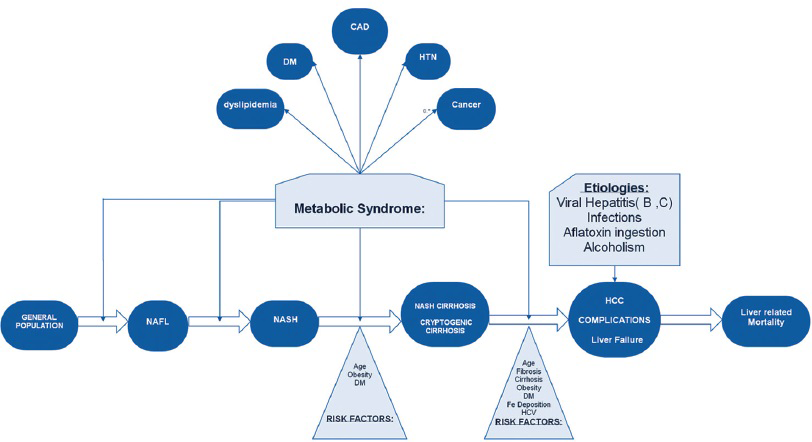
- Metabolic syndrome as a risk factor in the NAFLD spectrum.
PATHOGENESIS
3-hit hypothesis: A 3-hit mechanism is proposed in the pathogenesis and progression of NAFLD. The “1st hit” consists of hepatic triglyceride accumulation that increases the susceptibility to injury mediated by “2nd hit,” such as pro-inflammatory cytokines, which in turn lead to steatohepatitis and/or fibrosis.[2628] The impaired proliferation of hepatocyte progenitor cells represents the “3rd hit” in NAFLD pathogenesis.[132930]
Diets rich in saturated fat and cholesterol, low in polyunsaturated fat and fiber, and more recently, intestinal microbiota are linked to NAFLD pathogenesis, independent of body mass index (BMI).[13273031] Figure 4 is a summary of the “3-hit hypothesis” in the pathogenesis and progression of NAFLD.
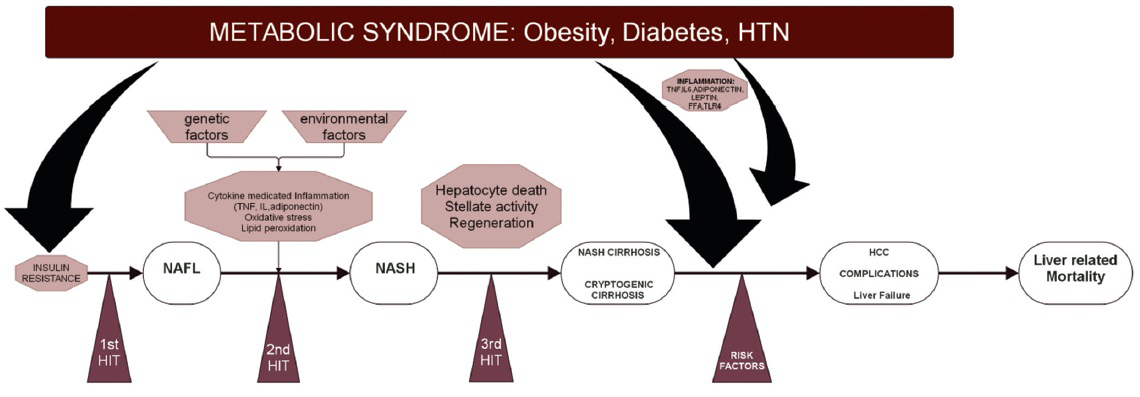
- Summary of pathogenesis of NAFLD.
DIAGNOSIS AND STAGING
Clinical presentation
Although the disease can be present from childhood, the “typical” NAFLD patient is an overweight, middle-aged adult. Majority of patients will have met at least three of the five NIH criteria for metabolic syndrome[32] [Figure 5]. Screening for NAFLD in adults attending primary care clinics or high-risk groups attending diabetes or obesity clinics is not advised at this time.[4]
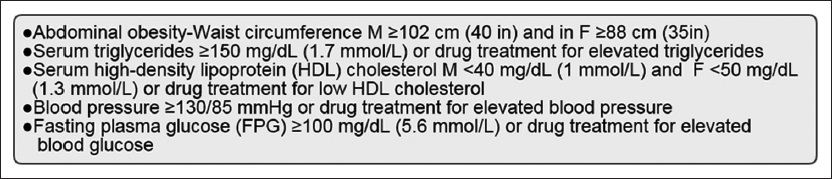
- NIH criteria in the diagnosis of metabolic syndrome.
Though fatigue, malaise, and vague right upper quadrant pain may occur, most patients with NAFLD are asymptomatic. Most cases of hepatic steatosis are incidentally discovered as modestly elevated liver aspartate aminotransferase (AST)/alanine transaminase (ALT) uncovered on routine testing or fatty liver on imaging [ultrasound (US) or computed tomography (CT)], which is called unsuspected hepatic steatosis (UHS). While the algorithm for symptomatic patients is better defined, the approach to the incidental detection is less clear.[4]
An approach to diagnosis of the spectrum of NAFLD is depicted in the flowchart [Figure 6].
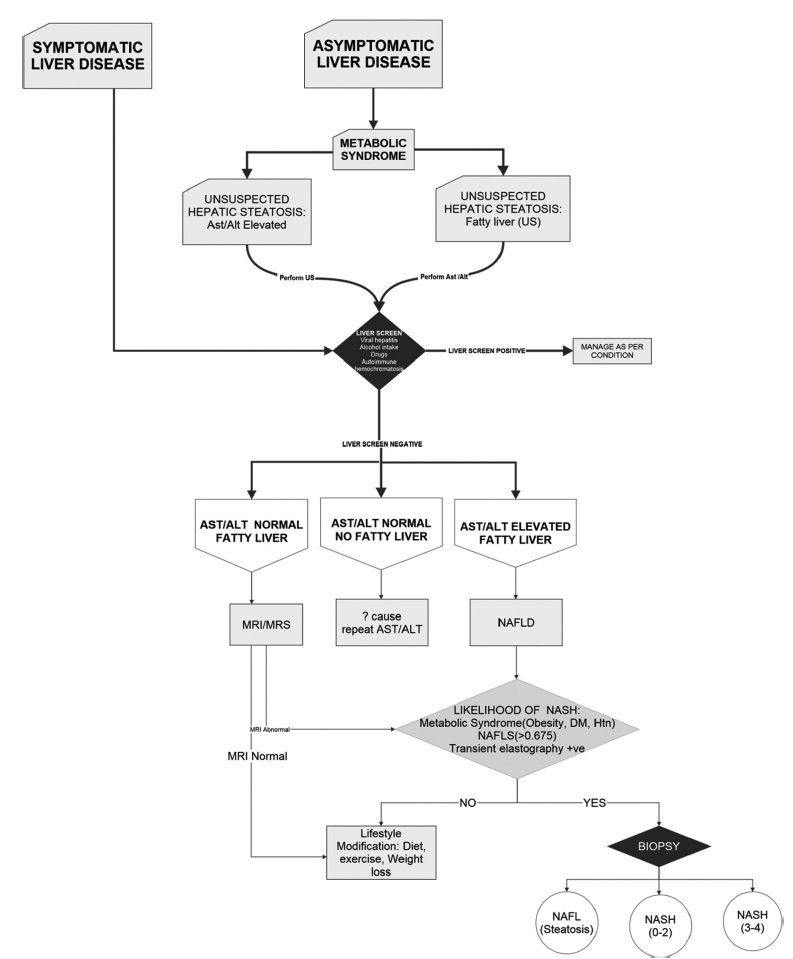
- Management algorithm in the detection of NAFLD.
Confirming diagnosis
The definitive diagnosis of NAFLD requires the following: (a) There is hepatic steatosis identified by imaging or histology and (b) there are no competing or co-existing etiologies for hepatic steatosis (significant alcohol consumption, hepatitis C, medications, parenteral nutrition, Wilson's disease, and severe malnutrition).[4]
Liver biopsy
Liver biopsy remains the Gold Standard for characterizing liver histology, reliably differentiating NASH from simple steatosis or fibrosis in patients with NAFLD. However, a biopsy is limited by cost, sampling error, procedure-related morbidity, and mortality.[4]
-
When to biopsy? Biopsy should be performed in those who would benefit the most from therapeutic guidance.[3334] Current recommendations for a biopsy are where competing etiologies are not excluded clinically and in those with risk factors for advanced fibrosis (like metabolic syndrome).[4]
In Figure 7 is shown a 46-year-old asymptomatic man, who presented with elevated liver enzymes, of unknown etiology. US-guided liver biopsy in the left lobe of the liver was performed.
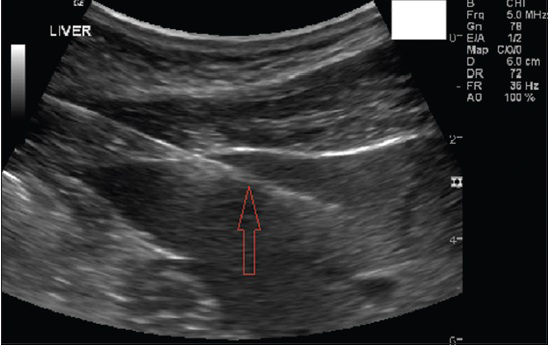
- 46-year-old asymptomatic man presented with elevated liver enzymes. Serological markers for other causes of elevated liver enzymes did not reveal any pathology. US-guided liver biopsy in the left lobe of the liver was performed. Gray-scale US longitudinal view shows the left lobe of liver with the needle in the parenchyma (seen as white echogenic line).
Staging
Once diagnosed, it is essential to grade and stage the disease, as the prognosis of NAFLD depends on the severity of liver injury and fibrosis.
-
Invasive method: Although liver biopsy has been the Gold Standard in this situation, it has its limitations.
The Brunt Classification is the predominant “grading and staging” system that determines management based on hepatic histologic findings. Depending on the degree of steatosis, lobular inflammation, and hepatocyte ballooning, the NAFLD is graded from 0 to 8. Depending on the degree, extent, and location of fibrosis, four stages (0–4) are assigned in the NASH fibrosis staging[35] [Figure 8]. This staging system serves as a guide to management, as will be discussed later.
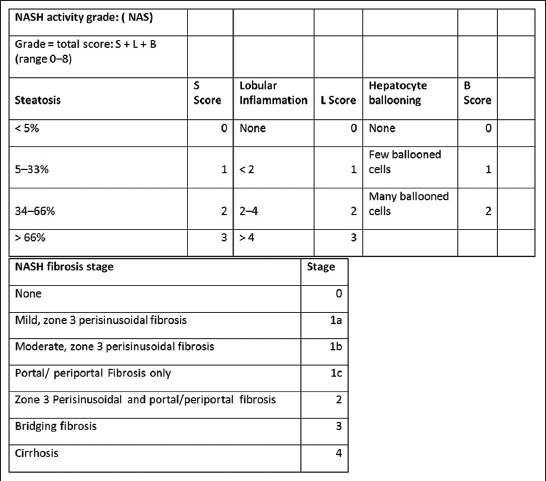
- NASH activity grade and fibrosis stage based on Brunt classification.
-
Noninvasive method: Both the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) guidelines have endorsed the use of the NAFLD fibrosis score (NAFLS) routinely in patients to determine the need for liver biopsy. The NAFLS panel assesses selected laboratory values (serum glucose, platelet count, albumin, AST/ALT ratio) and readily available patient characteristics (age, BMI, and diabetes status). Patients with a high NAFLD fibrosis score may be in need of additional studies such as elastography or liver biopsy. The score also identifies patients at low risk, who should be reassured and followed at periodic intervals.[436]
In an effort to develop noninvasive methods, two complementary approaches, one using a biological approach (serum biomarker levels) and the other using a physical approach [liver stiffness- transient elastography (TE)], are evolving.
Herein the authors discuss these as well as the value of routine imaging modalities like US, CT, and magnetic resonance imaging (MRI).
Laboratory variables
Of the biochemical markers available, cytokeratin-18 (CK-18) levels are validated and may help in distinguishing between simple steatosis from NASH, but the test has low overall sensitivity and specificity.[133738]
Imaging
To date, various imaging methods like US, CT, MRI, and magnetic resonance spectroscopy (MRS) have been used to detect and quantify noninvasively hepatic steatosis and detect cirrhosis. However, they are far less reliable at detecting NASH and the associated stages of fibrosis.[13] More recently, several imaging methods that measure liver stiffness have been investigated for their usefulness in assessing inflammation and fibrosis in patients with NAFLD.
The authors review the imaging methods currently utilized for the evaluation of NAFLD and discuss their practical applicability. Figure 9 summarizes the role of imaging modalities in the NAFLD spectrum.
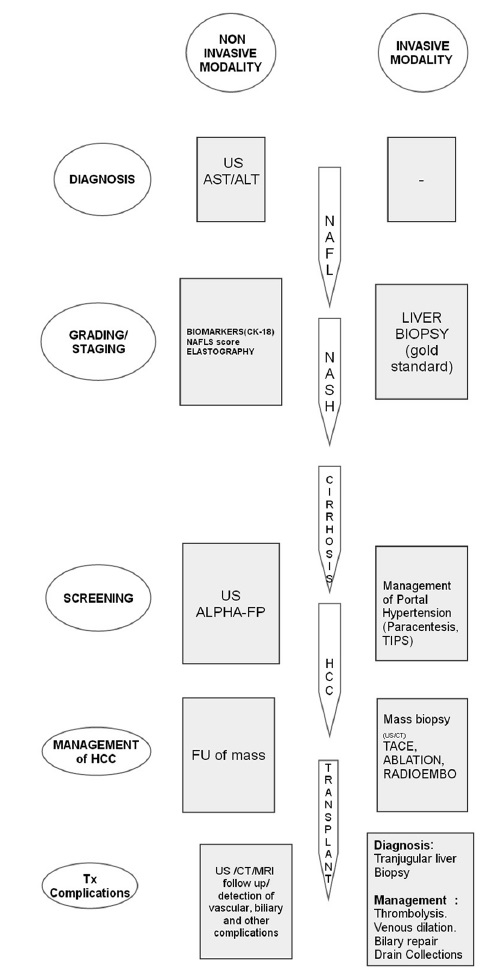
- Role of imaging (invasive and noninvasive) in the grading and staging of NAFLD.
Diagnosis (qualitative) and staging (quantitative) of NAFLD by US, CT, and MRI
Ultrasound
Qualitative: Characteristic findings on US are “bright liver,” loss of definition of the diaphragm due to posterior beam attenuation, and a “featureless or bland appearance” [Figure 10a].
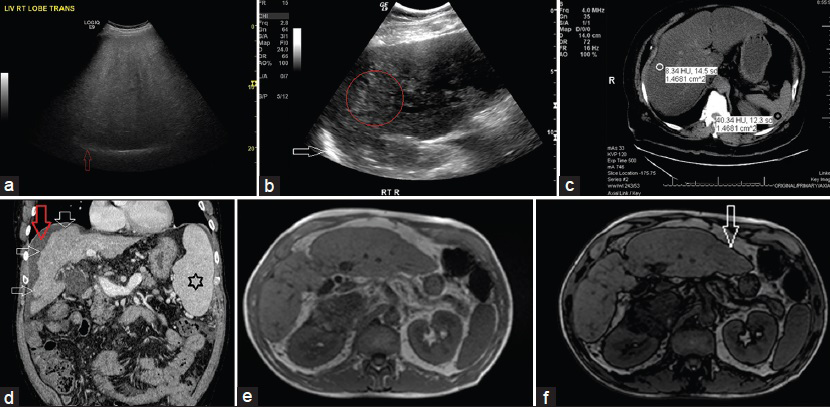
- (a) 56-year-old man with metabolic syndrome and mildly elevated liver enzymes. Gray-scale US transverse view through the right lobe of liver shows the following: 1. Hyperechogenic liver tissue with fine, tightly packed echoes on US examination (the so-called “bright liver”), characteristic of liver steatosis; 2. Decreased sonographic visualization of portal and hepatic veins giving rise to a “featureless or bland appearance;” and 3. Decreased ability of the US beam to penetrate the liver tissue causing posterior darkness and loss of definition of the diaphragm (posterior beam attenuation) (red arrow). (b) 68-year-old woman with metabolic syndrome. Gray-scale US longitudinal view of right lobe of liver shows the following: 1. Coarse echo pattern (within the red circle), different from fine, packed echoes of steatosis; 2. Lack of posterior beam attenuation with definite visualization of diaphragm (white arrow); 3. Fibrosis with steatosis seen as coarse echoes (“pin-head echoes”) within the fine echo pattern of steatosis (differentiation is difficult as fibrosis and steatosis have similar sonographic appearance, “fatty-fibrotic pattern”); and 4. Later there was volume loss, nodular contour, and ascites. (c) 48-year-old asymptomatic man with mildly elevated liver enzymes. Unenhanced CT scan, axial section of the liver shows HUliver 8 (white circle) and HUspleen 40 (black circle), CTL-S of −32, and hepatic attenuation index (HAI) of 0.2. Fatty infiltration is diagnosed when the criteria of HUliver <48 HU, CTL-S of −2, and HAI of 0.8 are met. (d) 53-year-old man previously diagnosed as NASH presented with US features of cirrhosis. Contrast-enhanced triple-phase CT was performed to evaluate for portal hypertension and hepatocellular carcinoma. Contrast-enhanced CT-venous phase, coronal images reveals a cirrhotic liver as evidenced by the characteristic features of right lobe atrophy, nodular surface (multiple white arrows), spenomegaly (black star), and ascites (red arrow). (e and f) 51-year-old woman with an ovarian cyst presented for evaluation by MRI. Abdomen and pelvic MRI was performed. (e) In-phase T1-weighted gradient-echo images of areas with a significant amount of intracellular fat shows the liver to be brighter in signal intensity than the spleen and paraspinal muscles. (f) Out-of-phase images shows lower signal intensity of liver than on the corresponding in-phase images. Characteristically, out-of-phase images are identified by a thick black rim at fat–water boundaries, an artifact that has been termed the “India ink effect” or “boundary artifact” (white arrow).
Quantitative: US may not be an appropriate tool for monitoring NAFLD patients. Liver fibrosis and steatosis can have similar sonographic appearances (termed the “fatty-fibrotic pattern”) [Figure 10b].[39404142]
Summarizing the role of US: US is a well-established and cost-effective imaging technique for screening subjects at risk of NAFLD.[43] However, it is not very effective in grading NAFLD.
Computed tomography
Qualitative: Unenhanced CT is considered the best CT method for estimation of liver fat since it involves simple measurement of liver attenuation in Hounsfield units (HU). Liver attenuation values decrease by about 1.6 HU for every milligram of triglyceride deposited per gram of liver tissue. In spite of this, measurement of absolute liver attenuation to diagnose fatty infiltration has proven impractical. On unenhanced CT, qualitative estimation of liver fat is performed by comparing the attenuation of the liver with that of the spleen. The spleen is good for comparison because splenic attenuation is unaffected by various diffuse pathologic processes and, also, the spleen is located in the same cross section as the liver. On unenhanced CT, a normal liver has higher attenuation than a normal spleen. If the attenuation is lower, a diagnosis of hepatic steatosis may be considered. Various indices have been described below.[44]
Quantitative: Several quantitative CT indices have been used to assess hepatic steatosis. While hepatic attenuation HUliver is an absolute value, difference in attenuation between liver and spleen (CTL-S) and hepatic attenuation index (HAI), the ratio of hepatic attenuation to splenic attenuation, are relative.[39444546]
Dual-energy CT may be used to evaluate focal and diffuse fatty infiltration of the liver by measuring the changes in hepatic attenuation between the images acquired at lower and higher energy levels[44] [Figure 10c and d].
Summarizing the role of CT: Although CT is quite accurate for the diagnosis of moderate-to-severe steatosis, it is not accurate for detecting mild steatosis and due to the potential radiation hazard, it is inappropriate for longitudinal follow-up. However, it is used to detect complications of cirrhosis like portal HTN and HCC, as well as moderate-to-severe hepatic steatosis in donor candidates for liver transplantation (LT).[474849]
Magnetic resonance imaging
Qualitative:
-
Conventional techniques: On conventional T1-weighted MR images, severe fatty infiltration of the liver appears as increased hepatic signal intensity (T1: hyperintense T2: mildly hyperintense). However, these standard spin-echo (SE) pulse sequences have relatively low sensitivity for the detection and quantification of fatty infiltration.
-
Chemical shift imaging: The chemical shift effect principle is used in both MRI technique and MRS. It utilizes the difference in resonance frequencies of water and lipid to differentiate the tissues containing only water from those containing water and lipid. This is known as the Dixon method. Although the Dixon two-point method with the use of an SE sequence for chemical shift imaging was first described in 1984, this was later replaced by the three-point Dixon method to overcome magnetic in-homogeneities. The three-point technique involves the acquisition of a third image in addition to the in-phase and out-of-phase images and results in increased scanning time. Modified fast gradient echo sequence (GRE) techniques using modified Dixon method are now in use, which have reduced magnetic in-homogeneities and also reduced the scanning time further, making breath-hold acquisitions possible.[505152]
The sequence is made of in-phase and opposed-phase imaging, such that the signal from fat protons is added (in-phase) or subtracted (opposed-phase) from the signal from protons in water. In-phase T1-weighted gradient-echo images of areas with a significant amount of intracellular fat show the liver to be brighter in signal intensity than the spleen and paraspinal muscles. Out-of-phase images show lower signal intensity of the liver than on the corresponding in-phase images, and this difference in signal intensity establishes the diagnosis of fatty liver. Characteristically, out-of-phase images are identified by a thick black rim at fat–water boundaries, an artifact that has been termed the “India ink effect” or “boundary artifact” [Figure 10e and f]. Of note, on in- and out-of-phase imaging, the maximum signal loss occurs when there is 50% fatty infiltration of the liver. In situations in which there is >50% fatty infiltration, the out-of-phase sequence paradoxically becomes less hypointense than at 50% fatty infiltration. Chemical shift artifact at the parenchyma–vessel interface aids in detecting this situation.[5354]
Quantitative: Precise fat quantification requires correction for T2* decay, which can be obtained by using triple-echo, gradient-echo sequence. Various methods to quantify fat are used, such as fat signal percentage (FSP), fat signal fraction (FSF), and hepatic fat fraction.[5055]
With fast SE imaging, the fat percentage in liver (HFP) is calculated from the percentage decrease in hepatic signal intensity on T2-weighted fat-saturated fast SE images in comparison with that of T2-weighted non–fat-saturated fast SE images as follows: A decrease in the signal intensity of the liver on T2-weighted fat-saturated fast SE images in comparison with T2-weighted non–fat-saturated fast SE images is suggestive of fatty infiltration.
Detection of cirrhosis: Liver cirrhosis is usually associated with increased hepatic iron content, which has a paramagnetic effect that may cause local field in-homogeneities and affect T2* relaxation. Hence, GRE sequences may be unreliable for fat quantification in cirrhotic patients. By using T2-weighted fat-saturated and non–fat-saturated fast SE sequences, signal loss caused by a T2* effect may be avoided. The underlying concept has potential for improving the detection of steatosis against the background of cirrhotic liver.
Magnetic resonance spectroscopy
The principle of MRS is to measure the chemical composition of tissue on the basis of the frequency composition of the signal arising from the voxel. Most of the identifiable peaks are derived from water and fat, with water appearing as a single peak at 4.7 ppm and fat appearing as multiple peaks. MRS can detect a very low quantity of fat, which is not possible with other MRI techniques, and is hence considered to be the most sensitive method. Proton density fat fraction (PDFF) can also be calculated as the ratio of the sum of the signal intensities of the fat-derived peaks divided by the sum of all peaks.[5556] MRS has often been used as the reference standard in a number of clinical studies and has determined the prevalence of NAFLD in the general adult population. However, MRI is not routinely used and is time consuming.
Summarizing the role of MRI: MR imaging, one of the most sensitive imaging modalities, is increasingly being used in the diagnosis, treatment, and follow-up of NAFLD. The use of MR imaging has benefits of technical simplicity, coverage of the entire liver, and absence of radiation exposure. Limitations to the routine use of MR imaging in liver fat quantification include potential variability of results due to differences in MR imaging systems, scanning parameters, and methods of analysis. In addition, MR imaging is relatively expensive, a factor that limits its applicability for repeated evaluations and monitoring of treatment response. Both MRS and MRI are reproducible and accurate, and have the potential to replace liver biopsy as the reference standard for research studies.[44]
Innovative radiological modalities
New imaging technologies, such as the US elastography (fibroscan; Echosens, Paris, France) and MR elastography (MRE), are emerging as promising methods to diagnose NASH. They evaluate the liver stiffness by measuring the velocity of shear wave using US or MRI. They may play a potential role in screening for NASH and/or advanced fibrosis in patients with NAFLD.[57585960]
Fibroscan, also known as TE, has been originally validated for the hepatitis C population. TE sends a pulse through the skin, which is circulated through the liver. The velocity of the wave correlates with liver stiffness.[61626364]
In MRE, shear waves are generated in the liver tissue by a driver attached to the abdominal wall. Magnetic resonance images are then obtained depicting the propagated shear waves, and finally, images of the shear waves are analyzed and used to generate quantitative maps of tissue stiffness, referred to as elastograms.[626566]
TREATMENT AND MANAGEMENT OF NAFLD
The goals of managing NAFLD are as follows:
-
Elimination of risk factors: Preventing fibrosis by treating diabetes, HTN, hyperlipidemia, and obesity.
-
Targeting the components of metabolic syndrome: This includes treatment of DM, hypertension, dyslipidemias, and reduction of patients’ cardiovascular risk profile.
-
Management of complications: This includes management of HCC, cirrhosis, and complications of liver transplant.
A comprehensive management approach is summarized in Figure 11.
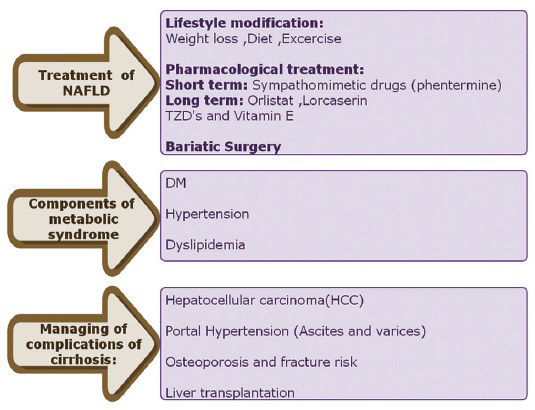
- Comprehensive management of NAFLD.
I. Elimination of risk factors
The primary approach to treat NAFLD involves elimination of the underlying risk factors. It has been reported that community-based lifestyle modification programs are effective in reducing and normalizing liver fat in NAFLD patients.[67] Treatment options are based on the BMI and “grading and staging” based on liver histology. These are summarized in Figures 12 and 13.
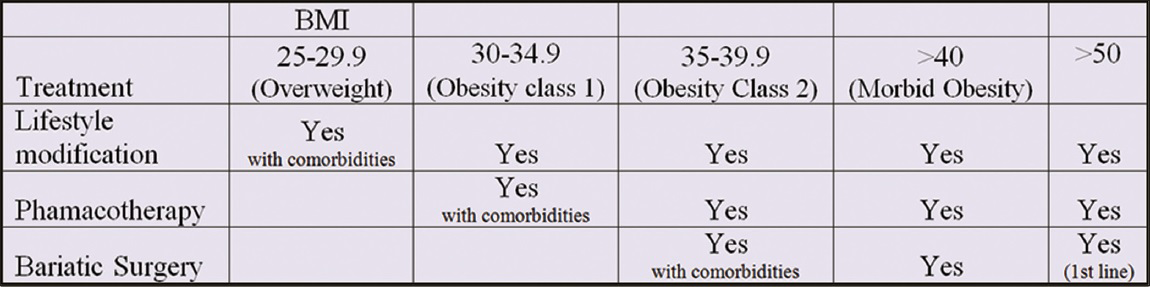
- Chart showing treatment modalities available based on BMI.

- Algorithm in the management of NAFLD based on histological staging and grading.
Lifestyle modification
Weight loss can be achieved either by a hypocaloric diet alone or in conjunction with exercise.[4] Fibrosis regression occurs in the majority of patients after weight loss.[6869] Also, weight loss is useful in the treatment of the metabolic component of obesity. A loss of 3–5% is necessary to improve steatosis, but up to 10% loss may be needed to improve necroinflammation.[41370717273]
-
Diet regimens to induce weight loss have been shown to be difficult to sustain due to the increase in hunger. Dieting produces a 24% increase in the 24-h ghrelin profile. This elevated ghrelin secretion may, therefore, be a reason why dieting is so difficult to sustain in the long term[74757677]
-
Exercise may reduce hepatic steatosis as it improves the markers of apoptosis and insulin sensitivity in NAFLD.[478]
Medical treatment
-
Targeting liver fat and inflammation: Despite a better understanding of the mechanisms of NAFLD pathogenesis, there are few effective therapies available. Pioglitazone can be used to treat steatohepatitis in patients with biopsy-proven NASH, although long-term safety is not established, especially in diabetics.[79] Vitamin E is the main antioxidant that has emerged as a treatment for NASH with the hope that it can help improve oxidative stress in the liver.[48081] Several other therapies are emerging, such as inhibitors of inflammation (e.g. GS-9450),[82] glucagon-like peptide-1 (Glp-1),[8283] and probiotics.[84]
-
Targeting metabolic syndrome/obesity: In spite of significant efforts in this area, current FDA-approved agents can achieve only modest levels of weight loss.[77]
Sympathomimetic drugs (phentermine), although useful, are not used in long-term pharmacotherapy because of their potential for abuse. Orlistat, an FDA-approved enteric lipase inhibitor, leads to malabsorption of dietary fat and can aid weight loss in conjunction with lifestyle modification.[8586]
Lorcaserin is a selective agonist of the serotonin 2C receptor that reduces food intake, appetite, and thereby reduces body weight. Adverse effects of lorcaserin are mild, but in patients with type 2 diabetes on oral agents, lorcaserin-induced weight loss may increase the risk of symptomatic hypoglycemia, necessitating a reduction in the dose of diabetes medications.[87]
Bariatric surgery
Lifestyle interventions, particularly weight loss, are often very difficult for patients to achieve and sustain. Bariatric surgery has an increasing role in the management of patients with obesity. Various types of bariatric surgeries are available.[88]
In carefully selected patients, weight loss after bariatric surgery has beneficial effects on the components of the metabolic syndrome and has demonstrated histological improvement in hepatic steatosis and fibrosis, reducing long-term mortality.[6889909192] Bariatric surgery should be avoided in subjects with advanced cirrhosis, as there is a risk of hepatic decompensation with rapid weight loss, besides other risks of surgery.[93] High mortality rates are seen secondary to bariatric surgery.[8694] A recent Cochrane review concluded that there is insufficient data to determine if bariatric surgery is an effective treatment for NAFLD.[95]
Surgery should be considered as the first-line option if the BMI is >50 kg/m2, and also in those with BMI >40 kg/m2 or 35–40 kg/m2 and having obesity-related comorbidities.[495]
Figure 14 is a synopsis of the role of bariatric surgery in obesity.
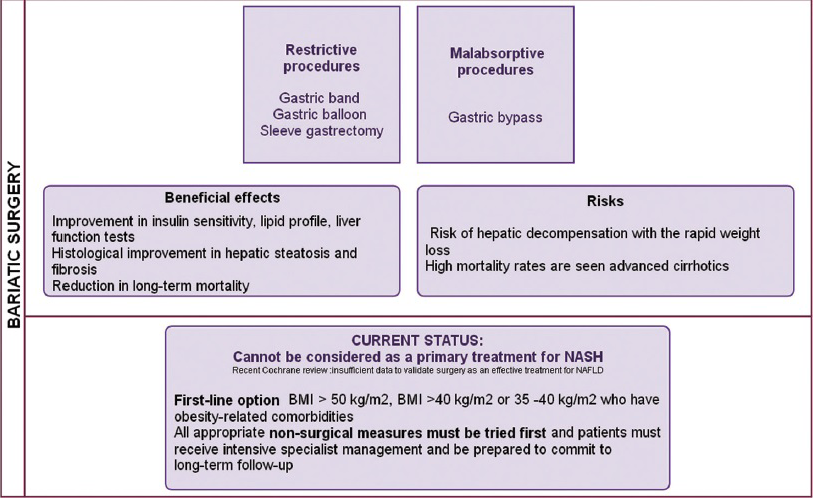
- Bariatic surgery in the management of obesity and NAFLD.
Left gastric artery embolization: An emerging technique
The growing obesity epidemic suggests that current therapeutic alternatives like lifestyle modifications, medical therapies, and bariatric surgery are insufficient. Moreover, invasive bariatric surgery carries a host of potential complications for the patient.[96]
Recent studies performed in animal models have demonstrated that body weight can be modulated via percutaneous, catheter-directed, transarterial embolization of the left gastric artery (LGA), the artery that preferentially provides blood flow to the fundus of the stomach. The hypothesis is that selective LGA embolization could cause relative ischemia in the mucosa of the gastric fundus, which could, in turn, suppress the production of the hormone ghrelin. Ghrelin, which is primarily secreted from the mucosa of the gastric fundus, has a powerful orexigenic effect, stimulating food intake and weight gain in both animal and human models.[979899100101102] Ghrelin directly stimulates appetite and induces positive energy balance, resulting in body weight gain.[103104105]
Early data in humans: The data is limited in humans, although a single retrospective study and a single prospective study have been performed.[106107]
In a historical fashion, Oklu et al., reported good results in a retrospective study of 15 patients who underwent transcatheter embolization of the LGA for the indication of gastric hemorrhage. Although they suggested further refinements of the procedure, they reported that if effective, transarterial embolization of the LGA would undoubtedly provide obese patients with a much less morbid therapeutic option.[2697108]
Klipshidze et al., reported the results of a first-in-human prospective study of LGA embolization in five patients. The mean weight, BMI, and ghrelin levels had decreased at 1 month, 3 months, and 6 months post-procedure.[106]
In a patient with HCC from NASH cirrhosis who was not a liver transplant candidate due to obesity, a transarterial embolization of the LGA and transarterial chemoembolization (TACE) of the left hepatic HCC was performed [Figure 15].

- 68-year-old female with a past medical history of morbid obesity, Type 2 diabetes, hypertension, and ultrasound features of cirrhosis was referred from an outside facility for evaluation. (a) Gray-scale US of the liver, longitudinal view through the right lobe of the liver shows a coarse echo pattern (within red circle) with a nodular surface suggestive of cirrhosis. (b) Also seen is a well-encapsulated echogenic lesion in the left lobe of the liver (between yellow marks). (c) This was confirmed (white arrow) on triple-phase contrast-enhanced axial MRI. A left gastric artery embolization was performed using using 500–700 μm embolic particles. (d) Pre-embolization angiogram shows a normal-appearing left gastric artery (black arrow) and fundal branches (white arrow). (e) Post-embolization angiogram shows absence of flow into the left gastric artery (white star) with normal flow into common hepatic and splenic arteries (white arrows). A transarterial chemoembolization (TACE) was performed via segment 2 and 3 hepatic branches. (f) Pre-TACE angiogram of the left hepatic artery reveals a small rounded hypervascular blush (black arrow). (g) Post-chemoembolization left hepatic angiogram reveals stasis (white arrow) in the segment 2/3 hepatic branch with no hypervascular blush.
II. Targeting the components of the metabolic syndrome
This includes management treatment of DM, HTN, and effective treatment of dyslipidemias, vital to reduce patients’ cardiovascular risk profile.[109]
III. Managing complications
Hepatocellular carcinoma
Surveillance for HCC: Since NASH is associated with HCC, patients with NASH cirrhosis should be considered for HCC screening, according to the AASLD/American college of gastroenterology (ACG) practice guidelines.[4] Surveillance with US and α-fetoprotein should be performed every 6 months.[110111] CT or MRI could be considered in subjects where US has failed to produce adequate examination of the liver.[112]
Since obesity is associated with HCC, aberrant genes involved in metabolic pathways are emerging as therapeutic targets in cancer treatment. The use of metformin or thiazolidinediones in diabetic patients is associated with risk reduction of HCC.[113114]
Management of HCC: Once HCC develops, the management protocol is similar to that in viral etiology of HCC. NASH HCC has less-severe liver dysfunction and better overall survival after curative treatment compared to its counterparts with HCV and alcoholic liver disease (ALD).[51]
Although the preferred therapy for HCC is surgical resection, many patients are not eligible because of the tumor extent or underlying liver dysfunction. For patients who are not surgically resectable, LT is the only other potentially curative option. For patients who are not eligible for resection or LT, treatment options include local nonsurgical methods of tumor ablation [radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), TACE, radiation therapy] and systemic therapy. The selection of treatment is determined by the severity of underlying liver disease, the size and distribution of the intrahepatic tumors, the vascular supply, and the patient's overall performance status.[115] Figure 16 gives an overview of the management algorithm for treatment of HCC based on the Barcelona Clinic Liver Cancer (BCLC) Staging System.

- Overview of the management algorithm for treatment of HCC based on the Barcelona Clinic Liver Cancer (BCLC) Staging System.
Screening for varices
Cirrhotic patients with NASH are at risk of varices. Therefore, patients with NASH cirrhosis should undergo endoscopic screening for esophageal and gastric varices.[4]
Liver transplantation
NASH cirrhosis is now the third most common indication for LT in the US and accounted for 12% of patients listed for transplantation in the UK in 2009.[116] Although recurrence of NASH is common post-transplant, occurring in 4–25% of patients, it does not appear to impact graft survival.[117118]
While LT has become more prevalent with improvement in both the surgical technique and postoperative care, there remain serious complications from the procedure.[119] Several of the common complications can present with deranged liver function tests.
-
Diagnostic modalities: US with Doppler, CT, MRI, and magnetic resonance cholangiopancreatography (MRCP) remain the first-line tool in assessing the LT. Despite this, there are situations when these tests cannot explain the altered liver function, and a diagnostic liver biopsy is performed. Either an US-guided percutaneous or a transjugular liver biopsy can be performed[120]
-
Minimally invasive treatment
Vascular complications remain a significant problem within the first 3 months following LT. The most common complication is with the hepatic artery stenosis/thrombosis, but problems also arise from the hepatic vein, portal vein, and inferior vena cava (IVC). These can be successfully treated using interventional radiology techniques like thrombolysis, thrombectomy, and balloon dilatation.[121]
Biliary complications like strictures, leaks, and bilomas can occur in 10–40% of patients following LT. Although some are treated by endoscopic retrograde cholangiopancreatography (ERCP), others need a percutaneous transhepatic cholangiography (PTC) approach.
Although there is limited experience of the role of Transjugular intrahepatic portosystemic shunt (TIPS) in liver transplant patients, it is used in case of liver failure due to graft failure.[122123]
We provide a summary of the key points in the clinical presentation, risk factors, pathogenesis, diagnosis, and management of NAFLD [Figure 17].

- Recap of key points in the clinical presentation, risk factors, pathogenesis, diagnosis, and management of NAFLD.
CONCLUSION
With the progressive epidemics of obesity and DM, the prevalence of NAFLD and its associated complications is expected to increase dramatically, with an enormous health and economic impact.
An awareness of the clinical presentation, risk factors, pathogenesis, diagnosis, and management is of paramount importance to a radiologist, both from the clinical perspective as well as from the imaging standpoint. Transarterial embolization of the left gastric artery (LGA), a novel method, promises to have an enormous potential in the minimally invasive management of obesity. With expertise in imaging modalities as well as minimally invasive percutaneous endovascular therapies, radiologists play an essential role in the comprehensive management of the entire NAFLD spectrum.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2015/5/1/32/157860
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Prevalence of and risk factors for nonalcoholic fatty liver disease: The Dionysos nutrition and liver study. Hepatology. 2005;42:44-52.
- [Google Scholar]
- Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-85.
- [Google Scholar]
- Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology. 2011;140:124-31.
- [Google Scholar]
- The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-23.
- [Google Scholar]
- Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1-16. vii
- [Google Scholar]
- The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722-8.
- [Google Scholar]
- Hepatology outpatient service provision in secondary care: A study of liver disease incidence and resource costs. Clin Med. 2007;7:119-24.
- [Google Scholar]
- Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682-98.
- [Google Scholar]
- Liver biopsy without clear indication or informed consent. Am J Gastroenterol. 2000;95:1588-9.
- [Google Scholar]
- Liver biopsy in elevated liver functions tests. An old question revisited? J Hepatol. 2001;35:290-4.
- [Google Scholar]
- Non-alcoholic fatty liver: Another feature of the metabolic syndrome? Clin Nutr. 1999;18:353-8.
- [Google Scholar]
- Concepts and treatment approaches in nonalcoholic fatty liver disease. Adv Hepatol. 2014;2014:1-7.
- [Google Scholar]
- Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806-14.
- [Google Scholar]
- National Task Force on the Prevention and Treatment of Obesity. Overweight, obesity, and health risk. Arch Intern Med. 2000;160:898-904.
- [Google Scholar]
- Annual medical spending attributable to obesity: Payer-and service-specific estimates. Health Aff (Millwood). 2009;28:w822-31.
- [Google Scholar]
- A potential decline in life expectancy in the United States in the 21 st century. N Engl J Med. 2005;352:1138-45.
- [Google Scholar]
- Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: A case‐control study. Hepatology. 2000;32:689-92.
- [Google Scholar]
- Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-38.
- [Google Scholar]
- Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst. 1996;88:1472-7.
- [Google Scholar]
- Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89:1360-5.
- [Google Scholar]
- Role of diabetes mellitus in the etiology of hepatocellular carcinoma. J Natl Cancer Inst. 2000;92:1096-9.
- [Google Scholar]
- Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-8.
- [Google Scholar]
- Systematic review: The diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2011;33:525-40.
- [Google Scholar]
- Fibrosis in genotype 3 chronic hepatitis C and nonalcoholic fatty liver disease: Role of insulin resistance and hepatic steatosis. Hepatology. 2006;44:1648-55.
- [Google Scholar]
- Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370-9.
- [Google Scholar]
- Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140:976-86.
- [Google Scholar]
- Non alcoholic steatohepatitis a precursor for hepatocellular carcinoma development. World J Gastroenterol. 2014;20:16464-73.
- [Google Scholar]
- Clinical management of metabolic syndrome: Report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation. 2004;109:551-6.
- [Google Scholar]
- Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-906.
- [Google Scholar]
- Pitfalls in interpreting liver biopsy results: The story of the blind men and the elephant. Liver Transpl. 2002;8:1200-1.
- [Google Scholar]
- Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-21.
- [Google Scholar]
- Serum markers of hepatocyte apoptosis: Current terminology and predictability in clinical practice. Hepatology. 2010;51:717-8.
- [Google Scholar]
- Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47:455-60.
- [Google Scholar]
- Non-invasive assessment of hepatic steatosis: Prospective comparison of the accuracy of imaging examinations. J Hepatol. 2010;52:579-85.
- [Google Scholar]
- The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745-50.
- [Google Scholar]
- Diagnostic value of a computerized hepatorenal index for sonographic quantification of liver steatosis. AJR Am J Roentgenol. 2009;192:909-14.
- [Google Scholar]
- Sonographic hepatorenal ratio: A noninvasive method to diagnose nonalcoholic steatosis. J Clin Ultrasound. 2013;41:18-25.
- [Google Scholar]
- What about non-alcoholic fatty liver disease as a new criterion to define metabolic syndrome? World J Gastroenterol. 2013;19:3375-84.
- [Google Scholar]
- Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:7392-402.
- [Google Scholar]
- Macrovesicular hepatic steatosis in living liver donors: Use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105-12.
- [Google Scholar]
- Biopsy-proven nonsteatotic liver in adults: Estimation of reference range for difference in attenuation between the liver and the spleen at nonenhanced CT. Radiology. 2011;258:760-6.
- [Google Scholar]
- Unenhanced CT for assessment of macrovesicular hepatic steatosis in living liver donors: Comparison of visual grading with liver attenuation index. Radiology. 2007;244:479-85.
- [Google Scholar]
- Association between body composition and pulmonary function in elderly people: The Korean Longitudinal Study on Health and Aging. Obesity (Silver Spring). 2011;19:631-8.
- [Google Scholar]
- Advances in the application of imaging methods in applied and clinical physiology. Acta Diabetol. 2003;40(Suppl 1):S45-50.
- [Google Scholar]
- Accuracy of liver fat quantification at MR imaging: Comparison of out-of-phase gradient-echo and fat-saturated fast spin-echo techniques--initial experience. Radiology. 2005;237:507-11.
- [Google Scholar]
- Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55:1809-19.
- [Google Scholar]
- Measurement of liver fat content using selective saturation at 3.0 T. J Magn Reson Imaging. 2007;25:743-8.
- [Google Scholar]
- Nonalcoholic fatty liver disease: Rapid evaluation of liver fat content with in-phase and out-of-phase MR imaging. Radiology. 2009;250:130-6.
- [Google Scholar]
- Hepatic fat quantification: A prospective comparison of magnetic resonance spectroscopy and analysis methods for chemical-shift gradient echo magnetic resonance imaging with histologic assessment as the reference standard. Invest Radiol. 2012;47:368-75.
- [Google Scholar]
- Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:spcone.
- [Google Scholar]
- Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422-31.
- [Google Scholar]
- Real-time tissue elastography for evaluation of hepatic fibrosis and portal hypertension in nonalcoholic fatty liver diseases. Hepatology. 2012;56:1271-8.
- [Google Scholar]
- Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008;48:442-8.
- [Google Scholar]
- Liver elasticity in NASH patients evaluated with real-time elastography (RTE) Ultrasound Med Biol. 2012;38:537-44.
- [Google Scholar]
- Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011;55:666-72.
- [Google Scholar]
- Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012;55:199-208.
- [Google Scholar]
- Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: Comparison between M and XL probe of FibroScan®. J Hepatol. 2012;56:833-9.
- [Google Scholar]
- Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD) Dig Liver Dis. 2008;40:371-8.
- [Google Scholar]
- Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749-56.
- [Google Scholar]
- Community-based lifestyle modification programme for non-alcoholic fatty liver disease: A randomized controlled trial. J Hepatol. 2013;59:536-42.
- [Google Scholar]
- Effect of bariatric surgery on nonalcoholic fatty liver disease: Systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396-402.
- [Google Scholar]
- Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009;137:532-40.
- [Google Scholar]
- Comparative review of diets for the metabolic syndrome: Implications for nonalcoholic fatty liver disease. Am J Clin Nutr. 2007;86:285-300.
- [Google Scholar]
- Prolonged saturated fat-induced, glucose-dependent insulinotropic polypeptide elevation is associated with adipokine imbalance and liver injury in nonalcoholic steatohepatitis: Dysregulated enteroadipocyte axis as a novel feature of fatty liver. Am J Clin Nutr. 2009;89:558-67.
- [Google Scholar]
- Dietary trans-fatty acid induced NASH is normalized following loss of trans-fatty acids from hepatic lipid pools. Lipids. 2012;47:941-50.
- [Google Scholar]
- Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: A randomized trial. JAMA. 2010;304:1795-802.
- [Google Scholar]
- Treatment of obesity by very low calorie diet, behavior therapy, and their combination: A five-year perspective. Int J Obes. 1988;13(Suppl 2):39-46.
- [Google Scholar]
- Competing dietary claims for weight loss: Finding the forest through truculent trees. Annu Rev Public Health. 2005;26:61-88.
- [Google Scholar]
- When prevention fails: Obesity treatment strategies. Am J Med. 2009;122(Suppl 1):S24-32.
- [Google Scholar]
- Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623-30.
- [Google Scholar]
- Treatment of nonalcoholic fatty liver disease: Role of dietary modification and exercise. Clin Liver Dis (Hoboken). 2012;1:117-8.
- [Google Scholar]
- NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-85.
- [Google Scholar]
- Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485-90.
- [Google Scholar]
- Pathogenesis and therapeutic approaches for non-alcoholic fatty liver disease. World J Hepatol. 2014;6:800-11.
- [Google Scholar]
- A phase 2, randomized, double‐blind, placebo‐controlled study of GS‐9450 in subjects with nonalcoholic steatohepatitis. Hepatology. 2012;55:419-28.
- [Google Scholar]
- Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678-93.
- [Google Scholar]
- The potential role of prebiotic fibre for treatment and management of non‐alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int. 2012;32:701-11.
- [Google Scholar]
- Centre for Public Health Excellence at NICE (UK); National Collaborating Centre for Primary Care (UK). National Institute for Health and Clinical Excellence: Guidance. Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London: National Institute for Health and Clinical Excellence (UK), National Institute for Health and Clinical Excellence: Guidance; 2006.
- [Google Scholar]
- Non-alcoholic fatty liver disease: A practical approach to treatment. Frontline Gastroenterol. 2014;5:277-86.
- [Google Scholar]
- Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: The BLOOM‐DM Study. Obesity (Silver Spring). 2012;20:1426-36.
- [Google Scholar]
- Liver: Does bariatric surgery reduce the severity of NAFLD? Nat Rev Gastroenterol Hepatol. 2010;7:11-3.
- [Google Scholar]
- Lifestyle, diabetes and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683-93.
- [Google Scholar]
- Effects of weight loss induced by bariatric surgery on hepatic adipocytokine expression. J Hepatol. 2009;51:765-77.
- [Google Scholar]
- Effects of bariatric surgery on nonalcoholic fatty liver disease: Preliminary findings after 2 years. J Gastroenterol Hepatol. 2007;22:510-4.
- [Google Scholar]
- Bariatric surgery in patients with liver cirrhosis. Surg Obes Relat Dis. 2013;9:1-6.
- [Google Scholar]
- Liver transplantation for subacute hepatocellular failure due to massive steatohepatitis after bariatric surgery. Liver Transpl. 2008;14:881-5.
- [Google Scholar]
- Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:897-901.
- [Google Scholar]
- Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010;20:CD007340.
- [Google Scholar]
- Bariatric procedures: An update on techniques, outcomes and complications. Curr Opin Gastroenterol. 2013;29:684-93.
- [Google Scholar]
- Measurement of free testosterone in normal women and women with androgen deficiency: Comparison of methods. J Clin Endocrinol Metab. 2004;89:525-33.
- [Google Scholar]
- Catheter-directed gastric artery chemical embolization for modulation of systemic ghrelin levels in a porcine model: Initial experience. Radiology. 2007;244:138-43.
- [Google Scholar]
- Ghrelin suppression and fat loss after left gastric artery embolization in canine model. Cardiovasc Intervent Radiol. 2012;35:1460-6.
- [Google Scholar]
- Bariatric embolization for suppression of the hunger hormone ghrelin in a porcine model. Radiology. 2013;266:471-9.
- [Google Scholar]
- Endocrine regulation of energy metabolism: Review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50:1511-25.
- [Google Scholar]
- Clinical perspectives for ghrelin-derived therapeutic products. Endocr Dev. 2013;25:157-66.
- [Google Scholar]
- First-in-man study of left gastric artery embolization for weight loss. J Am Coll Cardiol. 2013;61:E2056.
- [Google Scholar]
- A preliminary observation of weight loss following left gastric artery embolization in humans. J Obes 2014 2014 185349
- [Google Scholar]
- Bariatric embolization for suppression of the hunger hormone ghrelin in a porcine model. Radiology. 2013;266:471-9.
- [Google Scholar]
- Angiotensin-receptor blockers as therapy for mild-to-moderate hypertension-associated non-alcoholic steatohepatitis. World J Gastroenterol. 2009;15:942-54.
- [Google Scholar]
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2012;56:908-43.
- [Google Scholar]
- Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J J Gastroenterol. 2009;44:1190-4.
- [Google Scholar]
- Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938-46.
- [Google Scholar]
- Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167-74.
- [Google Scholar]
- Hepatocellular carcinoma: Management of an increasingly common problem. Proc (Bayl Univ Med Cent). 2008;21:266-82.
- [Google Scholar]
- Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-53.
- [Google Scholar]
- Recurrent disease following liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Transpl. 2009;15:1843-51.
- [Google Scholar]
- Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or nonalcoholic fatty liver disease. Liver Transpl. 2010;16:431-9.
- [Google Scholar]
- A case-controlled study of the safety and efficacy of transjugular intrahepatic portosystemic shunts after liver transplantation. Liver Transpl. 2011;17:771-8.
- [Google Scholar]
- Diagnosis and treatment of arterial steal syndromes in liver transplant recipients. Liver Transpl. 2003;9:596-602.
- [Google Scholar]
- Interventional radiology in living donor liver transplant. World J Gastroenterol. 2014;20:6221-5.
- [Google Scholar]
- Transjugular intrahepatic portosystemic shunts in liver transplant recipients: Technical analysis and clinical outcome. AJR Am J Roentgenol. 2013;200:210-8.
- [Google Scholar]
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
- [Google Scholar]






